Some Cerrado areas are suppressed by pine tree cultivation. These monoculture processes can exclude the fire presence and inhibit native species development. In Southeastern Brazil, thousands of hectares were planted with these exotic trees 44 years ago, and nowadays, efforts to remove these plantations and restore the native vegetation are being implemented. However, little is known about the regeneration of Cerrado after pine removal. Thus, the aim of this study was to analyze the native plant community of Cerrado, using some techniques to enhance species natural regeneration three years after pine trees removal in areas where plantations existed since 1966. Before treatments application, surveys of the herbaceous and woody community were conducted, followed by the treatment application (fire and the removal of needles) as a management intervention. Moreover, we established control plots, with no intervention. Four and 30 months after treatment application, the herbaceous and woody vegetation, as well as the dead biomass and bare soil components were monitored to observe their regeneration. The pine removal contributed to species development and both techniques contributed to soil exposition, opening space for colonization and species to resprout. The woody and herbaceous group increased in cover, mostly in fire plots, due to the soil exposition increasing light and contributing to species development.
Changes in land use have become very common in grassland and savanna areas, where native vegetation has been threatened by afforestation of exotic species (Veldman et al., 2015), due to an increasing demand for timber extraction and woody products with high economic returns, provided for example, by pine tree cultivation (Buisson et al., 2019; Chen et al., 2008; Rudel et al., 2005). However, afforestation in old-growth grassland systems directly impacts the ecosystem services, such as mineral soil, carbon stocks, hydrology and herbaceous species establishment due to changes in light conditions, limiting the vegetation productivity and richness (Chen et al., 2008; Davis and Condron, 2002; Veldman et al., 2015). Therefore, planting trees where formerly was an open system can lead to drastic changes in the system, being even more difficult to restore these ecosystems after tree removal (Buisson et al., 2019). However, restoration of old-growth grasslands are still an enormous challenge, because most of these areas had their fire regime altered (e.g. fire exclusion,; Durigan and Ratter, 2016), and thus, some of them might have lost their resilience (Buisson et al., 2019) also due to the long-term land use conversion (Abreu et al., 2011; Overbeck et al., 2015). Even though many parts of the tropics are covered by open ecosystems, they are in most cases neglected (Parr et al., 2014), being necessary integrated efforts to restore and to conserve these systems (Veldman et al., 2015).
Rehabilitation of grassy ecosystems may be fast (e.g. return of grass cover and biomass), however, restoration of these ecosystems is too difficult, mostly restoring both taxonomic and functional diversity (Zaloumis and Bond, 2011). Areas of grasslands that were replaced by afforestation with Pinus sp. in the past and left to natural regeneration after the plantation removal (secondary grasslands) do not show a heterogeneous species composition as natural grasslands (Zaloumis and Bond, 2011, 2016), being usually dominated by few species, with the presence of some invasive species and loss of functional diversity and carbon (Zaloumis and Bond, 2016). The afforestation process involves usually ploughing and shading, which could eliminate resprouter species from the system, which can affect vegetation regeneration after pine removal (Zaloumis and Bond, 2011). Efforts to restore these grassy biomes have to consider the different functional groups and some of them may be a more difficult challenge than others. Pilon et al. (2018) showed that the use of hay transfer was not as effective as top soil removal to restore the herbaceous layer of woody savannas invaded by African grasses, and also that this technique was successful in restoring open savannas and wet grasslands in areas of former pine plantations (Pilon et al., 2019). However, they pointed out that plant communities were still different from the reference ecosystem and some areas were later invaded by African grasses imposing thus, challenge for restoration in invaded areas (Pilon et al., 2018).
Other studies in the Cerrado showed that in the plantation areas, light availability and the thick litter layer (composed mostly by needles) affect native species establishment from the surrounding species pool and only few woody species and a scarce herbaceous layer are able to persist (Abreu et al., 2011; Brewer, 1998; Brewer et al., 2018). Thus, interventions are sometimes necessary to restore native plant communities in these former afforestation areas, removing the trees and adding a combination of management techniques, contributing to the regeneration process of these old-growth ecosystems (Buisson et al., 2019).
