Small forest fragments may play a major role in fragmented areas, but there is scarce empirical data to test this hypothesis. To understand in which context birds can use small Atlantic Forest fragments, we tested the presence of 11 bird species in 30 small fragments (4–10ha), in a range of matrices (eucalyptus-pasture), and in different landscape configurations. The results showed that landscape composition is a good predictor for presence of birds in small fragments and their use can be further associated with matrix type. Considering the number of species, and the species Chiroxiphia caudata, we found a pattern in which models that consider the matrix composition are the most plausible. Relative importance of the variables indicates that matrix is the most important single variable among the selected species (five among eight). This suggests that small fragments are effective for increasing connectivity, mainly in landscapes with a higher percentage of permeable matrix.
Habitat loss and fragmentation are the most important threats to biodiversity (Fahrig, 2003; Fischer and Lindenmayer, 2007). These threats are generally anthropogenic, and result in reduction of native vegetation, low landscape connectivity and habitat isolation. Additionally, species more sensitive to the effects of fragmentation become more susceptible to environmental and demographic stochasticity (Fahrig, 2003). As well, intra- and interspecific competition for resources are affected (Fischer and Lindenmayer, 2007), genetic variability decreases and long-term metapopulation persistence is reduced (Hanski and Gilpin, 1997). All of these processes may cause local extinctions.
Landscape connectivity can influence the dynamics of species in fragmented environments. Increasing habitat fragmentation leads to increased distances between the patches, and when the matrix is impermeable reduces the functional connectivity (Baum et al., 2004). Thus, the landscape composition can play a role facilitating or impeding the species’ movements depending of the species’ capacity to use the landscape structures (Baum et al., 2004). Conversely, to reduce the effects of fragmentation is to improve connectivity between fragments, through forest corridors, stepping-stones (Baum et al., 2004) or small patches, although this last approach as a connecting structure is still poorly understood (Turner and Corlett, 1996; Renjifo, 2001; Schleuning et al., 2011).
Fragments smaller than 20ha are generally unable to support viable populations of birds in the long-term given the scarcity of resources (Bierregaard and Lovejoy, 1989), but they can reduce the functional distances between larger habitat remnants (Ribeiro et al., 2009). These forest fragments may also benefit species able to cross the inter-habitat matrix (Fischer and Lindenmayer, 2002) and migratory birds, by providing temporary shelter and food (Robbins et al., 1992). The use of small remnants may be strongly influenced by the landscape context and how the species perceive different landscape elements (Uezu et al., 2008). Moreover, in fragmented areas a matrix composed of more complex structures, such as exotic plant species, can facilitate species movement in the landscape compared to open areas (e.g. pasture), even for less sensitive species (Renjifo, 2001). Thus, in more connected landscapes (Baum et al., 2004) or in a matrix with higher permeability (Uezu et al., 2008), small fragments may play a major role, although there is still scarce empirical data to test this hypothesis.
Our study focuses on the value of small Atlantic Forest fragments for birds in anthropogenic landscapes. The Atlantic Forest is one of the most threatened biodiversity hotspots (Myers et al., 2000), and has a total of 217 bird species are endemic to this biome and at least 98 species are threatened by extinction (Bencke et al., 2006). We expect that birds use small fragments more when the fragments are connected and embedded in a more permeable matrix. Our aim is to understand in which landscape context, level of connectivity, matrix type and percentage of forest, the small fragments (4–10ha) are being used by 11 bird species.
