The restoration of terrestrial ecosystems damaged by human activities is an urgent priority worldwide (Higgs et al., 2014). Reforestation is one of the most direct and efficient methods for reversing ecosystem degradation (Lamb et al., 2005) and contributing to biodiversity conservation. Reforestation is also seen as a fundamental tool for mitigating greenhouse gas emissions, and has inspired programs such as REDD+ (Reducing Emissions from Deforestation and Forest Degradation) and CDM (Clean Development Mechanism) (Canadell and Raupach, 2008). While reforestation implies planting trees on deforested land, afforestation is the planting of trees where they do not occur naturally (Putz and Redford, 2009). The term afforestation remains widely used in reference to this phenomenon, although the products, such as monoclonal or monocultures, cannot be considered forests in the ecological sense of the word (Putz and Redford, 2010). In their natural condition, many ecosystems are not dominated by trees (i.e., open ecosystems), including savannas and grasslands, for which reforestation is inappropriate (Veldman et al., 2015b,c). Savannas, in particular, cover approximately 33 million km2 at mostly tropical latitudes, and are one of the planet's largest non-forest biomes (see Beerling and Osborne, 2006, and references therein).
The so-called reforestation agenda that targets these naturally non-forested ecosystems is actually not reforestation at all, but a different process – afforestation. Afforestation has been applied massively in Asia, Africa, and South America, with the main focus being short-term economic benefit from rapidly obtaining timber, charcoal, pulp, oil or fruits (Jaiyeoba, 2001; Epron et al., 2009; Xu, 2011; Jagoret et al., 2012; Koh and Wilcove, 2008; Romero-Ruiz et al., 2012; Tang et al., 2013; Vargas et al., 2015). Afforestation of open ecosystems is an incongruous disturbance and, indeed, an impending ecological disaster. The practice of afforestation is in need of urgent evaluation of its causes and its consequences, and the need for policies to address it. Here we review what is known about the ecology and biodiversity of savannas and document current trends of afforestation and discuss its future, with particular emphasis on South America.
Afforestation in savannasWe define a savanna as a phytogeographic domain comprised of a complex of physiognomies (Bourlière and Hadley, 1983; Coutinho, 1978, 2006). Savanna ecosystems are increasingly becoming recognized for their essential ecosystem services, including provisioning of water, production of livestock forage and carbon storage, that latter being comparable to that of forests when above and belowground biomass is considered (Overbeck et al., 2015). However, savannas around the world have been impacted by anthropogenic activities and are currently threatened by many factors, including afforestation (Veldman et al., 2015b,c; Bond, 2016). The practice of afforestation of savanna areas is rapidly growing, such as the planting of exotic eucalypt and pine monocultures, especially in Brazil, Colombia, Nigeria, Congo, and China. In other countries, savannas are being afforested for the production of other commodities, such as palm oil in Latin America, and fruit in China (Jaiyeoba, 2001; Xu, 2011; Jagoret et al., 2012; Tang et al., 2013). In Colombia, for instance, the area occupied by oil palm plantations more than doubled over the last ten years (Vargas et al., 2015) largely through the afforestation of savannas (Romero-Ruiz et al., 2012).
Since savannas are largely located in developing countries, where the economy is fundamentally based on primary activities (e.g., agriculture, livestock, mining and silviculture), they have suffered considerable historical conversion (Myers et al., 2000; Silva and Bates, 2002; Klink and Machado, 2005; Fernandes et al., 2016a,b). Such conversion is translated into a major impact on biodiversity and ecosystem services, which we summarize here based on an analysis of afforestation of the Brazilian savanna known as cerrado, with the hope of raising awareness of the impending disaster that this practice represents for all the world's savannas.
The Brazilian savanna: concepts and threatsAs is the case with many savannas, the cerrado is a rich ecological mosaic with more than a dozen different formations or physiognomies ranging from open natural grasslands where tress are absent (e.g., open grasslands, rupestrian grasslands) to savannic vegetation (cerrado stricto senso), to partly forested vegetation under some particular local ecological conditions (e.g., cerradão, gallery forests) (Sano et al., 2007; Fernandes et al., 2016a). While the non-forest formations (grasslands and savannas) are fire-tolerant, the forested formations are not (Dantas et al., 2013a,b).
