The subject of biological invasions is well-recognized, especially due to the associated impacts, but different interpretations exist about the concept of invasive species. These are usually known as exotic species that proliferate intensely, spread rapidly and persist as dominant in the new community. However, some native species may behave the same way and bring serious ecological and economical losses. Nonetheless these native species may not attract management efforts and specific policies, partially because of the assumption that native species are harmless. We review the concepts of invasive species and show the potential harm of overabundant populations of native species, which we name “super-dominant” species. Based on literature review we demonstrated the lack of information on the Brazilian super-dominant plant species. Considering all kinds of published material and knowledge from our own experience we selected 16 Brazilian native terrestrial plants that most frequently show unexpectedly intense growth and dominance in their original habitats. We discuss the factors that may have triggered atypical dominance, negative impacts of these species on the native biodiversity and ecosystems, and future trends. Anthropogenic disturbances are the main drivers of the explosive population growth of these native species, especially habitat fragmentation, forest gap formation, and wildfires. The absence of legal support to deal with super-dominant native species is probably the main reason for the lack of disclosure of the subject. In the future scenario of climate change we expect the intensification of the phenomenon. Strategies for early detection and control need to be fast developed.
In the last decades the scientific community has given great attention to exotic invasive species due to the huge ecological, economic and social impacts they may cause (Mooney and Cleland, 2001; CBD, 2002; Charles and Dukes, 2008). However, there are also native species whose populations are released from controlling mechanisms, proliferate intensely and disproportionally, and may result in serious damages similarly to the exotic invasives (Garrott et al., 1993). Disturbances resulting from land use changes are the primary causes of unusual overabundance of native plants; future global scenarios point to the intensification of habitat disturbances, due to both land uses and climate change, thus further increasing the emergence of overabundant plant populations.
Although the impacts caused by overabundant native species are perceived by environmental managers, the scientific literature on the subject as well as researches devoted to their study are still scarce (Carey et al., 2012). Even a framework of these species in the context of biological invasions is lacking. In this sense, the main purposes of this paper are: (i) to point the existing diversity in the interpretation of the concept of invasive species and the need to consider the super-dominant natives into the context of biological invasions; (ii) to reflect about reasons that cause the release of native plant populations; (iii) taking the Brazilian case, to show the paucity of published information regarding native species that became overabundant; (iv) to highlight main species of Brazilian terrestrial plants that have established overabundant populations and the factors that may have triggered their atypical dominance, as well as their negative impacts; (v) to emphasize the potential of native super-dominant species on causing negative impacts on ecosystems, and therefore, to stimulate policy-makers, scientists and managers of protected areas to develop specific policies and management actions for them based on solid research, in order to maintain natural biodiversity and ecological processes.
Different definitions for invasive speciesCharles Darwin in The Origin of Species (Darwin, 1859) already recognized that some species can show explosive growth and the ability to spread rapidly over great distances, but the concept of invasive species became explicit only after the publication of Elton's book Ecology of Invasions by Animals and Plants (Elton, 1958). The understanding of the meaning of invasive species, however, remained very inconsistent for several more decades (see Richardson et al., 2000), perhaps because the notion of invasive species brings together a series of concepts from different fields, such as biogeography, demography, ecological succession, and community ecology. Still, a utilitarian sense of good/useful or bad/harmful has usually been associated to such species.
An initiative to organize the concepts, definitions and terminology related to the processes of biological invasion came only in a conceptual paper published in 2000 (Richardson et al., 2000). Focusing on plants, the authors define invasive species as being necessarily exotic (alien, non-native), with a great ability to reproduce and self-sustain populations over many life cycles, spread individuals/propagules over large areas, and whose introduction or process of spreading in the novel environment is human-mediated. This definition is currently adopted by most plant ecologists. Although a number of cases show that invasive species can transform the environment, change the community composition and structure, and alter ecosystems processes (Vitousek et al., 1997; Pimentel et al., 2005; Hejda et al., 2009), Richardson et al. (2000) did not require an implication of impact in their definition, and they possibly avoided the term “harmful” because of the judgement of values implicit in it.