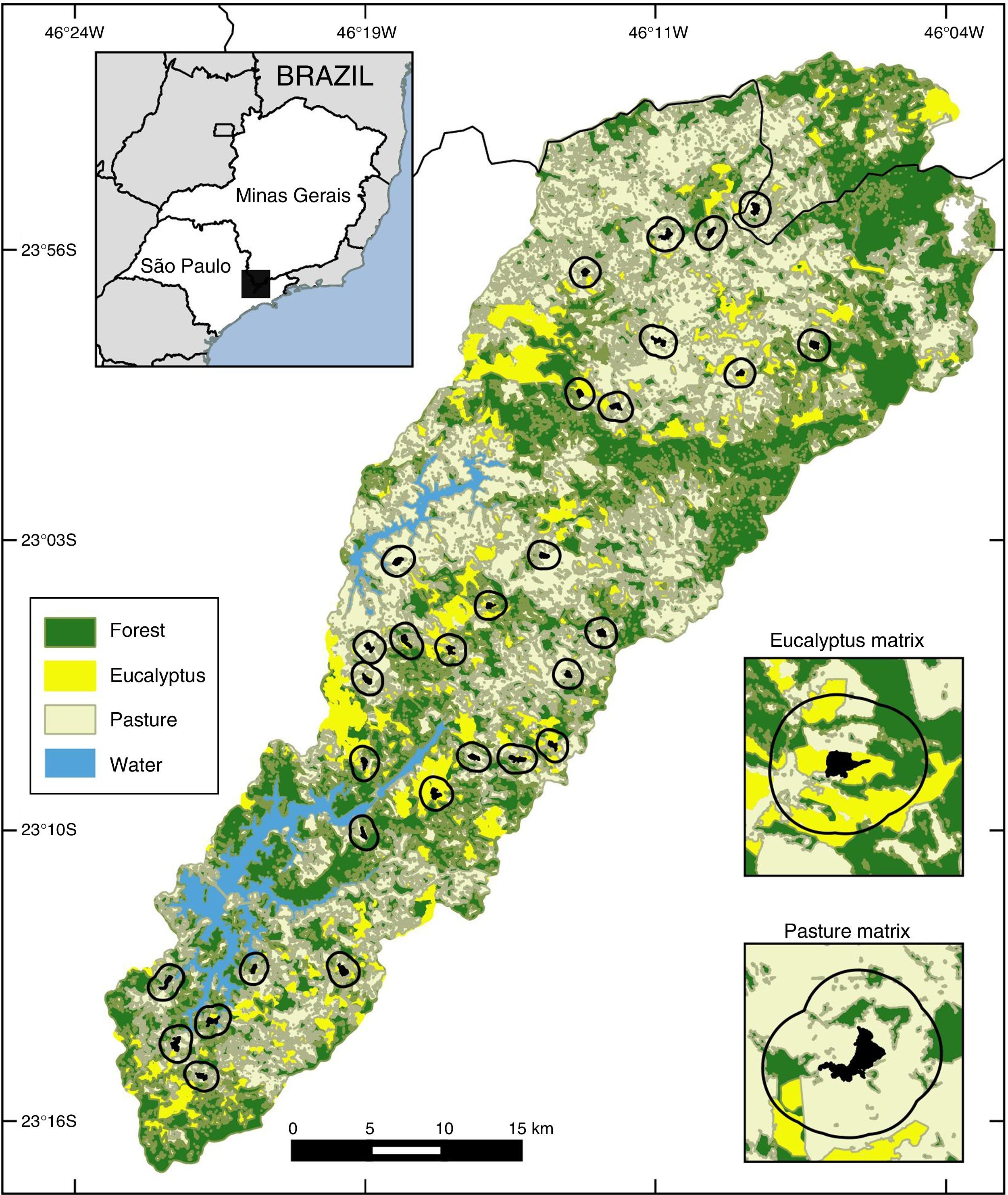
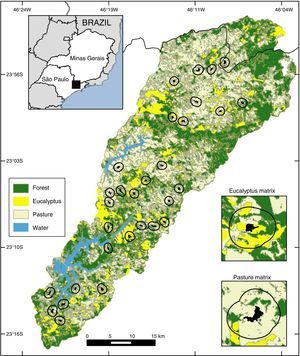
Materials and methodsThe study was conducted in the Atlantic Forest (ombrófila densa type) in the Cantareira-Mantiqueira mountainous corridor, specifically in the Atibainha and Cachoeira watersheds – Nazaré Paulista, Piracaia and Joanópolis municipalities (São Paulo state), and Camanducaia municipality (Minas Gerais state). Altitudes are 700–2000m with a wet season during September–March and a drier season during April–August. We mapped the region using ArcGIS and a mix of hi-resolution images (WorldView, QuickBird, OrbView) from 2010 to 2011 into four categories: pasture, water, forests and eucalyptus plantations. The land cover map coupled with field observations allowed us to identify that approximately 33% of the Atibainha and Cachoeira watersheds landscape were comprised of Atlantic forest (mainly in secondary forest) and 27% of commercial plantations (mainly eucalyptus).
Landscapes were defined using 500m buffers around the small forest fragments (Fig. 1). We selected 30 small fragments, embedded in a gradient of two types of non-habitat (pasture and eucalyptus matrix) and in different percentages of forest cover. Ten of these small fragments were connected to other fragments by narrow forest strips of less than one hectare, and 20 were not connected (open areas under 5m were disregarded). The vegetation of the selected forest fragments shows intermediate and advanced successional stages with similar internal structures at altitudes between 800 and 1080m.
