Soil granulometric composition can impose constraints on ant species living in ground habitats, being an important factor in defining the habitat templet, which describes how certain animal life histories, including the trait of body size, can be selected. The ant fauna plays a central role in soil formation, and a vast literature describes such influence, but not the converse. Along with termites, worms and other invertebrates, these organisms promote the formation of channels, pores, and aggregates that influence gases and water moving through the soil profile. On the other hand, it is important to understand whether soil traits constrain insect colonization, so we here ask how soil traits can influence niche specificities, which seems to be a neglected ecological issue. A literature search using the key words ‘ants or Formicidae’ and ‘soil structure or pedogenesis’ revealed numerous references dealing with the influence of ants on soil, but not conversely. We here present a novel geomorphologic approach to habitat templets for two distinct riparian Neotropical ecosystems, based on the amalgamation of soil/sediment analysis with ecological processes and ant species biology. We found that predominance of fine grains favoured the preponderance of small ant species at a threshold of <5mm in body length. Based on this, we propose the use of a quantitative, theoretically sound, statistical approach to bioindication.
Since Hutchinson's classical consideration of evolution as a play performed in the ecological theatre (1965), ecologists have tried to incorporate any type of role within the species and forcing an adaptative explanation, not always valid, to their interactions. However, whether neutral and random (Strong et al., 1984; Hubbell, 2001), or a product of directional evolutionary forces imposed by interspecific competition (Cody and Diamond, 1975’s first and second rules of competition and community structuring), species composition and species relative densities are fingerprints of the environment. As such, species and population traits may lead to a practical use, namely bioindicating the existence of certain conditions in ecosystems or their habitats. It follows that species community parameters should reflect ecosystem health and integrity. The baseline for this approach comes from Southwood's (1977) Presidential Address to the British Ecological Society, when he speculated whether habitat would be the “templet for ecological strategies”. In other words, he elegantly explained how habitats constrain and define what kind of community must be found in them, based on species specificities.
Further explored by Greenslade (1983), the habitat templet concept was used to better explain life history distributions in nature, and was refined to a simplistic r- to k- to A (for Adversity) strategies triangle. This theoretical framework should be an obvious foundation for the concept of bioindication, since by monitoring what species are found in certain habitats, one could provide a rapid diagnosis of impacts on these habitats. The absence of expected species, or the presence of opportunistic, unexpected species are both facts related to disturbance, and the monitoring of such species for bioindication is likely to be cheaper and quicker to carry out than a full environmental diagnosis.
Despite this promising idea, the principles of the habitat templet have not been widely adopted as baseline for applied studies of species populations or communities, except in the case of benthic aquatic fauna (Marques and Barbosa, 2001; Marques, 2004). There are two possible reasons for this. On the one hand, the idea of a habitat templet erroneously assumes constancy in the abiotic conditions, and does not consider the counter effect of, for instance, how a species can change the habitat during natural succession. Neither does it consider which species cause the most definitive changes in habitat conditions (Jones et al., 1997). We acknowledge that the difficulty of obtaining data on abiotic and biotic matching conditions renders a direct theoretical approach less palatable for applied ecologists. Still, none of these impediments should hinder attempts to incorporate the habitat templet concept into bioindication procedures.
Members of the soil fauna are among the most diverse components of an ecosystem (Vargas and Hungria, 1997). The soil macrofauna, including ants, spiders, termites, earthworms and others (Bachelier, 1978; Berthelin et al., 1994; Lavelle et al., 1994) are representatives of key functional guilds. Through digging, foraging, building nests, galleries, chambers and corridors, or transporting excavated soil materials, they redistribute organic and inorganic matter across the soil profile. It follows that the macrofauna can be used as a bioindicator of soil quality, since it plays an important role in the regulation of pedogenetic development (Brussard, 1998).
Likewise in a species-engineering (Jones et al., 1997) or a habitat templet (Greenslade, 1983) approach, ants, amongst all taxa, ought to be taken into account when considering soil traits that select species occurrence. However, soil fauna is not always considered in studies of soil beyond a description of community patterns (Andersen and Majer, 2004). Indeed, as far as we know, beyond our work only Yanoviak and Kaspari (2000) considered ant species traits (namely body size) based on habitat templet effects on competition between species. Notwithstanding this, invertebrates are widely used in impact and environmental restoration studies, especially in a bioindicative approach to human impacts (Abbott et al., 1979; Majer, 1983; Pérès et al., 2011; Simmons et al., 2015).
