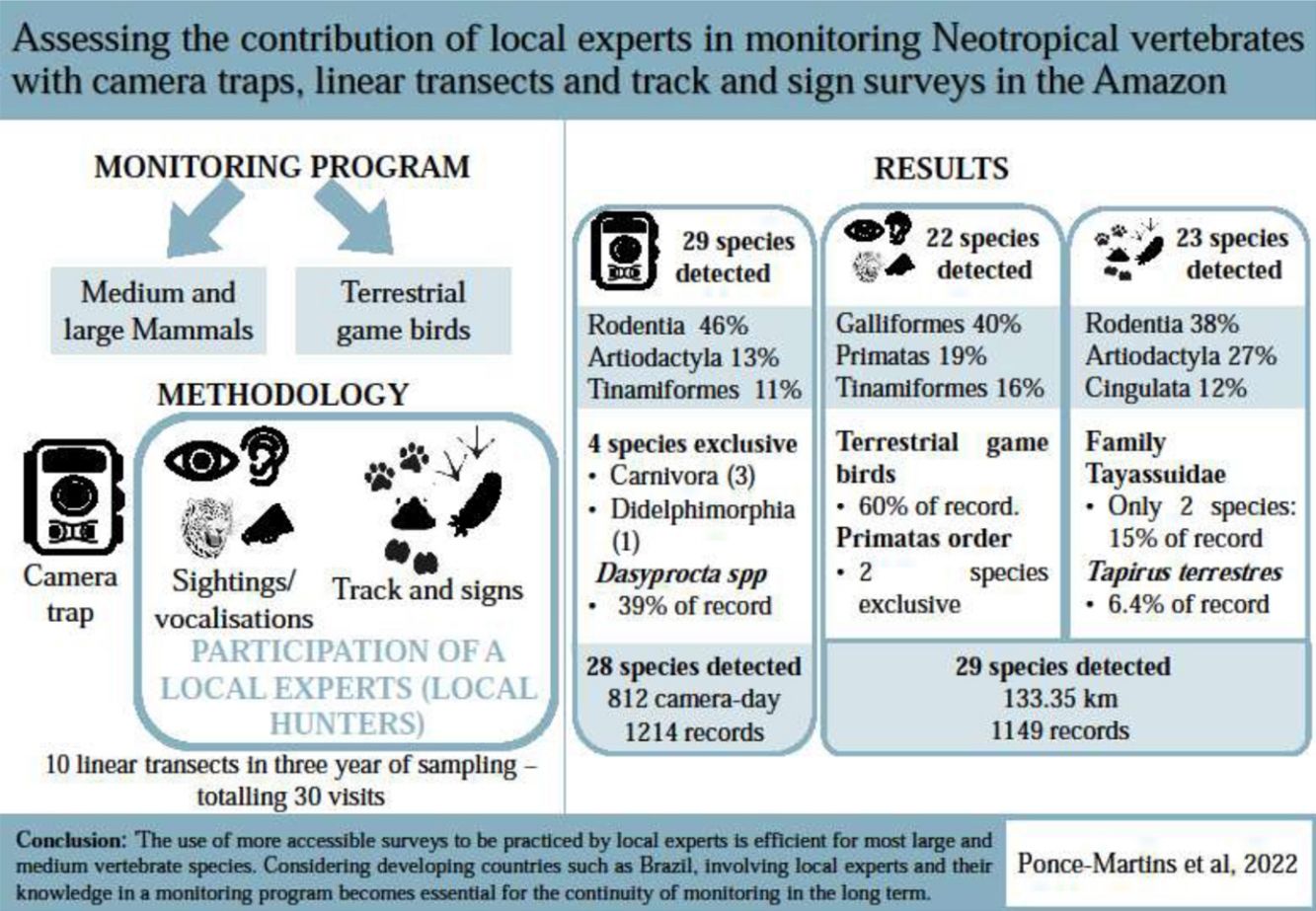
Given the need for consistent databases for conservation planning and management in protected areas, a challenge is to develop more accessible approaches, while ensuring that the data are robust and fit for purpose. We compared the assemblage of medium and large vertebrates using three techniques, camera trap, diurnal line-transect census (sightings/vocalisations) and track and sign surveys, the latter two being carried out with the participation of local experts (local hunters). We observed that the methods employed were selective in detecting groups of species and, therefore, their use in combination is recommended for a more comprehensive assessment of wildlife biodiversity, as well as for detecting population trends. When evaluating the sightings/vocalisations and tracks and signs data together (given they can be applied concomitantly) in comparison to the camera traps, we verified that broadly the same numbers of species were detected, recording 29 and 28 species, respectively. The sightings/vocalizations were more efficient for detecting primates; track and signs allowed detection of most nocturnal and cryptic or evasive species (e.g., Artiodactyla and Cingulata); camera traps are most effective for rare carnivores and rodents. Thus, in an ideal scenario, the three methods should be used to monitor these species, however, the use of more accessible surveys to be practiced by local experts are efficient for most large and medium-sized vertebrate species. Considering developing countries such as Brazil, involving local experts and their knowledge in a monitoring program becomes essential for the continuity of monitoring in the long term.