There is currently common agreement in the academic domain that to be named “invasive” a given species must reproduce and spread fast, disproportionately when compared to the native species amongst which it now finds itself, hence may rapidly come to dominate the community. However, controversies concerning a precise definition of invasive species still exist in the current literature of biological invasions: despite the above mentioned initiative of Richardson et al. (2000), there is no complete consensus regarding the inclusion of species geographic origin (exotic or native) in the concept definition, or its transport vector to the novel environment (human-mediated or not), nor the potential to cause impacts in the novel habitat(s). For example, authors and especially environmental organizations and instruments of administration (IUCN, 2000; CBD, 2002; GISP, 2016) state in their definition of invasive species the requirements of causing, or having the potential to cause, severe negative impacts – ecological, economic or social – besides the requirement of necessarily being exotic to the environment, and introduced by humans. Others (Simberloff, 2011; Carey et al., 2012; Heger et al., 2013) defend the use of the term “invasive” for every species – exotic or native – that spreads and dominates human mediated disturbed habitats in an unexpected way, where they cause negative impacts. Yet another group of authors (e.g., Valéry et al., 2008, 2013; Webber and Scott, 2012) support the minimum criteria for defining invasive species: they proliferate intensely, spread very fast, have competitive advantage and dominate the “invaded” community. For these last authors what really matters are the ecological mechanisms involved and the outcomes; the species geographic origin is not relevant as both native and non-native species can develop similar “invasive” behaviour. The impacts they may cause should not come as prerequisite, but as a consequence of the species ecological and demographic characteristics. Thus, the discrepancy in the perceptions of the concept held by researchers, managers and politicians remains considerable.
We believe (as do Heger et al., 2013) that these different points of view derive from the wide variety of interests and perspectives encompassed by the theme of biological invasion, from pure ecological science to environmental management. However, good policies and suitable management practices emerge from the perfect understanding of science, and for that, both scientists and practitioners must count on precise definitions and unmistakable meanings. We also consider that from the perspective of biological conservation it is necessary to focus on every species – being either exotic or native – that threatens the environment, the biological community and ecological processes. In the case of the native species that behave as exotic invasives, several terms have been used: native-invasives (e.g., Valéry et al., 2008, 2013), weeds (e.g., Richardson et al., 2000), overabundant (e.g., Jose et al., 2016) or super-dominant species (e.g., Callaghan et al., 2005; Silva Matos and Pivello, 2009). We chose the term super-dominant to name the native species whose populations are released from controlling mechanisms, so they proliferate intensely and unexpectedly, causing negative impacts by changing the community composition or structure, transforming the environment, or altering ecosystem processes. Even though less frequently used, this term does not involve anthropogenic implicit judgement (as weed, a “harmful” species) and it best reflects a strong demographic imbalance of the community (stronger than overabundant) instead of suggesting an external origin of the species (as native-invasive).
Why a native population becomes super-dominant?Compared to exotics, native species are much less likely to develop invasive behaviour in a community. In North America and Europe, for example, it has been verified that non-native species are much more prone to become invasive and cause impacts than native species (Simberloff et al., 2012; Hassan and Ricciardi, 2014). Likewise, biotic interactions generally prevent uncommonly high dominance of native species, as the co-evolutionary history shared with other species of the community – including the coexisting with natural enemies (herbivores, pathogens) – tends to shape species requirements and attenuate competition (Callaway and Aschehoug, 2000; Rausher, 2001; Paolucci et al., 2013). However, anthropogenic disturbances may trigger population explosions of native species. As well as co-evolution, recurrent mild disturbances are important vectors on regulating species populations, by shaping ecological niches and structuring species distribution in the community (Sheil, 2016). However, when the magnitude of disturbances (characterized by frequency, duration, intensity, spatial extent), timing or variability (Catford et al., 2012) are different than usual or normal conditions, and promote uncommon changes in habitat conditions or biotic interactions they may generate outbreaks and unusual proliferation of some native species, which may completely disrupt community interactions, generating new dynamics.