Cerrado has a significant number of endemic species, and it is one of the five South American biodiversity hotspots (Myers et al., 2000; Joppa et al., 2011), with 4.8% of the world's plant species (Fig. 1) (Ratter et al., 1997). Almost 40% of its 13,140 angiosperm species are endemic. Eighty-five percent of the plant species are shrubs and herbs, with trees representing only 15% (LEFB, 2015). In addition, cerrado landscapes hold the headwater springs of major Brazilian rivers that are responsible for the maintenance of critical hydrological dynamics of vast areas (Fernandes et al., 2016a). Yet, despite its critical biodiversity and the importance of its ecosystem services, the cerrado vegetation is being destroyed, with its natural plant cover being removed faster than that of any of the world's savannas. More than half of its 2 million km2 (an area about the size of western Europe, or slightly larger than Mexico) has been converted to agricultural land and pasture since the 1960s, and it is now ranked second among the Brazilian vegetation types in the number of threatened species (Klink and Machado, 2005). The expansion of agribusiness into the cerrado, which largely serves Chinese and European markets, was hailed as an economic miracle and shows no sign of attenuation (Liu and Diamond, 2005; Coelho et al., 2013; Gibbs et al., 2015). In light of this situation, this savanna was recently targeted as a potential “region for reforestation” to meet the Bonn Challenge target to globally “revegetate” 150 million ha by 2020 (Laestadius et al., 2011). An Atlas of Forest Landscape Restoration Opportunities (hereafter the Atlas) was published by the World Resources Institute and targeted 23 million km2 of forested lands to be restored (WRI, 2014). Surprisingly, the cerrado was listed for “reforestation” therein, under the premise that “all lands biophysically capable of supporting a tree canopy cover of at least 10% were included” (Rojas-Briales, 2015). The initiative of the Atlas to identify and map opportunities for landscape restoration is undoubtedly well intended. Nevertheless, the implementation of a reforestation program in savannic formations – that is, afforestation – of this large region would be an act of extraordinary folly. It is an ecologically incorrect and, indeed, hard to achieve initiative (Veldman et al., 2015b,c), for the cerrado is not, and never was, a forested ecosystem. Even scientists have been ignored this incongruence in some specific analysis (Beuchle et al., 2015). With the exception of riparian or otherwise very localized woodlands, most of the cerrado is grassland and scrub (Simon et al., 2009).