Medium and large Neotropical vertebrates are essential for maintaining the structure and composition of tropical forests (Bagchi et al., 2018; Bello et al., 2015; Fragoso et al., 2003; Galetti et al., 2013, 2006; Peres et al., 2016; Ripple and Beschta, 2012; Sobral et al., 2017). Moreover, they are considered good environmental indicators, owing to their potential to represent the conservation conditions of more than one biome or region of the country, or for comparisons between countries (Beaudrot et al., 2016; ICMBio, 2018). Thus, monitoring these animals is essential for understanding the compositional patterns of biological communities and the changes caused by human impact in these communities, and they are essential for decision making regarding species management and monitoring of natural habitats and Protect Areas (Beaudrot et al., 2016; Costa-Pereira et al., 2013; Pezzuti et al., 2022).
Different methods can be used to monitor populations and communities of medium and large Neotropical vertebrates; however, not all available methods can be applied effectively in all ecosystems, for all species, and in all situations (Fragoso et al., 2016, 2019; Silveira et al., 2003). In tropical forests, the diurnal line-transect census method (Buckland et al., 1993), is one of the oldest, simplest, cheapest, and most popular sampling techniques (Munari et al., 2011; Peres, 1999; Silveira et al., 2003); however, this requires favourable field conditions, trained personnel with experience, and the ability to detect animals (Fragoso et al., 2016; Silveira et al., 2003). Traditionally, line transects only involve direct records, for example, the visual or acoustic detection of animals. Alternatively, indirect records, which involve counting tracks and other signs (e.g., footprints, faeces, gnawed fruits, etc.) (Voss and Emmons, 1996), can be simultaneously collected, increasing the detection efficiency of rare or cryptic animals with nocturnal habits, such as many carnivores (Fragoso et al., 2016; Liebenberg, 2003; Smallwood and Fitzhugh, 1995).
Camera traps have become an increasingly popular tool for assessing, inventorying, and monitoring elusive forest vertebrates (Beaudrot et al., 2016; Benchimol and Peres, 2015; Srbek-Araujo and Chiarello, 2005; Trolle et al., 2008). This technique is less dependent on environmental conditions, does not require experienced field staff, and allows for non-invasive surveys (Munari et al., 2011; O’Brien et al., 2010; WWF, 2017). Compared to other sampling methods, camera traps are more suitable for standardisation because human influence and errors are reduced to installation/removal, maintenance of the camera traps, and photograph identification. This approach is more expensive, however, because the equipment requires constant maintenance (and such services are limited in many countries) and replacement. In addition, camera-trap sampling is subject to some degree of bias (i.e., manufacturer and model, installation site, battery life, etc.) and these devices are vulnerable to damage and theft (Jacobs and Ausband, 2018; Wearn and Glover-Kapfer, 2017).