Cerrado is the richest tropical savanna in plant species, being considered a hotspot of biodiversity (Klink and Machado, 2005; Myers et al., 2000), and fire is one of the most important ecological factors, that has been influencing species diversity and establishment for the last four millions of years (Simon et al., 2009). Although, fire has been suppressed in several areas due to fire exclusion policies (Durigan and Ratter, 2016), it could be used as an important tool for Cerrado restoration, because it contributes to the reduction of shrub encroachment and may increase plant diversity of grassy ecosystems (Buisson et al., 2019).
The afforestation practices in Cerrado need to be evaluated and new policies should be addressed to restore open ecosystems that were used for this practice (Fernandes et al., 2016), since their restoration is still a challenge (Buisson et al., 2019). Thus, studies investigating the role of ecological filters that influence Cerrado's regeneration after pine trees removal (e.g. the presence of a needle layer) are of crucial importance and should be considered. Moreover, we urgently need studies showing how we can overcome these filters (e.g. create gaps within the litter layer for plant establishment) to be able to restore these grassy biomes in the future (Buisson et al., 2019), using different management techniques to assure vegetation regeneration after pine removal. Therefore, we aim to evaluate the use of different techniques (fire, removal of needles, control) to enhance natural vegetation regeneration after pine plantation removal, in areas that were former pine plantation. Pine plantations led to the closure of the canopy, which altered the plant community structure and composition during the cultivation period. These areas were also excluded from fire and grazing for a long period. We hypothesize that fire will be the most successful management technique, since fire is very efficient in opening gaps within the vegetation and because it is a natural disturbance in the Cerrado and thus, it could enhance species establishment.
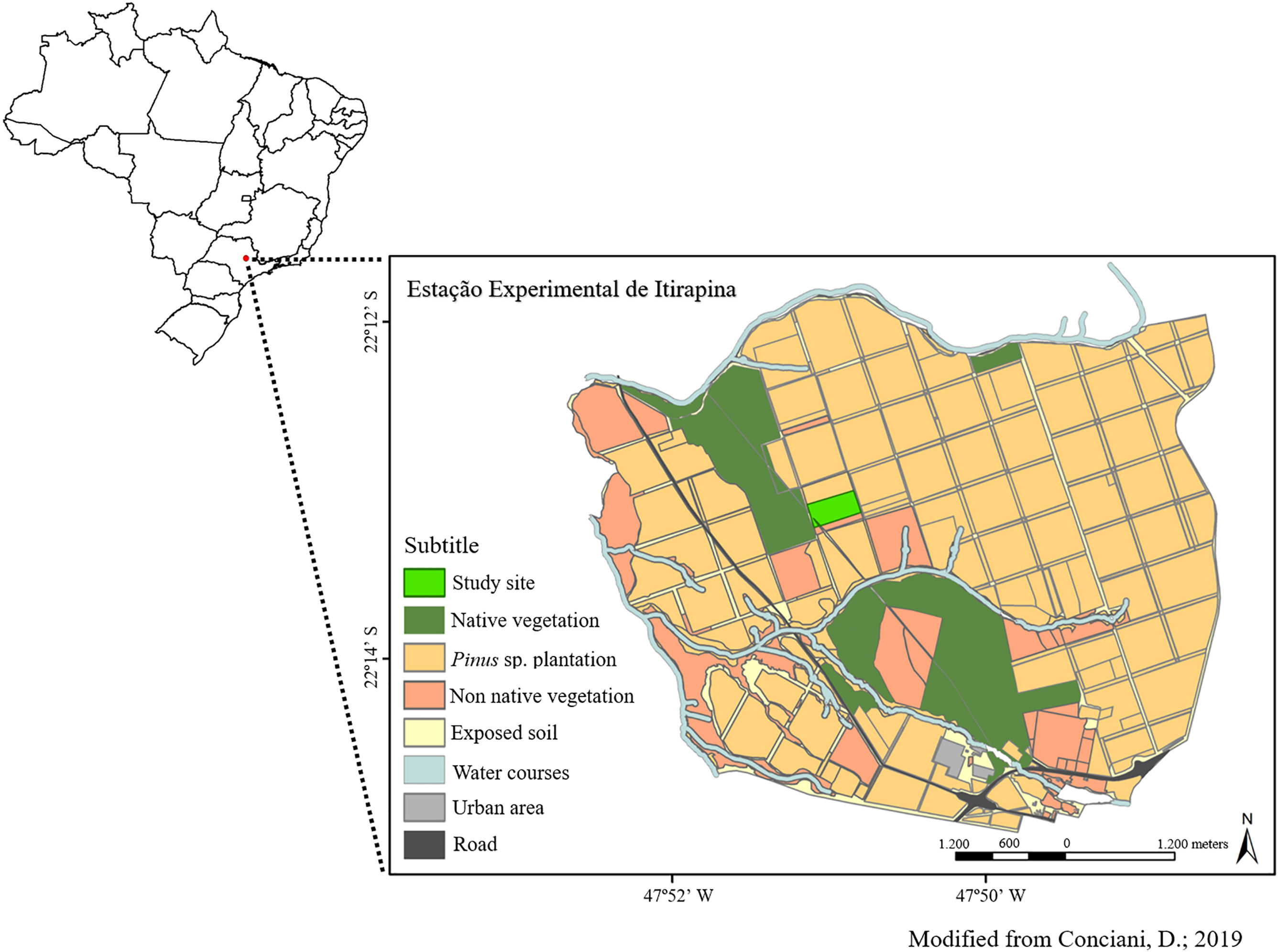