We here review disturbance in soil structure and the usage of ant assemblages as bioindication tools for diagnosing changes in habitats. Then, we illustrate the use of such an approach using two Brazilian examples of ant assemblage response to soil impacts. Finally, we discuss the relevance of adopting a theoretical approach for applied soil ecology studies, based on the assumption that soil granulometry is a key habitat filter, and thus a component of the habitat templet for soil fauna.
Disturbance and soil structureThe soil originates from rock degrading processes or structural rearrangement of a previously transformed matter that composes the earth's surface (Castro, 2008). These changes are caused by chemical, physical and biological factors that influence the rock matrix and cause soil horizon shaping, and thus, heterogeneity due to variations in mineralogical composition, colour, texture, and/or structure (Beljavskis and Juliani, 1986).
The mineral portion of the ground is composed of particles of various dimensions and arrangement in space. These sands, muds and gravels comprise individual particles that, along with organic matter, air and water, constitute what we know as ‘soil’. Determining the granulometric distribution of size and proportions of constituent particles is the basis for accurate description of soils. These traits directly affect physical processes taking place in soil, such as transport and deposition, porosity and permeability (Suguio, 1980). It is through knowledge of granulometric distribution that one can infer about the potential for compaction, aeration, transmission of heat, infiltration and water distribution in soil (Prevedello, 1996). Soil traits related to water retention and nutrient availability to plants are major components of edaphic stratification (Silva et al., 2005) and are essential for the diagnosis of soil functioning as well as effects of disturbance.
Various distinct soil features may appear after disturbance. Soils that have recently been exposed or impacted by activities such as mining may have a high infiltration capacity, since their grain structure becomes disarranged. However, if exposed for a long period, soil aggregates are broken up by the kinetic energy of raindrops when they touch the soil surface. These separated particles may form a dense crust a few millimetres thick on the soil surface, further reducing infiltration of water, transmission of heat and gas exchange between soil and atmosphere, and also impacting nutrient cycling (Al-Durrah and Bradford, 1982; Le Bissonais et al., 1989). The compaction process results in decreasing roughness and volume of macropores, thus increasing the density of the sediment (Leite et al., 2004). Soil macro- and micronutrients at the surface become blocked by the thin and compact crust and will not be washed down to the deeper levels. Due to this process, during the rainy season nutrients are leached and over time the process may result in soil nutrient impoverishment, which leads to slow or stagnated natural succession, an issue that may impede habitat restoration.
In disturbed areas, ants contribute to soil recovery, as they promote changes in chemical and physical properties, making soils richer in available nutrients such as nitrogen, phosphorus, potassium, magnesium and calcium (Carlson and Whitford, 1991; Wagner et al., 1997; Lafleur et al., 2005). Furthermore, they mix soil grains by the bioturbation process (Hole, 1961; Lavelle, 1993; Pielström and Roces, 2013). The galleries formed by ants are particularly important in environmental recovery, even after the colony has been abandoned. The network of galleries (or biopores) connecting nest chambers has a significant influence on water infiltration, on soil aeration process and on root penetration, particularly in areas where the upper layer of the soil is compacted (Lobry de Bruyn and Conacher, 1994; Nkem et al., 2000). In ecosystem rehabilitation projects, such as after mining or other severe types of long term disturbance, ants occupy an important role in the regulation of vital processes in soil, creating channels, pores, and aggregates (Coutinho et al., 2003; Lavelle et al., 2006), increasing fertilization (Wu et al., 2013) and causing protection from erosion (Hawksworth, 1991; Doran et al., 1994).
Bioindication by antsChanges in natural habitats may be detected by monitoring bioindicator species or functional guilds, which consist of those populations (or assemblages of species) that reflect the conservation status of the biotic and abiotic environment. In other words, a taxonomic bioindicator group can reflect changes in impacted habitats, communities or ecosystems, as their ecological requirements change. The more they are representative of overall community diversity and of distinct ecological functions, the better indicators they are (McGeoch, 1998). Ants are considered excellent bioindicators because they are an extremely abundant group, with relatively high numbers of species that are taxonomically treatable, with many specialist species occupying different trophic levels and niches, and many of those species react promptly to changes in the environment (Majer, 1983). Ant diversity is influenced by the diversity and density of plant species (Basset et al., 2012; Queiroz et al., 2013), vegetation physiognomy (especially stratification – Costa-Milanez et al., 2014), as well as by abiotic factors such as temperature, humidity, and soil composition (Cardoso et al., 2010; Costa et al., 2010; Philpott et al., 2010).