The association of invasive exotic species to disturbances not usually experienced by the system – especially the anthropogenic ones – has been extensively discussed (Vitousek et al., 1996; Mack et al., 2000; Byers, 2002; Gurevitch and Padilla, 2004; MacDougall and Turkington, 2005; Pyšek and Richardson, 2010; Sheil, 2016), but the fact that such disturbances can also trigger demographic explosions of some native species, similar to those observed with the exotic invasives (Carey et al., 2012; Simberloff et al., 2012) has been much less addressed. In such situations their population growth rates (λ) greatly increase, becoming much higher than those of the rest of the community, and new sites are achieved by replacement or displacement of other native species (Arim et al., 2006). Negative impacts on native communities are expected because the species at extremely high population sizes will consume the highest proportion of resources at the expense of other species, whose relative abundances will decrease. Eventually, λ of the released species will decrease and may return to values expected for stable populations (Arim et al., 2006), but it may take a long time. In some cases, λ may decrease by intrinsic population factors, such as density-dependence. Silva Matos et al. (1999) described the population dynamics of a dominant palm tree from the Atlantic Forest and how density can affect its population structure; in other cases, highly dense palm populations increase mortality of other species by leaf fall (Piñero et al., 1984). Arrested forest succession by super-dominant Guadua spp. bamboo (Griscom and Ashton, 2003, 2006; Lima et al., 2012) and lianas have been described (Schnitzer et al., 2000). As the λ decrease of the super-dominant species may take a long time, the effect on the whole community can be very severe, such as changes in food webs and ecological processes (pollination, seed dispersion, decomposition, nutrient cycling, productivity), and some species may be locally extinct; the ecosystem can change to a new state, where the resistance of the super-dominant species may prevent natural regeneration to occur. It is difficult to differentiate detrimental demographic explosions of super-dominant species from transient proliferations that occur after disturbances in natural processes of secondary succession (Garrott et al., 1993; Simberloff, 2011; Simberloff et al., 2012). Knowledge about ecological and demographical patterns of populations in their native habitats is essential to identify unusually high population growth, and to evaluate the necessity of control measures. Low species richness, low equability and high dominance of colonizer species are expected in the initial post-disturbance environments, and tend to reverse along the successional gradient (Odum, 1969). Thus, pulses of population expansion of pioneer species may occur after disturbance, but tend to return to initial conditions, and follow the natural ecological succession. The same applies to species that show a mast-year behaviour (sensuHarper, 1977), exhibiting massive and synchronized reproduction. In this case, peaks of population explosion are periodic, and so, masting events can be distinguished from invasion phenomena, as these are singular rather than recurrent events (Simberloff, 2011). Conversely, in processes of super-dominance and biological invasions an overwhelming dominance of a distinct species – usually in both abundance and biomass – lasts for several years or decades. It may be difficult, sometimes subjective, to define an expected time scale or the limiting time for reversibility in a successional process. The comparison of current species abundances and distributions with historical data (Carey et al., 2012), if they exist, is one direct possibility to assess unexpected species proliferations. However, due to the dynamic nature of ecosystems, the validity of historical data must be weighed in the light of the present conditions.
Consideration of the negative impacts caused by super-dominant species on the community also may allow distinguishing a process of ecological release from secondary succession (Simberloff et al., 2012). In this sense, it is possible to adapt the classification system developed for invasive alien species based on their impacts (Blackburn et al., 2014) to super-dominant native plant species. Impact mechanisms such as competition, flammability or toxicity (allelopathy) associated to native species and scored to major (that “causes changes in community composition, which are reversible if” the super-dominant species is controlled) or massive (that “causes…local extinctions and irreversible changes in community composition; …the system does not recover its original state” even if the super-dominant species is controlled) may be considered anomalous. At last, such impacts will result in measurable consequences for the ecosystem, for example, unusual proportion of biomass, land cover or seeds in the soil seed bank for the super-dominant species; lower species richness or diversity in the community compared to what is was before the super-dominance episode; lack or decrease of any ecosystem function (as decomposition, fruit production, etc.); signs of environmental degradation (sediments in water bodies, erosion, etc.). We are not focusing on social and economic impacts, but they may happen as well.
The case of super-dominant plants in BrazilPublished informationAlready in 1993 Garrott et al., referring mostly to animals, raise the issue of the overabundant native species and recognized the little attention given to such species even when they caused strong negative impacts over other natives. Later on, Valéry et al. (2013) reaffirm that “native species have de facto been ignored in the bulk of the literature devoted to invasions”, and Carey et al. (2012) state that “the impacts of native invaders are not well documented”. Therefore, it is recognized worldwide that super-dominant species have not received the same attention as the exotic invasives, and their impacts have not been well documented in the international literature.
In Brazil, the number of publications on biological invasions has increased fast, as show Frehse et al. (2016). In this recent review on exotic invasive species in Brazil, 112 plant species were found. However, few studies have focused on super-dominant species and on their harmful potential.
To verify the amount of available literature concerning plant species in situation of super dominance in Brazil we performed a systematic literature review that included journal articles, books, book chapters and theses. The literature search was completed in October 14, 2017.
We searched on the major electronic database ISI Web of Science, selecting all databases, all years and the English language. We used the following terms:
Topic (“native inva*”) OR (“non-exotic inva*”) OR (“non exotic inva*”) OR (superdominan*) OR (“super-dominan*”) OR (“unbalanced population”) OR (“explosive population”) OR (“native weed”) OR (overabundan*) OR (hyperabundan*) OR (hyper-abundan*) AND Topic (plant) OR (herb) OR (tree) OR (shrub) AND Topic: (Brazil) OR (Brasil). The resulting items were selected by title, abstract or, if necessary, by reading the entire text.
We also searched in the Brazilian MSc and PhD theses database (http://bancodeteses.capes.gov.br/), in the area of Biological Sciences, according to the same key-words, one at each time, for unlimited time period.