We selected 11 forest bird species representing a wide range of species that occur in the region, having dissimilar diet, stratum habitat, home-range sizes, abilities to move through the matrix and sensitivities to habitat loss and fragmentation (Stotz et al., 1996). Moreover, selected species are not rare, endangered or migratory and exhibit a territorial behavior responding to playback, which could influence the chances of detecting these species in small forest fragments (see supplementary data Table S1 for more information on the species). Previous visits were made to three forests fragments (>100ha) to confirm the presence of the species in the region.
Atlantic Forest pre-recorded vocalizations collected by the authors were used in playback experiments at central points inside each forest fragment, and at least 50m from the forest edge. For each species, the playback was reproduced three times with 1min intervals in between, followed by at least 30s of silent observation (Boscolo et al., 2006). We considered the species absent when neither a spontaneous vocalization nor a response after playbacks was heard or the species seen. The study was conducted in the breeding season (September–November) of 2011, with three visits to each sample area (at least 15 days between samplings), from 6am to 12pm on non-rainy days (Boscolo et al., 2006). During the breeding season, birds are more active in defending territories and, thus, respond better to playbacks.
To identify which characteristics of the landscape (percentage of each matrix type [pasture or eucalyptus], percentage of forest, connected or not connected, and fragment area) explains the species presence or total number of species in small patches. We created 26 regression models, with one or multiple variables (combining two or more variables) and a null model using generalized linear models (Table S2). The null model represents the uncertainty of the selected variables to the presence of the species. Each model represents a hypothesis on how the species respond to the patterns of the fragmented habitats. For model selection, we used the Akaike's Information Criterion adjusted for small samples – AICc (Burnham and Anderson, 1998) in the program R. The index of type of matrix was calculated through the formula based on the land cover map:
Thus, negative numbers indicate that there is more pasture than eucalyptus and positive numbers more eucalyptus than pasture in the landscape, so 1=100% eucalyptus matrix, −1=100% pasture matrix and zero=fifty-fifty. We used the weight of the variable to see which one is more relevant for the presence of the species or for the number of species in small patches, so we summed the weight of all models in which the variable appeared (Burnham and Anderson, 1998).
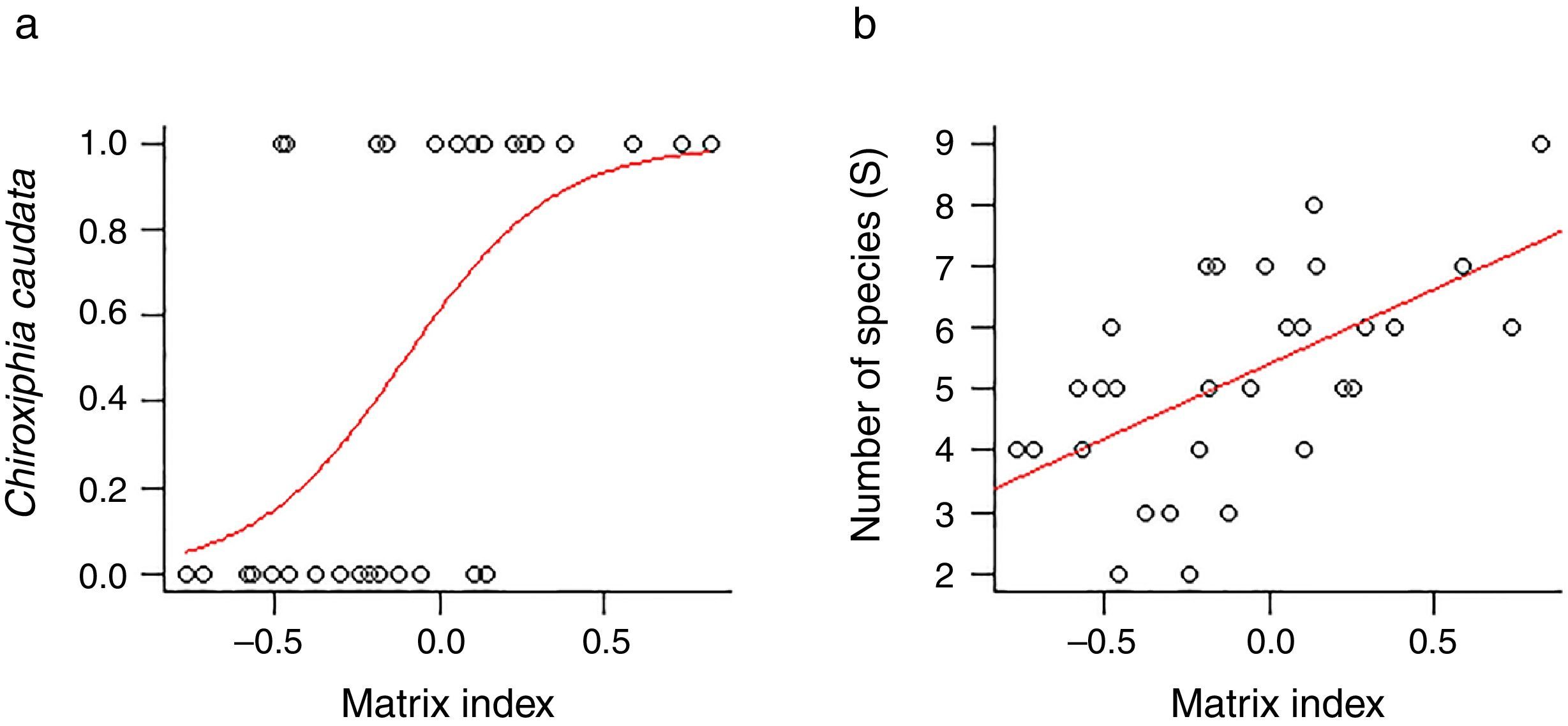
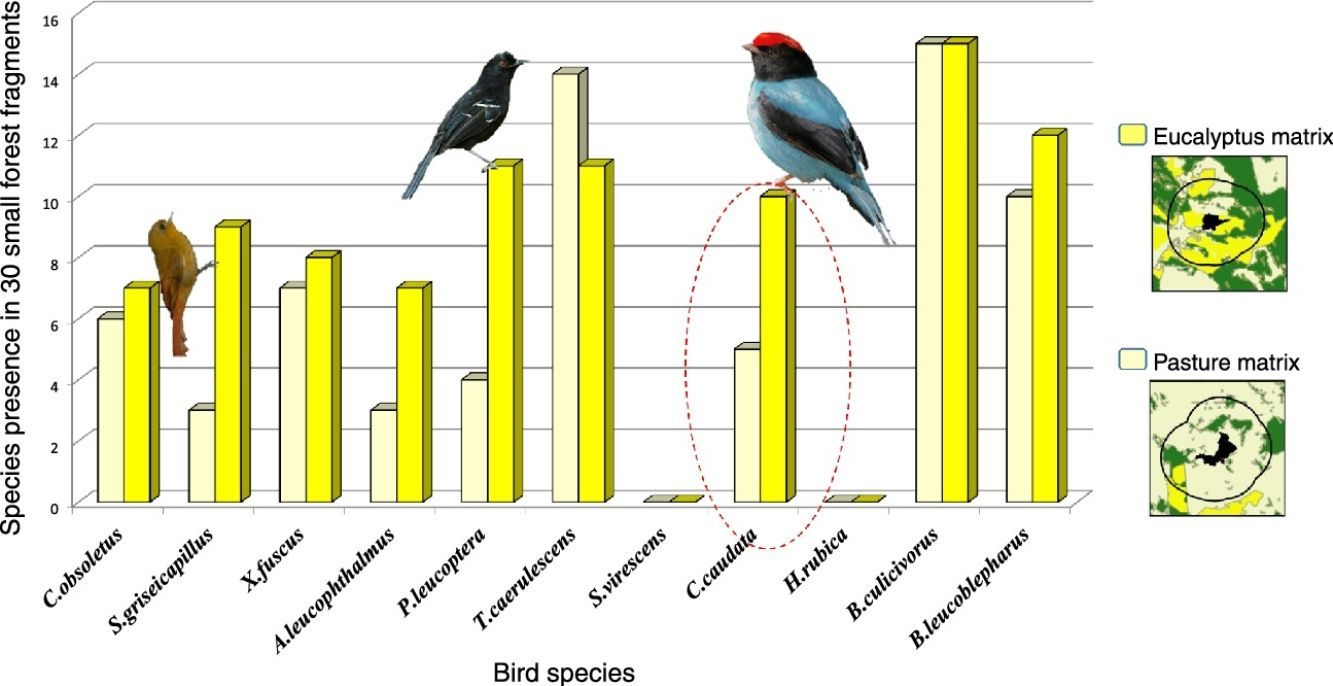
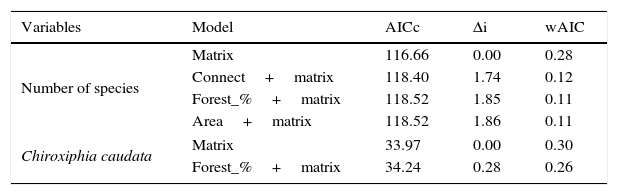
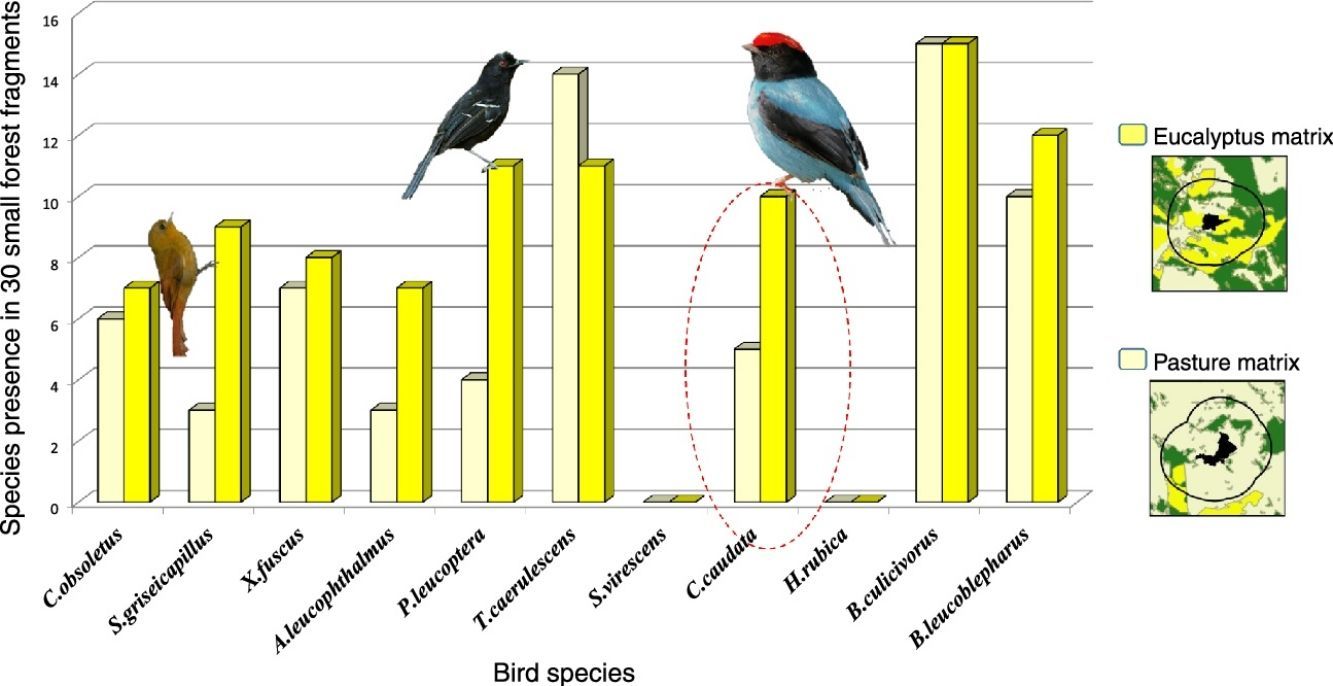
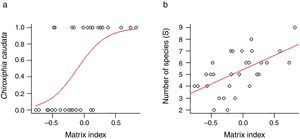