The concept of bioindication, though, has not been considered in relation to other ecological theories, some of which are essential for defining a proper role of species in a community. In order to reach a bioindicative conclusion, one must delimit a certain set of habitat conditions into which several niches can be accommodated, thus producing a templet of habitats where certain species should be found (Southwood et al., 1974; Greenslade, 1983; Yanoviak and Kaspari, 2000). For instance, the lack of expected species or functional guilds probably indicates some unperceivable impact that may have removed those species from their position in the templet. Hence, not just to have some species, but to have them in greater or smaller densities than expected, or to be not there at all, are ways to characterize an impacted ecosystem.
Furthermore though, it is necessary to consider the functional role of these species and guilds. Ants, for instance, serve perfectly to illustrate the concept of engineer species, as coined by Jones et al. (1997). These qualities as ‘engineers’ make ant assemblages important components for building bioindication tools. In this sense, soil-dwelling ants ought to be considered when attempting to understand soil protection or restoration after impacts such as those caused by mining. More than just reflecting good or bad conditions within a habitat, ants will change soil habitats into a better successional state, and thus are also predictable causes of changing directions in the qualitative state of the ecosystem.
Many studies have used ants as bioindicators of natural succession after human impacts, such as mining (Majer, 1983, 1984; Majer and Nichols, 1998), soil contamination by metals (Ribas et al., 2011), establishment of Eucalyptus monocultures (Costa-Milanez et al., 2014), or natural impacts including fire in the cerrado, which has increased in frequency over recent years (Frizzo et al., 2012; Caut et al., 2014; Andersen et al., 2014; Calcaterra et al., 2014; Anjos et al., 2015; Costa-Milanez et al., 2015). However, most of these studies have not dealt with how the ground dwelling ant assemblages are affected by impacts on soil physical traits, such as texture. The next section explores what is presently known on ants’ species responses to soil traits in natural conditions, and presents our most recent results on how subtle soil changes caused by impacts could be assessed based on soil ant species relative densities.
How soil modification can affect ant assemblagesTwo of our studies, one in riparian vegetation impacted by dredging in the Jequitinhonha River basin, and the other in the ‘veredas’ wetlands in the Brazilian Savanna, have investigated how soil granulometric distribution after disturbance affects the presence of ants. (Costa et al., 2010; Costa-Milanez et al., 2014; PhD unpublished data). In both studies, we found a significant positive relationship between the proportion of fine and very fine sand grains with the density of small ants (ant species with a body size smaller than 5mm). Here, we develop a rationale to support the hypothesis that ant body size is a life history trait that is likely to be useful for the bioindication of soil quality and ecological succession.
Soil ant nest and subterranean gallery construction are influenced by the ability to extract soil particles with the ant's mandibles, legs and psammophore (in some members of the Dolichoderinae, Formicinae and Myrmicinae), as they carry the load taken from the soil to another place (Fig. 1D). Camponotus punctulatus Mayr 1868, for example, can relocate 2100kgha−1 of soil during the construction process of its colony mounds (Folgarait, 1998), and Atta sexdens Linnaeus 1758 constructs its nest over an area of approximately 100m2, occupying a volume of 23m3, with almost 40 tonnes of soil (Autori, 1947).
However, if large-body sized soil ants, such as C. punctulatus and A. sexdens, are favoured by coarse grains, the same may be not true for small size ants, unable to manage large fragments. On the other hand, by having very small body sizes, some ant species could benefit by their potential ability to collect some hard-to-find resources, as well as to hide from harsh micro-climatic conditions in shelters found among the interstitial spaces of a sandy, very fine soil (Kaspari and Weiser, 2007). Besides, due to fine soil constraining traits against the presence and movement of large ant species, this very specific habitat component may result in an enemy-free space to the small ant species.
The soil is normally covered with leaves, twigs and other obstacles that can lead to the formation of a complex three-dimensional environment (Yanoviak and Kaspari, 2000). Ants with large body sizes can cross obstacles more easily than ants with small body sizes, and the latter may eventually choose to make their journeys through the subterranean interstitial spaces. Therefore, large ants usually recruit for foraging in the litter, while small ants recruit for foraging in the channels built in the ground and underneath the litter found in certain habitats (Costa et al., 2010).