Material and methodsStudy areaWe carried out our study at the Estação Experimental de Itirapina (EEI), Southeastern Brazil (EEI, S-22.225096 – W-47.852747; Fig. 1) (Zanchetta et al., 2006). The EEI is composed by areas with pine tree plantations and some fragmented areas of Cerrado vegetation, with cerrado sensu stricto (woody savanna) and cerradão (forest with savanna trees) physiognomies. The climate is mesothermic with a dry season during winter (April–September) and wet season during summer, with mean annual precipitation of 1459mm (Zanchetta et al., 2006).
From 1950 to 1960, São Paulo state government created a new development model and established exotic tree plantations in some native areas for commercial timber and resin extraction (Zanchetta et al., 2006). The main species used were Pinus elliotti, Pinus taeda and Pinus caribaea. The area where this study was carried out has 21ha and before the afforestation, it was an open savanna with the presence of cattle. In 1966, the cattle was removed and the area was planted with Pinus caribaea var. hondurensis (Sénécl.) W.H. Barret & Golfari (Zanchetta et al., 2006). Tree removal started in 2010 and we established the experiments in 2013.
MethodsWe randomly established 30 plots (10m×10m, 10plots/treatment) in the area, with at least 10m of distance among plots and considering a distance of 50m from the edge of the former plantation area to its center, to avoid the influence of the border. We applied the following treatments in the middle of dry season: control (C, no treatment); removal of needles (RN, manual removal of needles with no removal of native vegetation), and fire (F, plots were burned). In these plots we counted the number of woody individuals of each species with height ≥100cm, measured their height (cm) and their perimeter at the ground level (woody community, PGL, cm). Within each plot, we sorted five subplots (1m×1m) randomly to estimate plant cover (%) for each component of the herbaceous-shrub layer (shrubs<100cm, graminoids, forbs and palm trees). We did the same for the structural components of the plant community: dead biomass (litter+standing dead biomass) and bare soil.