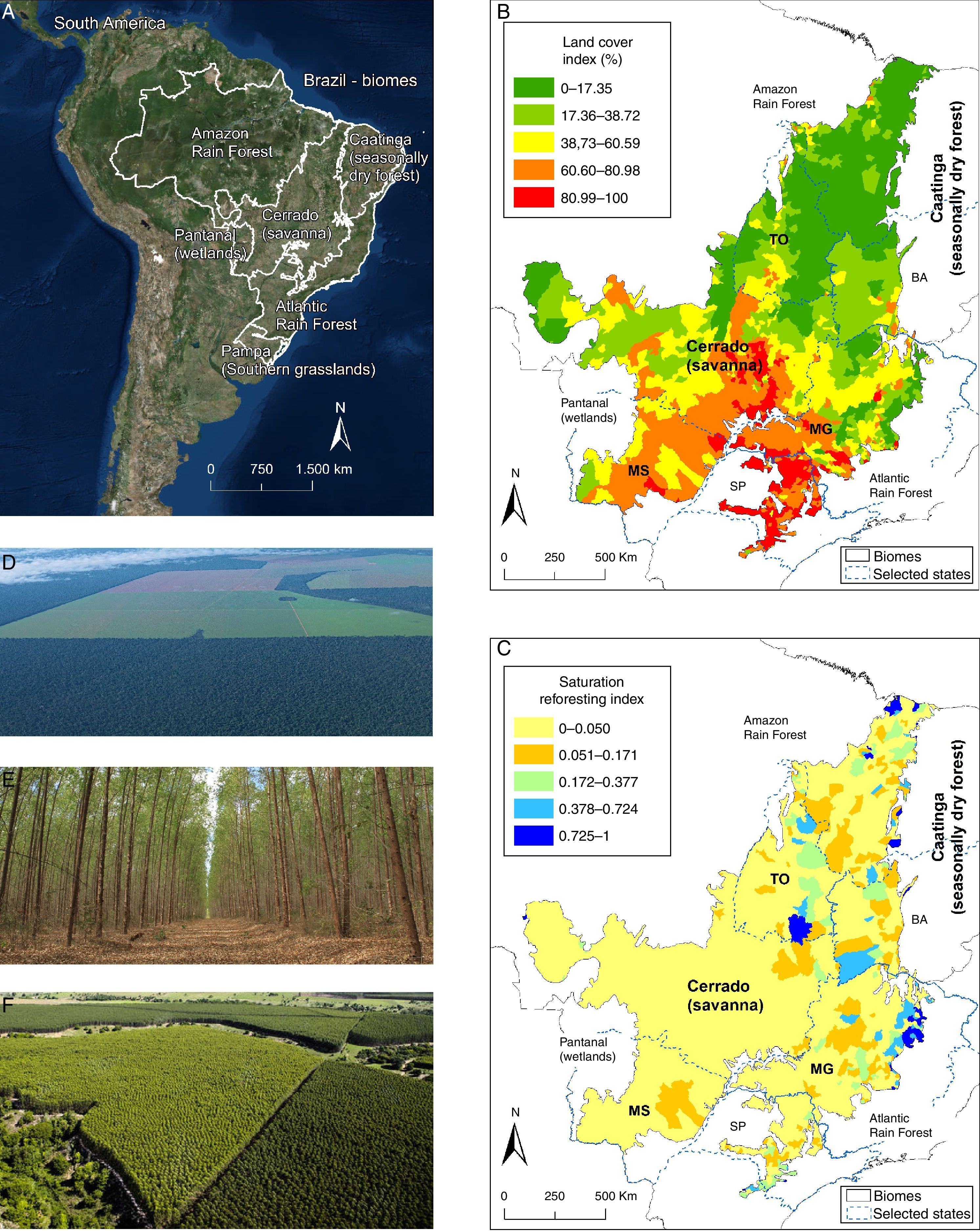
(A) The cerrado (savanna) and surrounding Brazilian physiognomies. (B) Map (year 2008) depicting the Cerrado land cover index. Maps B and C highlight the political contours of the states of Bahia (BA), Mato Grosso (MG), São Paulo (SP), Mato Grosso do Sul (MS), and Tocantins (TO) (see text for details). The lower the percentage value, the less anthropic conversion. (C) Cerrado saturation reforesting index (year 2006, see below) with eucalypt plantations. Lower values of the saturation reforesting index indicate the municipalities with lesser presence/area of eucalypts; saturation index 1 indicates maximum saturation (entire area converted to eucalypts plantation). Higher values of the saturation reforesting index indicate the municipalities with larger areas dedicate to eucalypt plantations. The expansion of eucalypts in such areas must be avoided because they coincide with municipalities with the highest remnant vegetation in the Cerrado, as seen in (B). (D) Land use change in the Cerrado. (E) Eucalypt plantation. (F) Aerial view of a Cerrado area converted to eucalypt stands. (Photo D, by J. Fragoso; photo E, by M. Andrade; photo F from http://www.portaldoreflorestamento.com.br/). See additional information in supplemental material.
In fact, and according to most recently published mapping of the cerrado biome – TerraClass Cerrado project (Brasil, 2015) – among the areas with remaining vegetation, about 418,840km2 were classified as a forest physiognomy, while approximately 700,000km2 are a savanna physiognomy. Given that almost 1 million km2 has already been deforested and occupied by pastures, agriculture and urban centers (in this order, mainly in flat areas), it can be assumed that the original area with savannas was at least double that which stands today. Unfortunately, from such a perspective many pasture areas (land use class dominant, and already practically tied with savanna in size, with just over 600,000km2) are being reforested with exotic woody species, aiming to increase income and beef productivity (applying livestock-crop-forest integration). Also, according to the TerraClass Cerrado report, the official area of planted forests in this biome is 30,607km2, which, as presented and discussed here, tends to spread through the remaining vegetation.
In addition, many of the species of this savanna are fire tolerant, and so fire plays an important role in its stability and complexity (Veldman et al., 2015a); one of the aspects that determines functional differences between forests and savannas (e.g., Dantas et al., 2013a).