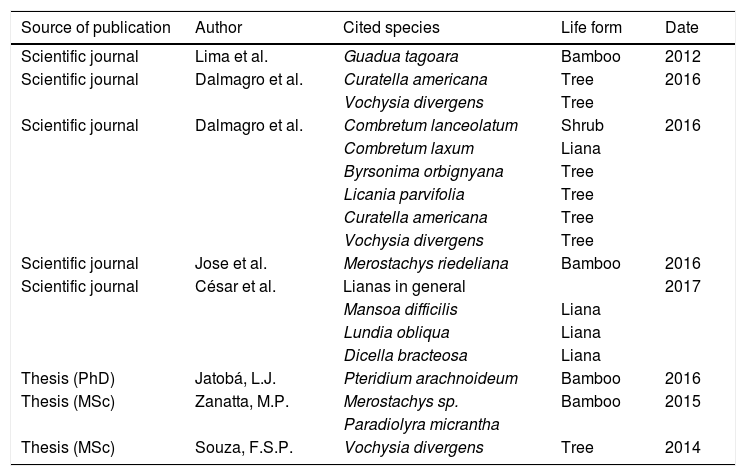
The search on ISI Web of Science resulted in 30 articles, and only five referred to super-dominant Brazilian plants (Table 1). We also found three theses that explicitly mentioned native species in the situation of “invasives” (Table 1). Although not identified in the Web of Science database (and therefore not included in Table 1) we found one paper through cross-reference (Nunes da Cunha and Junk, 2004).
Scientific papers and theses relating super-dominant plant species in Brazil, according to the systematic review carried out (see Methods and References for details).
| Source of publication | Author | Cited species | Life form | Date |
|---|---|---|---|---|
| Scientific journal | Lima et al. | Guadua tagoara | Bamboo | 2012 |
| Scientific journal | Dalmagro et al. | Curatella americana | Tree | 2016 |
| Vochysia divergens | Tree | |||
| Scientific journal | Dalmagro et al. | Combretum lanceolatum | Shrub | 2016 |
| Combretum laxum | Liana | |||
| Byrsonima orbignyana | Tree | |||
| Licania parvifolia | Tree | |||
| Curatella americana | Tree | |||
| Vochysia divergens | Tree | |||
| Scientific journal | Jose et al. | Merostachys riedeliana | Bamboo | 2016 |
| Scientific journal | César et al. | Lianas in general | 2017 | |
| Mansoa difficilis | Liana | |||
| Lundia obliqua | Liana | |||
| Dicella bracteosa | Liana | |||
| Thesis (PhD) | Jatobá, L.J. | Pteridium arachnoideum | Bamboo | 2016 |
| Thesis (MSc) | Zanatta, M.P. | Merostachys sp. | Bamboo | 2015 |
| Paradiolyra micrantha | ||||
| Thesis (MSc) | Souza, F.S.P. | Vochysia divergens | Tree | 2014 |
Comparing our results to Frehse's et al. (2016) on the exotic invasive species, who examined the same literature database (ISI Web of Science), the number of publications we found for Brazilian super-dominant species was extremely lower – only five – than the number found by Frehse et al. (2016) for exotic invasive plants: 121. Their search covered up to May 2014, and among the five publications of our search only one was previous to 2014 (Table 1), and therefore comparable to Frehse's et al. (2016) results. This comparison clearly shows that few studies have been devoted to the Brazilian super-dominant species, and almost all of the few available studies were published very recently.
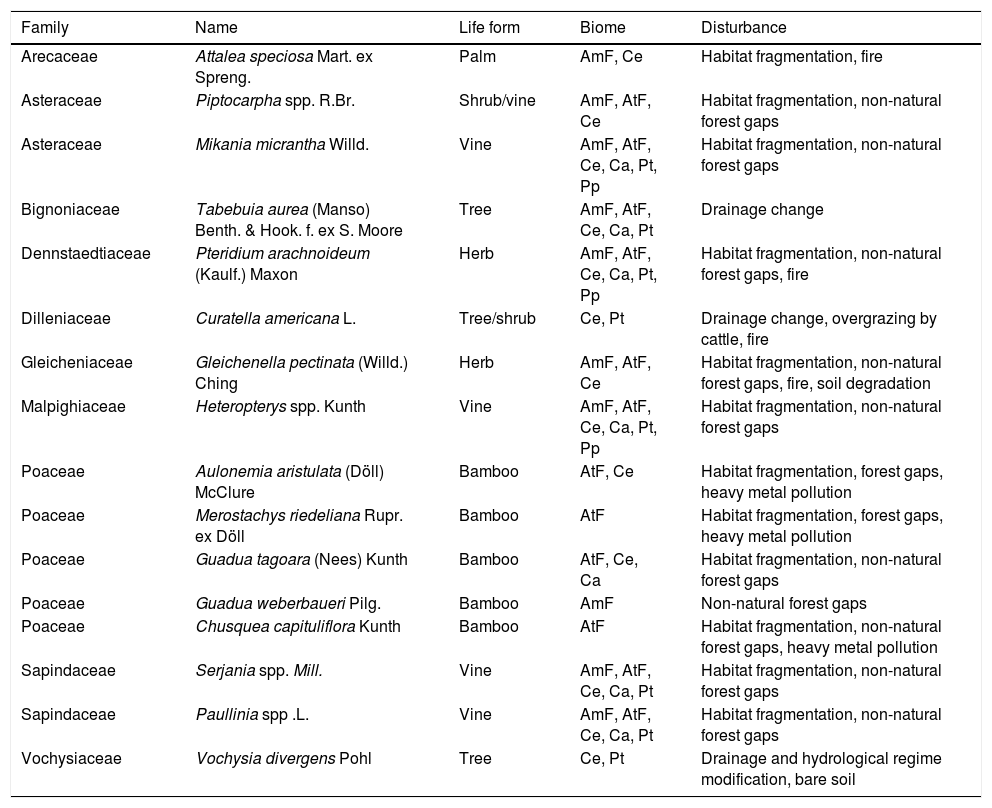
Examples of super-dominant speciesKnowing that a given species is able to develop explosive growth in its native range may serve to help managers avoid conditions that will trigger rapid growth and dominance by a single species. Thus, we produced a list of main examples on super-dominant species with the purpose of drawing the attention of environmental managers, scientists and policy makers about terrestrial plants that have been showing super-dominant populations in their native habitats – with extremely high growth rates – and causing serious negative impacts in different Brazilian ecosystems. This list was based on the above mentioned scientific literature (papers, books and theses, Table 1) as well as grey literature, papers and reports published in Portuguese – which are not easily accessible to an international audience – and our own experience. We elected 16 species (Table 2, Fig. 1), including their life forms, native biomes and the principal