Before treatments, vegetation and structural components were sampled. We sampled all plots again four and 30 months after treatments were applied.
Statistical analysesWe applied general linear mixed models (GLMM) to analyze differences for each of response variables (shrubs<100cm, graminoids, forbs, palm trees, dead biomass, bare soil, PGL and height of woody individuals and number of woody individuals≥100cm) among treatments and time. We considered treatment and time as fixed factors and plots as random factor, with a Poisson distribution. To test interactions between the treatments and time, least square means post hoc test was conducted.
All analyses were done in R environment (R Development Core Team, 2018) using the following packages: LME4 (Bates et al., 2015), LS means (Lenth, 2016), Multcomp (Hothorn et al., 2008) and PSCL (Jackman, 2017).
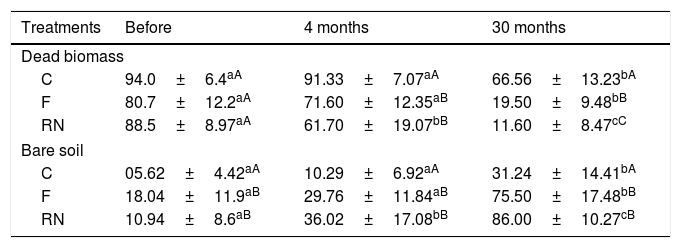
ResultsFour months after the removal of needles and fire, dead biomass cover started to decrease significantly in the removal of needles plots (p<0.0001; Table 1), while in the control and fire plots, dead biomass remained the same as at the beginning of the experiments (Control: p=0.99; Fire: p=0.36; Table 1). However, 30 months after the experiments, a significant decrease in dead biomass of ca. 50% in all treatments occurred (p<0.0001; Table 1), mainly in the fire and removal of needles plots (p<0.0001; Table 1). On the other hand, bare soil cover increased over time, being this evident in the plots where needles were removed at the beginning (p<0.0001; Table 1), and have increased in all plots, independently of treatment 30 months after the experiments were applied (p=0.0001; Table 1).