Afforestation in Brazilian savannaTypical cerrado plants have evolved long radicular systems enabling them to reach water up to 20m below the surface (Hoffmann and Franco, 2003). Exotic “reforestation species”, such as eucalypts and pines, are not able to absorb water in the same way and instead they exhaust superficial water. Consequently, this “enrichment” or replacement of savannas with such forest trees negatively impacts biodiversity and the functioning of the original ecosystem (Hoffmann and Franco, 2003), effectively leading to perverse conservation outcomes (Putz and Redford, 2009; Veldman et al., 2015c). This is particularly true when afforestation is done via tree monocultures, which seriously challenges the purported benefit of increasing carbon sequestration (Putz and Redford, 2009; ABRAF, 2013; Veldman et al., 2015c). Furthermore, the negative effects of afforestation are not only local, but regional as well, often causing substantial losses to stream flow in watersheds, and increased soil salinization and acidification (Jackson et al., 2005; Wang et al., 2015). On the other hand, plantations purported as cerrado restoration are misleading. Even when geographically native trees are planted, the negative consequences will likely be the similar to those exerted by plantations with exotic trees. Furthermore, from a practical perspective, afforestation is no easy task. Eucalypt cultivation, for example, requires great investment in machinery, fertilizers and pesticides, and planting is costly, requiring cloned seedlings to ensure stand uniformity and optimal performance. Considering a full harvesting cycle, until the first cut – 7 years on average – the cost of eucalyptus management can reach US$ 2500.00/ha, significantly higher than that for other commodities, including those related to food production such as soybeans (US$ 600.00/ha), or corn (US$ 1200.00/ha) (IBGE, 2013).
Although there are policies in place for the control and supervision of reforestation in the cerrado (Brazilian federal and state forest laws) (Soares-filho et al., 2014), there remain strong socioeconomic pressures that encourage its expansion. Afforestation in cerrado is gaining strength, stimulated by the demand for charcoal for the iron and steel industries, and for pulp for paper companies. Advances in infrastructure, such as increased rural electrification and new paved roads, plus credit/financing with subsidized rates, among other factors, are attractive but perverse incentives for the establishment of charcoal and wood-cellulose companies in this ecosystem. In the face of this threat, the cerrado is in need of a specific ecological restoration program, which must be based on the specific features of this ecosystem.
Pine and Eucalypt as the main threatsThe total land area planted with pines and eucalypts in Brazil has seen an 18.3% increase in seven years (from 5.6 million ha in 2005 to 6.7 million ha in 2012). Three Brazilian states – Minas Gerais (MG), São Paulo (SP) and Bahia (BA) (see Fig. 1B and C) – represent 25% of the cerrado (56 million ha), and contain 62.3% of the national eucalypt production. Eucalypt plantations alone grew by 44.2% (ABRAF, 2013), expanding from 3,745,794ha to 5,402,030ha during this period (IBGE, 2006; ABRAF, 2013). All Brazilian states that have cerrado vegetation now have eucalypt plantations, which represents 20.67% of the area planted with eucalypt in the entire country (IBGE, 2006; ABRAF, 2013) (Fig. 1). In the states of Tocantins (TO) and Mato Grosso do Sul (MS), which are predominantly covered by cerrado, eucalypt plantations increased 4.9- and 7.8-fold, respectively, from 2006 to 2012 (ABRAF, 2013), and there are no signs that this trend will attenuate. Indeed, the area planted with eucalypt increased by 40% in Tocantins and 19% in Mato Grosso do Sul in a single year (2011/2012). Between 1990 and 2010, 61% of the eucalypt plantations in Três Lagoas (MS) were established in areas formerly covered with native cerrado vegetation, while another 25% and 13% were on pastures and agricultural lands (including former tree plantations and soybean and corn crops), respectively (ABRAF, 2013).
According to these trends (depicted in Fig. 1), the eastern portion of the cerrado will be almost completely obliterated with eucalypt or pine plantations, with few available areas for additional afforestation. In this paper we calculated a “reforestation saturation index” which is the proportion of degraded area occupied by eucalypt or pine plantation at the level of municipality. Lower values of the saturation index (not degraded=0) indicate the municipalities with lesser presence/area of eucalypt or pine, whereas higher values (maximum saturation=1) indicate a greater presence/area converted to eucalypts or pine. Higher values of the saturation index, therefore, indicate the municipalities with larger areas dedicated to these plantations. As shown in Fig. 1C, many municipalities planted with eucalypts and other species have saturation index values ranging from 0.05 to 1 (see Fig. 1C). In cases where there is an extensive eucalyptus plantation encompassing a large part of a municipality, new plantations will necessarily replace crops or pastures, or require the destruction of the remaining native cerrado ecosystems, be they grasslands, savannas or even forest formations. In the same way, the northeastern portion of the cerrado contains extensive eucalyptus plantations, but also large areas of remaining native cerrado (see Fig. 1B). In this sense, native cerrado species (and eventually small agricultural or pasture lands) will be substituted with further afforestation.