causes of their super-dominance. In the text below we also describe the main impacts they may cause to the natural or anthropic resources. The original habitats of the listed plant species followed the records of the Brazilian flora in Flora do Brasil (http://floradobrasil.jbrj.gov.br/) and specific papers that refer to such super-dominant species (cited below, as the species are described). This was an effort to gather the largest amount of information possible to the scientific community, managers and policy makers about the potential harm of super-dominance, taking those species as examples.
Species of terrestrial plants detected as super-dominant in Brazil, their life forms, the biomes where they occur, and the main human-mediated disturbances that trigger their super-dominance. (AmF=Amazon Forest, AtF=Atlantic Forest, Ce=Cerrado, Ca=Caatinga, Pt=Pantanal, Pp=Pampa).
| Family | Name | Life form | Biome | Disturbance |
|---|---|---|---|---|
| Arecaceae | Attalea speciosa Mart. ex Spreng. | Palm | AmF, Ce | Habitat fragmentation, fire |
| Asteraceae | Piptocarpha spp. R.Br. | Shrub/vine | AmF, AtF, Ce | Habitat fragmentation, non-natural forest gaps |
| Asteraceae | Mikania micrantha Willd. | Vine | AmF, AtF, Ce, Ca, Pt, Pp | Habitat fragmentation, non-natural forest gaps |
| Bignoniaceae | Tabebuia aurea (Manso) Benth. & Hook. f. ex S. Moore | Tree | AmF, AtF, Ce, Ca, Pt | Drainage change |
| Dennstaedtiaceae | Pteridium arachnoideum (Kaulf.) Maxon | Herb | AmF, AtF, Ce, Ca, Pt, Pp | Habitat fragmentation, non-natural forest gaps, fire |
| Dilleniaceae | Curatella americana L. | Tree/shrub | Ce, Pt | Drainage change, overgrazing by cattle, fire |
| Gleicheniaceae | Gleichenella pectinata (Willd.) Ching | Herb | AmF, AtF, Ce | Habitat fragmentation, non-natural forest gaps, fire, soil degradation |
| Malpighiaceae | Heteropterys spp. Kunth | Vine | AmF, AtF, Ce, Ca, Pt, Pp | Habitat fragmentation, non-natural forest gaps |
| Poaceae | Aulonemia aristulata (Döll) McClure | Bamboo | AtF, Ce | Habitat fragmentation, forest gaps, heavy metal pollution |
| Poaceae | Merostachys riedeliana Rupr. ex Döll | Bamboo | AtF | Habitat fragmentation, forest gaps, heavy metal pollution |
| Poaceae | Guadua tagoara (Nees) Kunth | Bamboo | AtF, Ce, Ca | Habitat fragmentation, non-natural forest gaps |
| Poaceae | Guadua weberbaueri Pilg. | Bamboo | AmF | Non-natural forest gaps |
| Poaceae | Chusquea capituliflora Kunth | Bamboo | AtF | Habitat fragmentation, non-natural forest gaps, heavy metal pollution |
| Sapindaceae | Serjania spp. Mill. | Vine | AmF, AtF, Ce, Ca, Pt | Habitat fragmentation, non-natural forest gaps |
| Sapindaceae | Paullinia spp .L. | Vine | AmF, AtF, Ce, Ca, Pt | Habitat fragmentation, non-natural forest gaps |
| Vochysiaceae | Vochysia divergens Pohl | Tree | Ce, Pt | Drainage and hydrological regime modification, bare soil |
Examples of Brazilian superdominant species: (a) Gleichenella pectinata, in Fontes do Ipiranga State Park, São Paulo State; (b) Pteridium arachnoideum, in Gonçalves, Minas Gerais State; (c) Chusquea capituliflora, in Fontes do Ipiranga State Park, São Paulo State; (d) Aulonemia aristulata, in Fontes do Ipiranga State Park, São Paulo State. (Photos authorship: a, c, d – Maria Tereza Grombone Guaratini; b – Jean Paul Metzger.)