Given the different approaches available, monitoring medium-large vertebrates requires efficient and reliable data collection methods, with standardised protocols allowing comparisons over time. The individuals performing the monitoring are just as important as the methods used. Community-based citizen science has proven to be an increasingly useful tool in ecological and conservation studies (Liebenberg et al., 2017; MacPhail and Colla, 2020). Indeed, local experts can be as effective in data collection as trained scientists (Danielsen et al., 2014; Temple et al., 2020) and can play a significant role in monitoring programmes when properly trained as part of well-designed projects (Danielsen et al., 2005; Liebenberg, 2003; Luzar et al., 2011). Moreover, involving local communities in monitoring increases management responses at the local scale and the speed of decision-making in the face of environmental threats (Abrahams et al., 2017; Beirne et al., 2019; Danielsen et al., 2014, 2010; Pereira and Diegues, 2010).
For long-term monitoring, choosing the most appropriate method requires a full evaluation of the potential limitations, such as accessibility to the area, interaction with the local population, and available budget (Gaidet-Drapier et al., 2006; Lyra-Jorge et al., 2008). It is also important to evaluate a country’s political and social context. For countries such as Brazil, which presents some challenging environmental policies (Artaxo, 2019; Diele-Viegas et al., 2020), the search for efficient, long-lasting, and financially accessible monitoring methods is essential. The promotion of community-based citizen science offers significant potential in this search, and is seen as crucial to improve decision-making at all levels of resource management (Danielsen et al., 2014).
Here, we report an animal monitoring study in a Protected Area (PA) in the eastern Amazon with the aim of comparing the sampling effort, selectivity, and complementarity of different methods. With the participation of the local population, we counted sightings/vocalisation, undertook active searches for animal tracks and signs, and employed camera traps. Overall, we sought to contribute to strategies that guarantee the maintenance of monitoring programmes based on an integrated sampling effort.
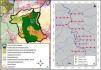
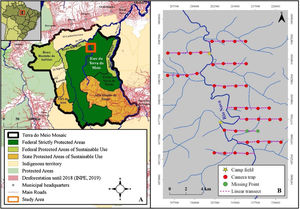
Material and methodsStudy areaThe study was conducted at the Terra do Meio Ecological Station (EETM) in the central-western region of the State of Pará. This Protected Area (PA) is approximately 3,373,110 ha and is located between the Xingu River and its tributary, the Iriri. The Terra do Meio Mosaic (Fig. 1A) is formed by Integral Protection Areas, Sustainable Use Areas, and Indigenous Lands (Law No. 9985), and represents the principal block of protected tropical forests in the eastern Amazon. The PA is located in an unconsolidated frontier of interest for agribusiness, illegal logging, and mining (Fearnside, 2005; Schwartzman et al., 2013). The climate is hot and humid, with a distinct dry season that extends from May to October (Camargo et al., 2004). The human population of the region is made up of small communities (between one and 26 houses) that descended from rubber tappers, forming the main non-indigenous traditional inhabitants (ICMBio, 2015). For subsistence, the majority of the population depends on hunting, fishing, plant extraction, and family gardens, and the communities are economically dependent on fishing trade and plant extractivism (Chase et al., 2020; Rezende and Sanches, 2020).
The EETM is included in the Brazilian in situ monitoring programme of Federal Protected Areas (the Monitora Program), managed by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) and the National Agency for Protected Areas. This initiative involves standardised biodiversity monitoring in over 80 federal protected areas distributed across all Brazilian biomes (ICMBio, 2018). The Monitora Program adopts standardised and simple sampling protocols based on a minimum modular common protocol, with the ability to collect basic information from essential bioindicators (ICMBio, 2018; Roque et al., 2018). Basic, advanced, and complementary protocols have been established for each global bioindicator. In all protocols, there is some involvement of the local community, either as field assistants (basic and advanced protocols) or monitors alongside interviews with other residents (complementary protocol). The Monitora Program has adopted the TEAM protocol for camera traps (Jansen et al., 2014; Rovero and Ahumada, 2017) as an advanced protocol for monitoring medium and large terrestrial vertebrates. The Tropical Ecology Assessment and Monitoring (TEAM) network is dedicated to monitoring temporal trends in biodiversity and changes in land cover, climate, and ecosystem services in tropical forests.