Cover (mean±SD, %) of dead biomass and bare soil, subjected to the treatments C=control; F=fire; RN=removal of needles, before treatments application, four and 30 months after treatments application. Lower case represents significant differences in each treatment over the time and upper case represents statistical differences between treatments at each time of observation (p≤0.05).
| Treatments | Before | 4 months | 30 months |
|---|---|---|---|
| Dead biomass | |||
| C | 94.0±6.4aA | 91.33±7.07aA | 66.56±13.23bA |
| F | 80.7±12.2aA | 71.60±12.35aB | 19.50±9.48bB |
| RN | 88.5±8.97aA | 61.70±19.07bB | 11.60±8.47cC |
| Bare soil | |||
| C | 05.62±4.42aA | 10.29±6.92aA | 31.24±14.41bA |
| F | 18.04±11.9aB | 29.76±11.84aB | 75.50±17.48bB |
| RN | 10.94±8.6aB | 36.02±17.08bB | 86.00±10.27cB |
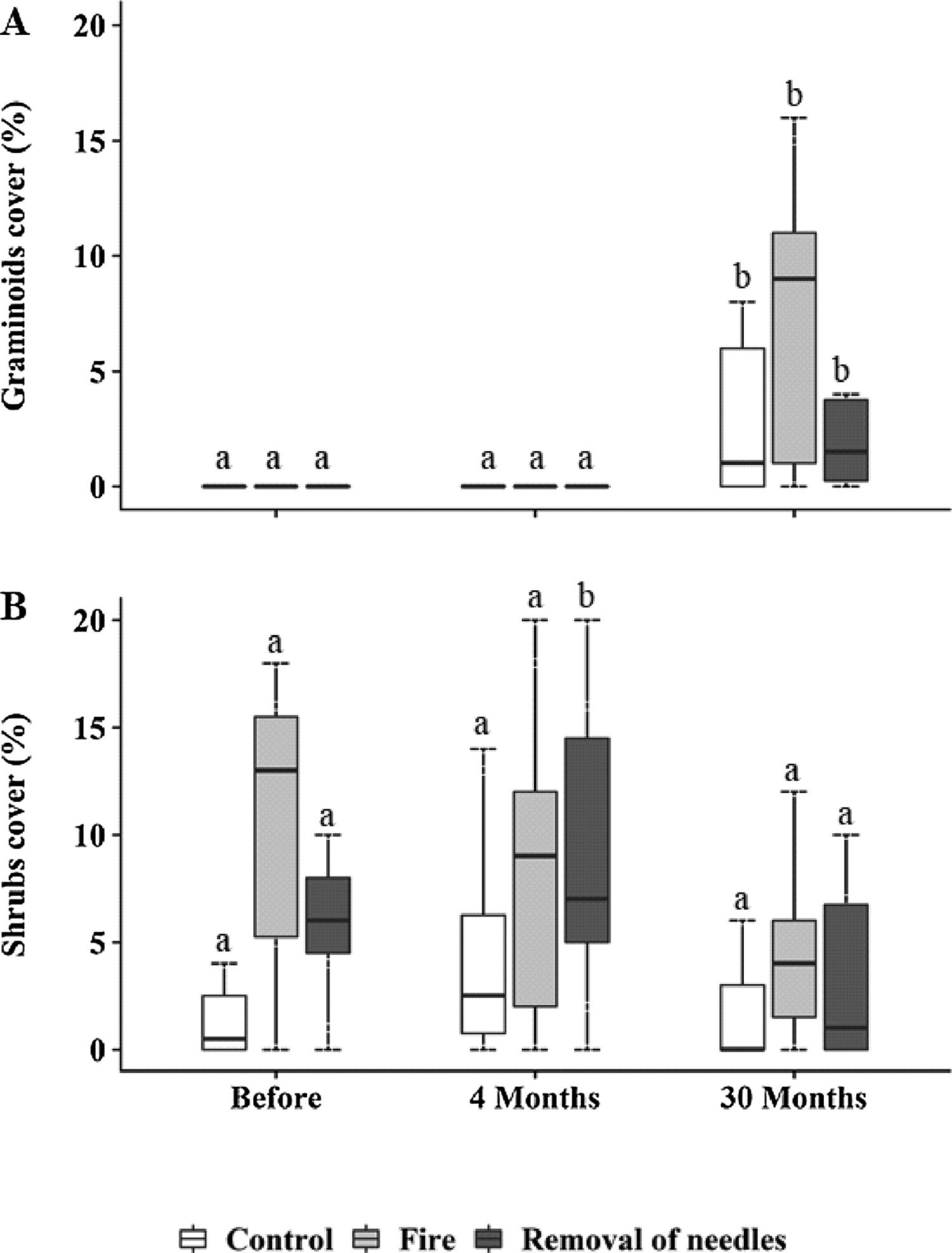
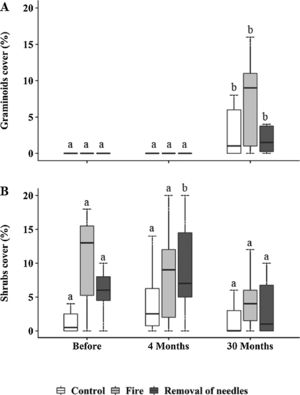
Graminoids and shrubs were the only groups that showed changes in cover over time (Fig. 2), while forbs and palm trees were not influenced neither by treatment nor by time (Fig. S1). Graminoids significantly increased in cover at 30 months in all plots (Control: p=0.03; removal of needles: p=0.009; Fig. 2A), mostly in the burned plots (before: 1.80±4.47% – 30 months: 11.74±14%; p<0.0001; Fig. 2A). However, no significant differences were observed among treatments. Shrub cover was only affected by the treatment where needles were removed, showing an increase at four months (before: 6.5±4.55% – 4 months: 13.6±11.84%; p<0.0001; Fig. 2B). However, 30 months after treatments implementation, all shrub cover showed no difference among treatments.