Woody bamboos are important elements that contribute to forest diversity in South and Central America (Londoño, 1996) and especially in Brazil, which has the highest bamboo diversity in the Americas, comprising 89% of the genera and 65% of the known species (Filgueiras and Santos-Goncalves, 2004). Bamboos generally have discontinuous distribution but are able to show invasive behaviour in disturbed habitats, especially in forest borders and gaps (Vinha et al., 2011). Several studies have shown that bamboos change the structure and composition of tropical forests by severely reducing tree density, total basal area, richness, seedling establishment, and functional groups of plants (Griscom and Ashton, 2006; Rother, 2006; Griscom et al., 2007; D’Oliveira et al., 2013; Montti et al., 2014). The natural die-back process of bamboos may represent an opportunity to foster the recruitment of their seeds into seedlings and saplings.
The genera Chusquea, Merostachys and Guadua have been reported in the Brazilian Amazon and southeastern Atlantic forests to shift a diverse forest into an impoverished and homogenized community (Nelson, 1994; Torezan and Silveira, 2000; Vinha et al., 2011; Lima et al., 2012). In the southern São Paulo State about one third of the forest remnants are dominated by woody bamboos, mostly Guadua tagoara (Fantini and Guries, 2007), and the massive mortality of this species after the flowering season of 2004–2005 together with the consequent changes in the forest structure intensified landslides, which affected the traffic on a main highway. Actions to avoid new landslides in the area costed about US$ 4.2 million to the State government. Another potential impact by super-dominant bamboos is the rise on rodents’ population rates due to resource input when the massive seed production occurs. These rodents may carry hantavirus that causes deadly haemorrhagic fever in humans (Jaksic and Lima, 2003). In 2007, the massive flowering of Aulonemia aristulata increased rats populations in a State Park in São Paulo city (Parque Estadual das Fontes do Ipiranga), and thus rose snake populations.
Most bamboos grow fast and produce high amounts of biomass, and some species (as shown for A. aristulata by Grombone-Guaratini et al., 2013) respond positively to CO2 concentration by producing extra biomass, that increases even more their dominance and brings harmful predictions on a global climate change scenario. In addition, the accumulation of litter on the ground greatly increases the risk of wildfires (Grombone-Guaratini, unpublished data).
Lianas are frequently referred as structural parasites by forest managers, but they comprise a very important component of tropical forests, especially lowland and seasonal forests, as up to 25% of the woody species (Gentry, 1991). Therefore, they contribute substantially to forest biodiversity, primary productivity and carbon sequestration (Laurance et al., 2001; Schnitzer and Bongers, 2002). They show fast stem elongation in their search for light, climbing quickly on the trees; also, they are very efficient at water absorption and transportation, and regulate transpiration, which favours their success in forest gaps and under seasonal rainfall regimes (Phillips et al., 2002; Schnitzer, 2005; Schnitzer and Bongers, 2002, 2011; Swaine and Grace, 2007). Therefore, when their populations grow out of control they start to compete with the trees both above- and below-ground for light, water and nutrients, and often lead them to death; when in high abundance, lianas tend to retard forest succession (Schnitzer and Bongers, 2002; Schnitzer and Carson, 2010) or direct the forest composition by influencing the seed rain (César et al., 2017).