The tropical and subtropical climate of the Brazilian savanna macroregion make it highly attractive for eucalypt monoculture because the time needed to reach harvest does not exceed 7 years, while in temperate regions it can take up to 50 years (Soares et al., 2010). The favorable climate and the short time period for rotation of these plantations represent a short-term economic advantage for Brazil, but this should not, however, be the only factor taken into account in consideration of the expansion of this industry. There have been major investments in this sector in the cerrado, particularly in the southeastern portion of Mato Grosso do Sul (MS), where two of the world's major cellulose companies (Fibria Cellulose SA and Eldorado Brasil Cellulose SA) are located. Their plans for expansion should have targeted areas previously used for pasture and agriculture – areas already environmentally impacted – rather than areas of native cerrado vegetation. Unfortunately, most of the afforestation in this region has been very close to rivers (within 1km, and in many cases even less than 100 meters), which poses a severe risk to regional water resources by affecting groundwater recharge, variation in water discharge and sediment load, thereby altering the local and regional hydrological cycles. Furthermore, the negative impacts of afforestation on water are not solely restricted to the riparian zones, as the whole watershed is impacted with negative effects on water production. Brown et al. (2005) examined published paired-catchment studies of catchments belonging to one four broad categories (e.g. afforestation, deforestation, regrowth and forest conversion experiments), and showed clearly that a 20% change in vegetation cover is a threshold level after which a significant change in water yield occurs. This impact is exacerbated by that of roads (less than 1km from plantations) developed to facilitate management, harvest and market flow (Brown et al., 2005).
Concluding remarksThe social impacts of afforestation must also be considered. Indigenous and local communities in the cerrado region meet their fundamental needs and earn monetary income from subsistence farming and harvesting of wild products (Scariot, 2013). These products vanish with industrial afforestation with monocultures and thus destroy the livelihoods of these residents (Cardinale et al., 2012; Pacheco, 2012). Afforestation in the cerrado should only be implemented following a thorough assessment of the status of the previous native vegetation, and after taking into account not only the economic but also the ecological, social and cultural costs.
Although afforestation of the cerrado ecosystem may be consistent with the aims of the previous-mentioned Bonn Challenge, revegetation of degraded areas of cerrado with native species at their historical densities represents a competitive alternative. Such an approach would not only contribute to the global revegetation target, but would simultaneously avoid biodiversity loss and socioeconomic disruption to local communities. The pressure from the exotic monoculture industry has increased significantly in the last twenty years (Coelho et al., 2013; Gibbs et al., 2015), and areas originally covered by savanna are giving way to pine and eucalypt plantations. A better understanding of the trade-offs between land use and conservation can provide insight to a common sense approach to unifying the financial gain of an important economic sector, dominated by a few companies, with the collective interest of society's demand for the maintenance of ecosystem services provided by the biodiversity of the cerrado (Klink and Machado, 2005). Failure to understand or misinterpreting the concepts of revegetation and afforestation can conceal, and indeed favor, the short-term economic interests of a few to the detriment of the global conservation targets advocated by the CBD (Convention on Biological Diversity) and defended by the majority of Brazilian society (Stickler et al., 2009). An important way to avoid this huge ecological mistake and correct erroneous policies is to foment research on the use and implementation of native plants in the proper ecological restoration of this ecosystem (Veldman et al., 2015b). Many of the 13,140 angiosperm species native to the cerrado, and their above- and below-ground carbon storage, would continue to contribute significantly to carbon sequestration and would help in the restoration of degraded areas of the second largest South American ecosystem. Urgent action from research institutions, government agencies and international organizations are needed to prevent the impending ecological disaster that afforestation with alien – or even geographically native – tree species would bring to the world's savannas.
Conflicts of interestThe authors declare no conflicts of interest.