Data collectionCamera trap data form part of the advanced protocol of the Monitora Program, which is based on the TEAM protocol for large vertebrates (Rovero and Ahumada, 2017). In all PAs where the advanced protocol is applied, only camera trap data are collected; however, in the EETM, the protocols for sightings/vocalisation sampling and active searches for tracks and signs were applied as part of this study. Data were collected during the dry seasons (June and August) of 2016, 2017, and 2018. Sampling was conducted along 10 linear transects approximately 4.5 km in length using three methods as follows: (i) camera traps, (ii) visual and acoustic sampling, and (iii) active searches for tracks and signs. Each transect was sampled by a biologist and a local expert.
Nine biologists participated in the sampling over the 3-year study period. Owing to logistical difficulties, the sampling was conducted by biologists with different levels of experience, including: three with more than 10 years of experience in the area as well as MSc students with limited knowledge of the area. During installation of the camera traps, the less experienced biologists were accompanied by more experienced biologists on their first transects to train and prepare them for data collection at trap removal. All local assistants, referred to as ‘local experts’, were subsistence hunters from the local communities of Boa Esperança and Rio Novo, with extensive experience and knowledge of the forest where the monitoring programme was conducted. Eight local hunters participated as assistants, aged between 18 and 60 years, with at least seven years of experience in hunting, specifically experience in detecting animals through direct and indirect signs — measured by informal conversations and questionnaires from other surveys in the region. Considering the studies that have proven the accuracy of experienced hunters (Ahmad et al., 2021; Braga-Pereira et al., 2022; Keeping et al., 2018), in our work, we started from this assumption. In addition, in the first year, the assistants received additional data collection training, with oral presentations on what would be carried out and how their experience in detecting animals would be used in the study.
Camera trapsThe arrangement of camera traps (Bushnell® Trophycam) was based on the TEAM protocol (Rovero and Ahumada, 2017) for large vertebrates. We selected 40 sampling sites, 1.4 km apart and distributed across 10 linear transects (Fig. 1B). The cameras were fixed to trees at knee height and programmed to operate continuously throughout the sampling period. In 2017 and 2018, two trap sites were lost because of flooding, leaving 38 points of effort. For the purpose of this study, the sampling interval for each camera was seven consecutive days. To ensure temporal independence between records in the camera trap data, we removed records of a species within 60 min of the last record of the same species in the same camera trap (Burton et al., 2015; Sollmann et al., 2013).
Counting and mapping sightings/vocalizations and active search for tracks and signsThe trails created to access the camera trap sites were used as linear transects (Fig. 1B). Therefore, when the camera traps were removed, a diurnal census procedure similar to that described by Peres and Cunha (2011) was carried out, and the active search for tracks and signs was performed. These sampling procedures were performed in pairs, with a trained biologist and a local expert at a constant speed of approximately 1.5 km/h. Considering that the trails were created at the time of camera trap installation, when performing these methodologies, the trails were already created, without a fixed width because they were created only for the passage of researchers.
We relied on the experience of the local hunters to detect animals through direct (sightings/vocalisations) and indirect (tracks and signs) signals. Indirect observations included footprints, faeces, animal carcasses, scratched trees, gnawed fruits, and other traces along the transects (Voss and Emmons, 1996). The correspondence between the different indicators based on local knowledge was checked based on specialised literature (Emmons and Feer, 1997). Only tracks and signs up to one week old were considered, with the approximate age being conservatively estimated based on the local hunters’ knowledge (Ahmad et al., 2021; Braga-Pereira et al., 2022; Keeping et al., 2018).
Tracks and signs of the same species crossing the same transect were considered independent if there was no clear evidence that they belonged to the same individual. Footprints of the same animal along the transects were considered single observations, as were tracks and direct observations of gregarious animals. Observers briefly deviated from the transects when there was a favourable substrate for track marking, such as mud or areas with wet soil. The start time of the monitoring activities was based on visibility, weather, and access conditions to the transect, respecting the peak activity time of most animals during the day between 6 and 8 am (Pardini et al., 2012; Peres and Cunha, 2011). At the end of the three-year sampling period, the sampling effort employed along the 10 transects totalled 133.36 km over 30 visits. The inclusion of sightings/vocalisations and tracks and signs along the camera access paths (Monitora Advanced Protocol) did not incur any additional costs for the monitoring program as they were carried out during the planned camera removal activities. The pairs of biologists and local expert did, however, spend considerably more time and attention undertaking additional monitoring activities compared to that if they had only removed the cameras.