ResultsWe found differing responses to landscape context, with some birds more sensitive to different landscape characteristics, although there was a tendency of species presence in small patches to be more influenced by matrix composition. Two species, Schiffornis virescens and Habia rubica, were present in all control areas but were absent in all 30 small forest fragments, and so were not considered further. Contrarily, Basileuterus culicivorus was recorded in all fragments and was considered only in the sum of the number of species in forest patches (Table S2). Considering the eight remaining species, only Chiroxiphia caudata revealed a strong evidence that matrix was the variable that better explained the presence of the species in small forest patches (Fig. 2a). The model with the single variable “Matrix” was the most plausible (Table 1) and this variable also presented the highest relative importance (Table 2). For the other seven species (Sittasomus griseicapillus, Myiothlypis leucoblephara, Automolus leucophthalmus, Pyriglena leucoptera, Crypturellus obsoletus, Xiphorhynchus fuscus, Thamnophilus caerulescens), the null model was among the selected models (Supplementary data Table S3, Table S3), indicating a high uncertainty in model selection. However, considering the relative importance of the variables, the “Matrix” presented the highest value for five of the eight species (Table 2). Furthermore, the model with the single variable “Matrix” was also the most likely to explain the number of species in small forest patches (Table 1) and presented the highest value of relative importance (Table 2). There is a tendency of an increasing number of bird species when the proportion of eucalyptus matrix increases (Fig. 2b, Table S4).