Percentage cover of (A) graminoids and (B) shrubs <100cm, subjected to the treatments C=control; F=fire; RN=removal of needles, before, four and 30 months after treatments application. Boxplots represent the median (black line), and the first and third quartiles (lower and upper lines, respectively). Lower cases indicate significant differences over the time of observation for each treatment. No significant difference among treatments in each time was found (p≤0.05).
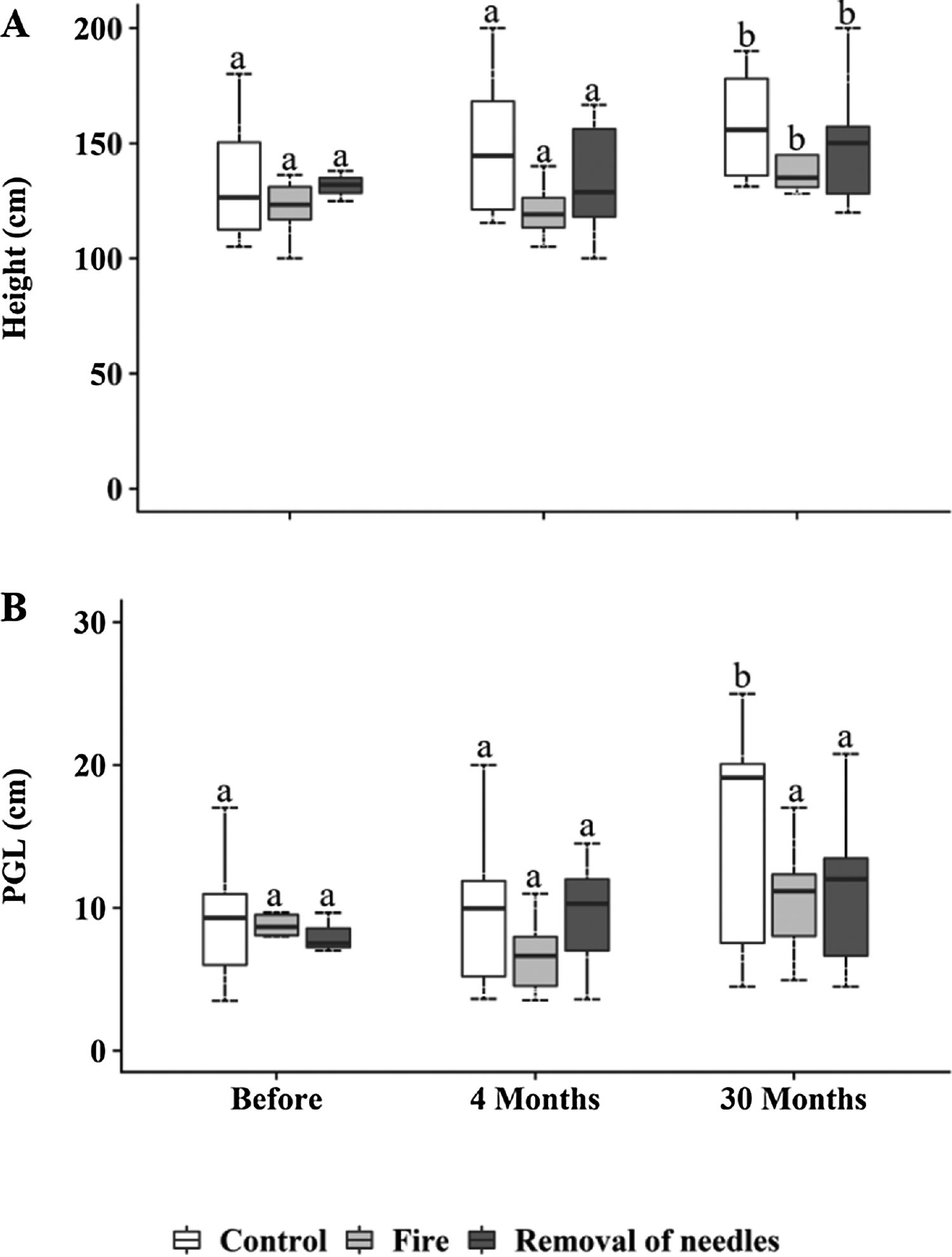
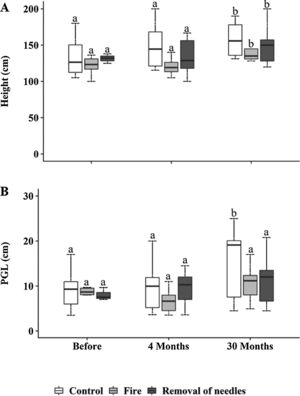
Bauhinia sp. and Memora sp. occurred in all plots and observation times (Table S1). Pouteria torta also had a higher number of individuals in all plots but did not occur in all observation times (Table S1). Woody plants significantly increased in height at 30 months in all plots (p<0.0001, Fig. 3A). Woody individuals showed no differences in perimeter among treatments. However, an increase in perimeter of woody plants was observed in control plots 30 months after treatments application (p<0.0001; Fig. 3B).
(A) Woody individuals (≥100cm) height (cm) and (B) perimeter at ground level (PGL, cm), subjected to the treatments C=control; F=fire; RN=removal of needles, before treatments application, four and 30 months after treatments application. Boxplots represent the median (black line), and the first and third quartiles (lower and upper lines, respectively). The lower cases indicate statistical differences over the time of observation for each treatment (p≤0.05).
The presence of Pinus changed local environmental conditions, due to the decrease in light availability and the presence of a thick needle layer, being both considered ecological filters driving the processes of native plant communities (Abreu et al., 2011; Lemos-Filho et al., 2010). Moreover, the pine plantation suppressed the native vegetation mostly due to shading, causing a homogenization of the community and cover reduction of native species that needed sunlight and open soils to establish and persist in the system (Brewer, 1998; Abreu and Durigan, 2011; Kortz et al., 2018; Zanchetta and Diniz, 2006). Therefore, after the removal of pine trees local environmental conditions were altered, and the thick needle layer must be overcome in order to plant community to regenerate again. We observed that just removing the Pinus tree improved the system by increasing light availability leading to an enhancement of the community regeneration, by the increase of woody species perimeter in control plots. Also, in control plots there was a decrease in dead biomass cover probably because