Data analysisAll statistical analyses were performed using the statistical program R (version 3.6.2) (R Core Team, 2020), and the ‘vegan’ package (Oksanen et al., 2020). Species rarefaction curves were generated to assess the relationship between sampling effort (number of visits) and the number of species recorded by each method (Gotelli and Colwell, 2001). Data from all transects and camera traps were grouped by visit. A visit included a seven-day period, with each single day involving walking on the transect for the sightings/vocalisations and track and sign methodologies. Further, we grouped the camera-trap records by transect, regardless of the number of active cameras in the transect. Thus, considering the three years of sampling in 10 transects, we undertook 30 visits in total. We assumed a one-hour interval for independence between camera-trap detection events (Beaudrot et al., 2016). Owing to the difficulty in species identification, Crypturellus, Tinamus, Penelope, Leopardus, and Dasypus were classified at the genus level. For comparative purposes, four treatments were considered as follows: (1) camera traps, (2) sightings/vocalisation counts, (3) active searches for tracks and signs, and (4) the sum of species detected during the sightings/vocalisations and active searches for tracks and signs.
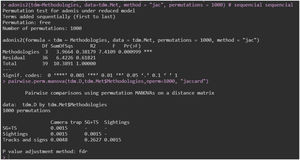
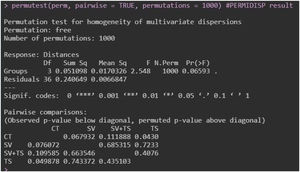
To determine whether there was variation in species composition between treatments (methods), we used principal coordinate analysis (PCoA) based on presence/absence data and the Jaccard similarity coefficient. We then used a permutational multivariate analysis of variance (PERMANOVA) to confirm possible differences in species composition between the treatments (Anderson and Walsh, 2013). PCoA is an unrestricted ordination approach that permits visualisation of the total pattern of dispersal and potential differences in the relationships of dispersal within each group. We also tested the differences in species composition among the four treatments using multivariate permutation of dispersal analysis (PERMDISP). This test compares the variation in the similarity of communities in the groups, where reduced dispersal indicates more homogeneous communities within the group, whereas more variable values indicate heterogeneous communities (Anderson, 2006).
The selectivity and complementarity were assessed through compositional analyses and by examining each methodology individually, through detection rates (DR), the number of records (RN) and the species or species group that was detected with each method. The sampling effort for the camera trap was defined as [number of camera traps × number of sampling days], totalling 812 cameras-days. For tracks and signs, the sampling was defined as the total kilometres walked, totalling 133.36 km. The number of records corresponded to the number of records for each methodology (independent record within 60 min for a camera trap or visualisation/tracks and signs where the animal could be identified, Table 1) and the detection rate was defined as the number of animal detections divided by the sampling effort (Table 2).
List of species of medium and large mammals and terrestrial game birds registered at the Terra do Meio Ecological Station, number of records (RN) by method.