Species occupancy models on standardized data according to Akaike's criterion for small samples (AICc). The best models (Δi<2.0) that explain the presence of Chiroxiphia caudata and number of species in the patches. The Δi (delta) relative difference to the lower value of AICc; wAIC (Akaike's weight) – chance that the model is selected.
| Variables | Model | AICc | Δi | wAIC |
|---|---|---|---|---|
| Number of species | Matrix | 116.66 | 0.00 | 0.28 |
| Connect+matrix | 118.40 | 1.74 | 0.12 | |
| Forest_%+matrix | 118.52 | 1.85 | 0.11 | |
| Area+matrix | 118.52 | 1.86 | 0.11 | |
| Chiroxiphia caudata | Matrix | 33.97 | 0.00 | 0.30 |
| Forest_%+matrix | 34.24 | 0.28 | 0.26 | |
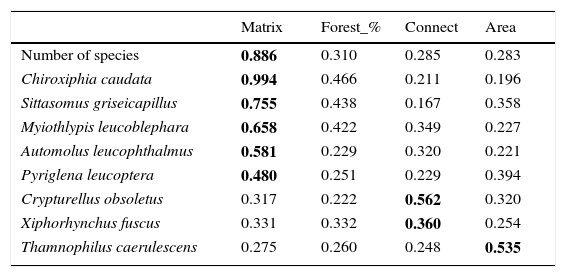
Relative importance of the variables, given through of the sum of the weight of the model where the variable appears. Matrix, index of matrix type (pasture or eucalyptus); Forest_%, percentage of forest in the landscape; Connect, 1 connected or 0 not connected; Area, size of the forest fragment in hectares.
| Matrix | Forest_% | Connect | Area | |
|---|---|---|---|---|
| Number of species | 0.886 | 0.310 | 0.285 | 0.283 |
| Chiroxiphia caudata | 0.994 | 0.466 | 0.211 | 0.196 |
| Sittasomus griseicapillus | 0.755 | 0.438 | 0.167 | 0.358 |
| Myiothlypis leucoblephara | 0.658 | 0.422 | 0.349 | 0.227 |
| Automolus leucophthalmus | 0.581 | 0.229 | 0.320 | 0.221 |
| Pyriglena leucoptera | 0.480 | 0.251 | 0.229 | 0.394 |
| Crypturellus obsoletus | 0.317 | 0.222 | 0.562 | 0.320 |
| Xiphorhynchus fuscus | 0.331 | 0.332 | 0.360 | 0.254 |
| Thamnophilus caerulescens | 0.275 | 0.260 | 0.248 | 0.535 |
The matrix composition appears to be the most important predictor variable for the presence of birds in small forest patches in comparison to other studied landscape variables. This is evident when we consider the variation in the number of species in small patches, although it is less clear when we consider the presence of each species separately. This might be because species occurrence is influenced by matrix composition, but it is also sensitive to other local and landscape factors (Boscolo and Metzger, 2011) not considered in this study, making this relationship harder to detect. However, when we combine all the species, counting the number of species per patch, this pattern was more consistent and it became apparent that landscape matrix composed mainly of eucalyptus favors a higher number of species in small patches than a matrix dominated by pasture. As detected by other studies, in more permeable matrices birds are more likely to use stepping-stones or small forest fragments (<10ha) (Fischer and Lindenmayer, 2002; Uezu et al., 2008; Goulart et al., 2015).