of the decomposition of needles with time and thus, new spaces were also available (although in less importance and amount) and as a consequence, an increase in grass cover after five years of pine tree removal could be observed. However, both forbs and grasses were mostly enhanced and favored in plots where the management techniques were applied (fire and removal of needles), because they were more efficient in opening gaps for plant establishment.
Few native woody species can establish and persist under pine tree canopies (Abreu et al., 2011; Abreu and Durigan, 2011). Since these woody species have plasticity, they can tolerate both shady and sunny environments, although some typical Cerrado species are still dependent on sunlight to develop (Abreu et al., 2011; Lemos-Filho et al., 2010). Thus, the removal of pine tree plantation might be important to enhance the growth of these species, due to a higher light availability, allowing their establishment and development in these areas, contributing to local diversity (Abreu et al., 2011) and serving as a propagule source for neighboring areas. We observed that woody species already present under the Pinus canopies could develop better after the removal of these trees, by growing in height and diameter, as found in our results, probably due to the higher availability of light. However, other species, mostly from the herbaceous layer, could not persist for a long time under shade conditions and thus, the establishment of these species might rely on the arrival of propagules of neighboring sites. Therefore, it is of crucial importance to have remnants of native vegetation close to the areas to be restored, as propagule sources for the recently managed areas. However, in São Paulo state most of the remnant Cerrado areas are fragmented, isolated and small and therefore, source of native species propagules would be restricted to these areas (Durigan et al., 2007). Moreover, these fragments are within a matrix of sugarcane plantations and pastures planted with African grasses (Durigan et al., 2007), which area a source of propagules of invasive species, being a threat to these areas under natural regeneration and a challenge to be overcome for Cerrado restoration (Buisson et al., 2019). Therefore, restoration areas far from protected areas would have an additional challenge, since there is a large source of invasive species propagules (coming from planted pastures) and lack of native species propagule source and thus, additional restoration techniques such as planting seedlings or using direct seeding to enhance Cerrado regeneration.