The relationship between soil texture and ant body size not only influences the process of nest foundation and establishment in certain soil textures, as discussed in Kaspari and Vargo (1995), but also influences the survival rate of colonies. Soils with a high clay concentration have higher capacity to retain water, reducing the risk of desiccation of ants. In the early stages of the colony, desiccation is one of the factors that results in colony mortality (Johnson, 2000).
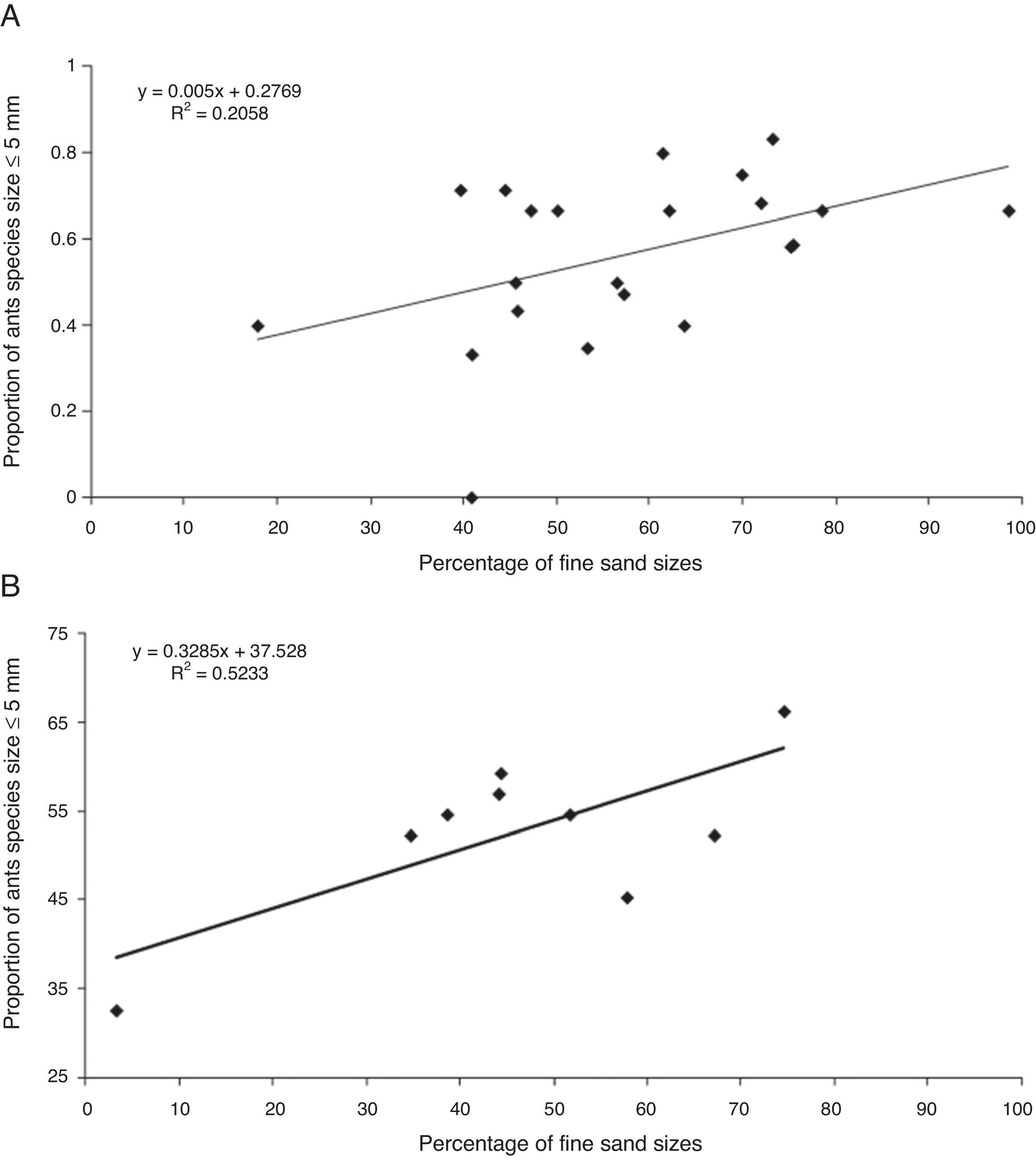
In the Jequitinhonha River study (Costa et al., 2010), which was undertaken in a transitional area from Brazilian savanna (cerrado) to semi-arid deciduous forests, the region also contains semi-deciduous forests (Fig. 1A and B). A diamond mining company dredged the bed and banks of the upper basin of the River over a distance of 11km. Before dredging the sediment bed, the surface soil was saved for later use in restoration. River banks are covered with several native tree and shrub species, such as Pilosocereus gounellei (xique-xique), Acacia alata (acacia), Tabebuia chrysotricha (ipê amarelo) and Solanum sp., amongst others (Ribeiro, 2002). Between 1991 and 1995 a restoration project was implemented in the study area and 10 years later we selected seven locations within the restored sites and two undisturbed areas (controls) to investigate the effect of sedimentary grain size on ant species diversity and abundance. Ant sampling was conducted in April 2005, using three methods, namely baits, pitfall traps, and hand collections. An increase in the proportion of small ant species of 5mm or less body length with the increase in percentage of fine grains in the sediment was observed (Fig. 2A – Table S1). This relationship very neatly explained the disproportionate occurrence of workers of small species, such as Dorymyrmex pyramicus Roger 1863, but the predominance of fine grains did not explain overall ant species richness and abundance. Dorymyrmex pyramicus is typical of open environments, impacted areas and is less sensitive to human disturbance (Santos et al., 1999; Costa et al., 2010). The zones where this species dominated had a slow rehabilitation recovery rate because of their proximity to Jequitinhonha river, which suffers from the direct influence of floods during the rainy season (Costa et al., 2010). The effects of flooding were significant but were also confounded by the underground water level, which may inhibit tree root development and ant colonization.
This result highlights a problem in the restoration methods used after dredging. The relocation of alluvial sediments resulted in large changes in soil granulometric makeup to a state that was quite different to how it was before the dredging (Costa et al., 2010). Most of the grains in the restoration area were between 0.2 and 0.06mm (fine sand and very fine sand classes) and were largely composed of quartz. The variation in ant species among the studied areas could indicate a differentiation in the distribution of resources and habitat structure, which conforms to our suggested habitat templet-niche response pattern (Southwood et al., 1974; Greenslade and Greenslade, 1984; Yanoviak and Kaspari, 2000). In particular, granulometric composition and soil structure seems to favour foraging and nesting by different sized ants (Farji-Brener et al., 2004). Because of this, if certain soil conditions constrain ant colonization, a negative feedback process may result in the further stagnation