The importance of the matrix has been discussed in others studies, which have revealed that a structurally more complex or a better quality matrix leads to an increasing functional connectivity for forest birds (Dario and Almeida, 2000; Renjifo, 2001; Baum et al., 2004; Goulart et al., 2015). However, it is surprising that the matrix composition is more important to determine species occurrence in the patches than other landscape variables, such as percentage of forest cover in the landscape. This is a valuable finding since it emphasizes the importance of the matrix management.
In general, populations of insectivore bird species are lost or tend to decline following forest isolation (Bierregaard and Lovejoy, 1989; Stouffer et al., 2011), being affected mainly by reduction of invertebrates in fragmented areas (Schleuning et al., 2011). Therefore, a more permeable matrix may facilitate the species dispersal through the landscape, helping them find resources in other areas. This, at least partly, seems to be the function of the eucalyptus plantations for the insectivore species S. griseicapillus, M. leucoblephara, A. leucophthalmus and P. leucoptera. Particularly, S. griseicapillus is a vertical climber species that needs to cross open areas in single flights, which can cause the species to avoid long distance movements. A study conducted in central Amazonian Brazil showed that S. griseicapillus has gone extinct in isolated, one-hectare fragments, which were not recolonized (Stouffer et al., 2011). Distinct from the insectivores, the omnivores, such as C. caudata, are favored with the possibility of shifting their food source when another is scarce (Stotz et al., 1996). In fact, C. caudata is less sensitive to environmental changes (Stotz et al., 1996) and even is able to cross open areas of more than 100m between forest patches (Uezu et al., 2005). Nonetheless, its presence in small forest fragments was strongly correlated with matrix, an indication that although it can cross forest gaps it prefers to use a matrix of eucalyptus to move through the landscape and reach the small patches.
For C. obsoletus and X. fuscus, the most important variable was the presence of a corridor. The former is a terrestrial species that forages on the ground, rummaging through the leaf litter and pecking their prey, so continuous connection among forest patches might be important to those species that have difficulty in crossing large inter-habitat distances, perhaps avoiding predation in open areas (Boscolo et al., 2008). Concerning X. fuscus, it has a limited dispersal capacity (Boscolo et al., 2008) but seems to be less sensitive to landscape structure in the study region in comparison to the other woodcreeper, S. griseicapillus, as it was present in 15 small fragments while S. griseicapillus in 12.
The insectivore species H. rubica was not present in any of the studied small fragments, perhaps because it is a central mixed flock species and its absence may be related to the decline of invertebrate populations (Develey and Peres, 2000). Conversely, B. culicivorus is an understory insectivore bird that, despite the apparent similarity with M. leucoblephara in body shape and morphology, shows distinct habitat requirements, being present in all studied small fragments (Stotz et al., 1996; Uezu et al., 2005). T. caerulescens also seemed to be less sensitive as it occurred in almost all (25 of 30) forest fragments. We also recorded breeding activities of M. leucoblephara, with nest and eggs, and performances of multi-male courtship displays of C. caudata in small forest fragments, corroborating the conclusion of Turner and Corlett (1996) that small fragments can be important for survival and habitat for bird species with different habitat requirements.
The absence of a given species in small forest fragments may be related to their sensitivity to habitat fragmentation, even in more connected fragments or in those embedded in matrices that are more permeable. For example, probably these fragments or the context in which they are inserted are not enough for H. rubica and S. virescens, as we did not detect them in these areas during the breeding season. However, we cannot reject the hypothesis that they can increase landscape connectivity, working as stepping-stones in other periods of the year, outside the reproductive season. Further long-term studies are necessary to test this function of small fragments for such a sensitive species.
Due to the increasing forest loss and fragmentation in the Neotropics, the knowledge of the role of small forest fragments is important for biodiversity conservation, mainly in biomes with high endemism and under intense anthropogenic pressure such as the Atlantic Forest. Our results suggest that the composition of the matrix can be important for the use of small forest fragments by birds. Changing the management of productive areas to improve permeability and avoiding bare pastures should be regarded as important biodiversity conservation tools, together with other practices, such as habitat restoration and corridor creation.
Conflicts of interestThe authors declare no conflicts of interest.
We are very indebted to IPÊ – Instituto de Pesquisas Ecológicas and partners, colleagues and collaborators for their support. We thank also the Conservation Leadership Program for their workshop, which improved the manuscript. Danielle Rappaport, Daniele Barcelos, Thiago VV Costa, Luke Powell, Norbert Cordeiro and Jeff Stratford provided valuable suggestions and corrections to the manuscript.