There is a positive association between liana overpopulation and human-caused disturbance, especially in Neotropical forests (Schnitzer and Bongers, 2002, 2011; Campanello et al., 2007; Swaine and Grace, 2007). Habitat fragmentation and the generation of borders, logging and the making of large gaps, and higher CO2 emissions, all contribute to increased liana abundance and biomass, as their physiological attributes (above mentioned) facilitate their growth under drier conditions of disturbed environments (Schnitzer and Bongers, 2011). However, lianas density starts to decrease when the level of disturbance limits the availability of host trees (Rice et al., 2004; Campanello et al., 2007). Species of the genera Mikania, Piptocarpha, Heteropterys, Serjania and Paullinia have been observed as developing explosive populations under disturbed conditions. Considering the intensifying environmental perturbation due to human activities and the adaptive characteristics of lianas to drier and warmer environments, a significant growth of abundance and biomass in this group is expected in Neotropical forests (Swaine and Grace, 2007; Gallagher et al., 2010; Schnitzer and Bongers, 2011), rising chances of super-dominance episodes with negative consequences to the overall biodiversity. Much of the Brazilian territory fits these predicted conditions of drier and warmer climate (IPCC, 2014).
Palms can become dominant in the community by many reasons: they are usually very fruitful and efficiently dispersed by birds, they can damage other species’ seedlings when their large and lignified leaves fall on the ground (Silva Matos and Watkinson, 1998; Queenborough et al., 2012), their seedlings tend to establish more successfully around the parental trees where the high number of seeds compensates the high rate of mortality (Silva Matos and Watkinson, 1998). An example of longstanding and irreversible state of super-dominance of palms is given by Attalea speciosa Mart. (Arecaeae) a tropical palm tree that occurs in the transition between the Amazon rainforest and the Cerrado of Piauí and Maranhão states, northeastern Brazil (Ribeiro and Walter, 1998; Mitja and Ferraz, 2001). These stands are so prominent that they are considered as a specific vegetation type, the Cocais Forest (Ferri, 1980). Its origin probably dates to the XVIII and XIX centuries, when large areas were deforested for pastures and crops (Santos-Filho et al., 2013). Fire, used for clearing land, was a decisive factor for causing and maintaining its super-dominance, which stimulates fruit opening and germination (Viveiros, 1943). Therefore, the origin of these mono-dominant forests was probably disturbance-mediated while biological characteristics, e.g., species-specific plant–soil relationship and high propagule production, may be responsible for their success.
Ferns can also develop huge populations and form continuous monospecific extents. For example, Pteridium arachnoideum (Kaulf.) Maxon. (Dennstaedtiaceae) may be considered a transformer species as it produces large frond and rhizome biomass, large amount of litter and allelopathic compounds affecting soil acidity and microbiota (Silva Matos et al., 2014). It dominates the soil seed bank in areas of the Atlantic Forest, and affects the natural process of secondary succession by outcompeting other species, especially in the seedling phase (Silva and Silva Matos, 2006; Silva Matos and Belinato, 2010; Xavier et al., 2016). This species also grows successfully in the Cerrado, negatively impacts the vegetation structure, and increases the risk of wildfires due to the accumulation of a great amount of necromass (Miatto et al., 2011; Silva Matos et al., 2014). As the area burns, the spread of P. arachnoideum is facilitated, and a feedback loop is created (Silva Matos et al., 2005, 2014; Silva and Silva Matos, 2006). Even in the surroundings of these highly dominated areas, where the aboveground biomass of P. arachnoideum is not so abundant, this species is super-dominant in the soil seed bank (Silva and Silva Matos, 2006). This represents a serious conservation problem, as the contaminated seed bank does not permit the recovery of natural diversity. The dominance of P. arachnoideum in Brazilian native grasslands also causes concern for animal health, as cattle that feed on it may become intoxicated with subcutaneous and internal bleeding, as well as carcinogenic mucosal ulcers (Hirono et al., 1973; França et al., 2002; Marçal et al., 2002). Milk consumption by humans from contaminated animals is not recommended since experiments showed the development of tumours in mice fed with milk from cows that consumed Pteridium sp. (Marçal et al., 2002).
Another fern, Gleichenella pectinata (Willd.) Ching (Gleicheniaceae), can also be considered super-dominant. The super-dominance of this species is mostly related to clonal reproduction besides the large amount of wind dispersed spores it produces, as well as strong phytotoxic and inhibitory compounds (Peres et al., 1998; Voltarelli et al., 2012). G. pectinata is mostly found in shallow and poor soils, usually in slopes, and the dense canopy formed by its fronds together with inhibitory compounds outcompete native plants. G. pectinata can also grow over P. arachnoideum stands and cause the death of its fronds (D.M. Silva Matos, personal observation).