of succession. Examples of ants found in the study that were absent in soils with substantial accumulation of fine sand quartzes include Atta sexdens rubropilosa Forel 1908, Nomamyrmex esenbeckii Westwood 1842, and Ectatomma permagnum Forel 1908. Both A. sexdens rubropilosa and N. esenbeckii were sampled in undisturbed areas and the former species requires some preserved and structured soil to maintain the colony (Moutinho et al., 2003). Furthermore, it forms the main dietary component of N. esenbeckii, which is a subterranean species (Souza and Moura, 2008). Ectatomma permagnum is an aggressive epigaeic predator that builds small nests in soil (Brandão et al., 2012). The nesting behaviour of these large ants is underground, so the proportion of fine grains and the fact that groundwater near the surface may not favour colonization is these environments, thus impeding the development of a more complex food web.
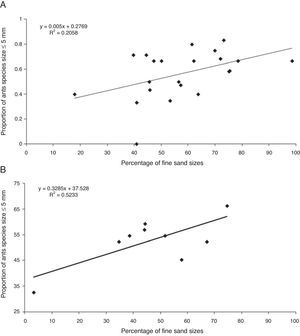
In the veredas wetlands study, Costa-Milanez et al. (2014) investigated the response of ant species to changes in wetland and cerrado ‘sensu stricto’ soil after introduction of Eucalyptus monoculture. The Brazilian savanna (cerrado) wetlands, so called veredas, are headwaters of shallow drainages. This ecosystem is formed on sandy soils with high concentrations of peat (Eiten, 1994; Alencar-Silva and Maillard, 2007), and is composed of soils of very fine particle size which is well structured and channelled due to large amounts of decaying organic matter. These characteristics create wet, black sediment that is colonized by hydrophilic grasses (Fig. 1C). However, during the years 1970–2000, 44% of natural Brazilian savanna, including that surrounding veredas, was replaced by Eucalyptus monoculture plantations. Such plantations tend to worsen the local water deficit, soil fertility and pH, as well as decreasing native fauna biodiversity (Souza et al., 2006; Alcides and Pereira, 2007). The fieldwork was carried out in two reasonably well-preserved veredas (control locations) and in two disturbed veredas (modified locations: one surrounded by a 3-year old Eucalyptus plantation and other surrounded by a 5-year old Eucalyptus plantation). Ant sampling was conducted in May 2010 (dry season) using three methods, namely baits (soil and arboreal), pitfall traps (soil and arboreal), and hand collections. In our study, there was a clear contrast between areas with greater proportions of coarse grain soil (in wetlands) versus areas where fine grains naturally occur (fine sand and very fine sand), such as in Eucalyptus and cerrado habitats (Costa-Milanez et al., 2014). Here, a significant positive correlation was found between the density of small ant species in areas where the proportion of fine and very fine grains was detected (Fig. 2B – Table S1). This relationship explained the occurrence of small, often aggressive species in the cerrado, such Dorymyrmex jheringi Forel 1912 and Solenopsis invicta Buren 1972, along with a disproportionate occurrence of Pheidole gertrudae Forel 1886 in the Eucalyptus habitats. Apparently, the natural granulometric condition, which is a templet for small ants, could be disturbed after plantation establishment, thus intensifying its filtering effect on the ant species assemblage. This modification allowed large colonies of the most aggressive ant species, such as Solenopsis invicta and Linepithema humile Mayr 1868, to colonize these areas which, as a result, lower ant species diversity.