Grassland systems replacement by tree plantations leads to changes in light availability and fire regime (e.g. fire suppression), that has an important function on these old-growth ecosystems, enhancing the herbaceous species that are fire-adapted (Coutinho, 1982; Fynn et al., 2004). However, as a consequence, the absence of fire leads to an accumulation of litter, which directly affect the herbaceous plant community (Buisson et al., 2019; Abreu and Durigan, 2011; Parr et al., 2014). For example, the natural regeneration in abandoned pastures is driven by the establishment and growth of woody species, changing thus the state of the system from grassland/savanna to a forest system (Cava et al., 2018). As a consequence, there is a loss of grassland/savanna species due to limited propagule availability and changes in other environmental filters, such as light availability, being very difficult to change the system back to open areas (Buisson et al., 2019; Cava et al., 2018). Therefore, efforts must be made in such areas in order to reestablish and enhance the regeneration of the herbaceous layer, because these grassy ecosystems are of high importance and should be conserved, but at the same time, they are usually neglected and less attention is directed to them in conservation issues (Zaloumis and Bond, 2016; Buisson et al., 2019). In the case of pine plantations, the removal of trees followed by the needles layer removal and fire treatments already showed a tendency to enhance the natural regeneration of grasses and shrubs, whilst in control plots, this regeneration was lower and slower.
The removal of pine tree plantation was fundamental to promote the development of species already present in the monoculture. However, most of these species were woody species and only the removal was not enough to guarantee the regeneration of the herbaceous layer (e.g. graminoids cover). Therefore, the use of techniques that helped to overcome the thick litter layer could be efficient to enhance species cover. Our study showed that fire and removal of needles techniques could be used, since both were efficient to expose the soil, creating new gaps and opportunities species establishment. We propose the use of fire, since the manual removal of needles is a more expensive and difficult technique. Moreover, fire is a natural disturbance in Cerrado Coutinho, 1982) and could also enhance the vegetative regeneration from the bud bank, if buds are still viable. We must consider the use of integrated techniques to enhance the restoration process (e.g. native plant reintroduction) together with fire treatment (Buisson et al., 2019). The use of fire has been poorly studied in restoration (Buisson et al., 2019), so this study can contribute to a better understanding of the role of fire as a management tool to enhance regeneration of old-growth ecosystems. And when we analyze all afforestation by Pinus spp. problems, it is possible conclude the importance to create conservation policies to decrease the impact caused by afforestation in native open areas and the necessity to develop management tools to contribute to native species improvement.
The authors would like to acknowledge the staff of Estação Ecológica e Experimental de Itirapina for all support during the experiments. Also, we thank Ana Carolina Ferreira, Karen Castillioni, Jonathan Galdi Rosa, Elizabeth Gorgone-Barbosa, Rafael Pinheiro, Caio Matheus Silva, Ademir Francisco, Rafael Ferreira de Andrade, Priscila Sperandio and Lucas Barbosa for their valuable help during the experiments. V. Zanzarini received scholarship from PIBIC/IF and PIBIC/UNESP. A. Fidelis receives productivity grant from CNPq.