Some trees that are frequent in the hyperseasonal savannas of the Pantanal may advance on the natural fields, massively encroach and form almost monospecific huge stands (Pott and Pott, 1994, 2009; Santos et al., 2006; Soares and Oliveira, 2009; Dalmagro et al., 2016a), therefore, being recognized as super-dominants, particularly the species Curatella americana L. (Dilleniaceae), Tabebuia aurea (Manso) Benth. & Hook. f. ex S. Moore (Bignoniaceae) and Vochysia divergens Pohl (Vochysiaceae) (Dalmagro et al., 2016a,b). The Pantanal is a complex of savanna-seasonal forest-grassland vegetation subjected to a natural flooding regime, and a mosaic of these phytophysiognomies vary spatially and temporarily according to the level of soil water saturation (Junk et al., 2006; Pott et al., 2011). Those above mentioned species are conditioned primarily by the plurennial cycles of drought and flood, but may also be determined by soil type and human action. C. americana and T. aurea spread out in the drier years, while in the wetter years V. divergens expands (Pott and Pott, 2009; Ferreira Júnior et al., 2016; Nunes da Cunha and Junk, 2004). Pott and Pott (2009) also attribute the establishment of C. americana and T. aurea according to soil texture, being the first best fit to sandy soils, and the second to more clayey soils (although this is not a consensus, see Nunes da Cunha and Junk, 2004). Anthropogenic activities mainly initiated by deforestation and agriculture (crop plantations and planted pastures) as well as the construction of roads and dams may bring erosion and silting, that changes the natural drainage and water flux, and thus the structure of the community, favouring the overabundance of such species. Also the inadequate management of cattle in native pastures, with frequent burnings and overgrazing may favour the encroachment of C. americana and T. aurea. The super-dominance of these trees not only seriously threatens the regional biodiversity but also modifies the complex and dynamic functioning of the ecosystems. Moreover, the massive tree encroachment decreases the productivity of cattle farms (Santos et al., 2006; Dalmagro et al., 2016a).
Concluding remarksThe issue of super-dominant species has not been given enough attention in the scientific literature worldwide (Carey et al., 2012; Valéry et al., 2013), and we could demonstrate this same gap in Brazil, where also a lack of legal support hampers management initiatives. Although a reasonable amount of laws and regulations have been created to establish clear procedures regarding the introduction and control of exotic species in the country (e.g., Law 9.985 of July/18/2000, Decree 4339 of August/22/2002, CONABIO Resolution 5 of October/21/2009), there is no specific legislation regarding cases of super-dominance, and the management of native species is not regulated. In addition, as the environmental legislation is severe against damages on native species, managers are reluctant to take any action against super-dominants even when severe impacts are evident, since such actions may be interpreted as non-compliance with the law and bring them penalties. However, super-dominant species bring negative impacts on natural ecosystems – which may be irreversible – and must be managed for the purpose of conserving the biodiversity, the natural resources (art.9, Law 9.985, of July/18/2000) and the ecosystem services they offer.
Among the 16 selected Brazilian super-dominant plants a variety of life forms is represented, and most of them naturally occur in wide-ranging habitats (Table 2), showing high ecological plasticity. Anthropogenic disturbances are the major drivers of them, as in other parts of the world (Simberloff et al., 2012). Habitat fragmentation and the associated edge effects, wildfires, human-made forest gaps, and the intensification of seasonality and extreme events (consequences of climate change), are the main drivers of super-dominance of terrestrial plants. Based on the highlighted species we can say that for all the biological groups mentioned, the species with the greatest chance of becoming super-dominants are those which can quickly and efficiently use the available resources, reproduce intensely and soon occupy the spaces, and settle well in human-altered environments – as it happens with most exotic invasives (Grotkopp and Rejmánek, 2007).
In future scenarios of intensification of land use, pollution and climate change – major anthropogenic disturbances – we may expect an increase in both the invasiveness of exotics and changes in the dynamics of native species, which may spread their range and threaten the persistence of other natives, even in areas currently not suitable for such processes (Bellard et al., 2014). Land use will be directly and indirectly affected by climate change, hence the prospect in any future scenarios is likely for super-dominant species to become more frequent. In this sense, it is essential to identify not only the situations of super-dominance but also how land use, habitat fragmentation and climate change interact to cause explosive population growth of a given species, and to define potential management strategies for them. Modelling the ability of native species to shift regions under climatic change contributes to the identification of potential super-dominant natives, as well as invasive aliens, and for the appropriate control and/or restoration actions (Webber and Scott, 2012).
For the conservation of diversity and ecosystem services, scientists and decision makers should work closer, combining ideas and future directions to define strategies. One important first step is to become aware of the problem that super-dominance by native species has negative impacts, and share information on super-dominant species. Evaluation of the risk of super-dominance cases, types of disturbances favouring them, and their prevention should be the focus of future studies.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are very grateful to Sidinei Magela Thomaz for his valuable comments, to Flávia Bottino for organizing Fig. 1, and to Kevin J. Murphy for reviewing English.
This research has not received any specific grant from funding agencies.