The whole process of preparing the plantation promotes changes that may be favourable for some species but not for others. Ants of approximately 5mm in size or less were favoured in areas with a higher proportion of fine grains, as exemplified by P. gertrudae in the Eucalyptus habitat. Over to four years of study, 69% of sampled ants belonged to the genus Pheidole. The small body sized ant species of this genus nest in diverse locations, including soil, litter or trunks, and have large colonies with massive recruitment (Lizidatti, 2006). All of these characteristics make them effective in colonizing vacant habitats and excluding competitors. Indeed, the dominance of this species was disproportionate, indicating high disturbance.
In contrast, some authors have found that wetland habitats had less fine grains due to the accumulation of organic matter. The organic material makes the sediment/soil lumpy, maintaining the moisture in the spaces between the grains. These granules form agglomerates by the process of agglutination, or by contraction/relaxation of soil muds. Curiously, in more mature Eucalyptus monocultures, the soil tends to evolve into this direction, and this process is further accentuated by the reduction in the groundwater level, which results in more and bigger agglomerates (Viana, 2006; Melo, 2008). As a result, plantations established on cerrado soils will end up with a structure most unlike, and coarser than the original. After a substantial period of time, the number of soil ant species and the average ant size may increase in Eucalyptus plantations but, in this case, this indicates a further soil change towards a even more distinct, although more diverse, habitat templet. The possibility of using the same assumptions of niche-habitat adjustments based on the habitat templet model to detect novel ecosystems seems to us to be an even stronger argument in favour of establishing a serious theoretical fundament while applying the bioindication concept.
Concluding remarksHere we have presented results from two independent locations, so the generalities of what we are suggesting need further investigation in other habitats and Palaeo-regions (for instance, Australasian ant fauna may not have such small species in opportunistic guilds with dominant abundances, and Rhytidoponera may replace Brachymyrmex). However, we suggest that a complete or partial change in the soil/sediment particle size is an important determinant of the ant species distribution patterns, which could therefore be used as a tool for the biomonitoring of degraded areas, at least for riparian and ecotone forest-freshwater communities. Further, the data discussed here may clarify the potential for using ants as bioindicators in processes of change in soil texture in various situations, as diverse as mining and planting of Eucalyptus monocultures. However, it is important to note that this tool is fundamentally ecological, and is therefore quantitative and theoretically sound. An important aspect for the construction of this analytical tool is the amalgamation of soil/sediment analysis with ecological processes and ant species biology. Also, we see an urgent need to consolidate a deeper, theoretically sound approach to the use of bioindication. This urgency has implications for the increasing unpredictability in the climate and land use change. Likewise, quite specifically for the Minas Gerais State where these studies were conducted, there is an urgency to improve bioindication tools as a response to the recent worst river disaster on Earth (the Samarco's mud flood on the whole Doce river basin until the sea), traversing this and also Espirito Santo State.
Conflict of interestThe authors declare no conflicts of interest.
We thank Rodrigo Feitosa for identifying the ants sampled in this project. Two anonymous referees, plus Grazieli Dueli and Brian Heterick improved the manuscript. The Financial support for CB Costa-Milanez was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq. SP Ribeiro and PTA Castro are granted researcher by CNPq. IBAMA provided the collect permits.