| TAXON | LOCAL NAME | Camera trap | Sightings/vocalizations | Tracks and signs | SG + TS | CT + SG + TS |
|---|---|---|---|---|---|---|
| AVES | 324 | 147 | 129 | 276 | 600 | |
| Galliformes | 108 | 100 | 60 | 160 | 268 | |
| Cracidae | ||||||
| Aburria cujubi | Cujubi | 2 | 2 | 1 | 3 | 5 |
| Crax fasciolata | Mutum-piníma | 15 | 11 | 0 | 11 | 26 |
| Pauxi tuberosa | Mutum-castanheira | 56 | 33 | 2 | 35 | 91 |
| Penelope spp. | Jacu | 12 | 35 | 11 | 46 | 58 |
| Odontophoridae | ||||||
| Odontophorus gujanensis | Uru | 23 | 19 | 46 | 65 | 88 |
| Gruiformes | 77 | 7 | 0 | 7 | 84 | |
| Psophiidae | ||||||
| Psophia dextralis | Jacamin | 77 | 7 | 0 | 7 | 84 |
| Tinamiformes | 139 | 40 | 69 | 109 | 248 | |
| Tinamidae | ||||||
| Crypturellus spp. | Nambu/Jaó | 53 | 14 | 17 | 31 | 84 |
| Tinamus spp. | Nambu | 86 | 26 | 52 | 78 | 164 |
| MAMMALIA | 890 | 102 | 767 | 869 | 1759 | |
| Artiodactyla | 162 | 21 | 239 | 260 | 422 | |
| Cervidae | ||||||
| Mazama spp. | Veado | 114 | 15 | 108 | 123 | 237 |
| Tayassuidae | ||||||
| Pecari tajacu | Caititu | 32 | 2 | 62 | 64 | 96 |
| Tayassu pecari | Queixada | 16 | 4 | 69 | 73 | 89 |
| Carnivora | 50 | 2 | 16 | 18 | 68 | |
| Canidae | ||||||
| Speothos venaticus | Cachorro-vinagre | 1 | 0 | 0 | 0 | 1 |
| Felidae | ||||||
| Leopardus spp. | Gato-do-mato | 27 | 0 | 5 | 5 | 32 |
| Panthera onca | Onça-pintada | 2 | 2 | 3 | 5 | 7 |
| Puma concolor | Onça-vermelha | 6 | 0 | 2 | 2 | 8 |
| Puma yagouaroundi | Gato-mourisco | 1 | 0 | 0 | 0 | 1 |
| Mustelidae | ||||||
| Eira barbara | Irara | 1 | 0 | 0 | 0 | 1 |
| Procyonidae | ||||||
| Nasua nasua | Quati | 12 | 0 | 6 | 6 | 18 |
| Cingulata | 47 | 2 | 108 | 110 | 157 | |
| Chlamyphoridae | ||||||
| Euphractus sexcinctus | Tatu-peba | 0 | 0 | 2 | 2 | 2 |
| Priodontes maximus | Tatu-canastra | 2 | 0 | 13 | 13 | 15 |
| Dasypodidae | ||||||
| Dasypus spp. | Tatu | 45 | 2 | 93 | 95 | 140 |
| Didelphimorphia | 4 | 0 | 0 | 0 | 4 | |
| Didelphidae | ||||||
| Didelphis spp. | Gambá | 4 | 0 | 0 | 0 | 4 |
| Perissodactyla | 56 | 2 | 57 | 59 | 115 | |
| Tapiridae | ||||||
| Tapirus terrestris | Anta | 56 | 2 | 57 | 59 | 115 |
| Pilosa | 5 | 1 | 1 | 2 | 7 | |
| Myrmecophagidae | ||||||
| Myrmecophaga tridactyla | Tamanduá-bandeira | 2 | 0 | 1 | 1 | 3 |
| Tamandua tetradactyla | Tamanduá-mirim | 3 | 1 | 0 | 1 | 4 |
| Primates | 11 | 48 | 4 | 52 | 63 | |
| Atelidae | ||||||
| Alouatta belzebul | Guariba | 0 | 2 | 1 | 3 | 3 |
| Ateles marginatus | Macaco-aranha | 0 | 3 | 0 | 3 | 3 |
| Cebidae | ||||||
| Sapajus apella | Macaco-prego | 10 | 32 | 3 | 35 | 45 |
| Saimiri collinsi | Macaco mão-de-ouro | 1 | 4 | 0 | 4 | 5 |
| Pitheciidae | ||||||
| Plecturocebus vieirai | Macaco zogue-zogue | 0 | 7 | 0 | 7 | 7 |
| Rodentia | 555 | 26 | 342 | 368 | 923 | |
| Caviidae | ||||||
| Hydrochoerus hydrochaeris | Capivara | 0 | 0 | 1 | 1 | 1 |
| Cuniculidae | ||||||
| Cuniculus paca | Paca | 82 | 4 | 88 | 92 | 174 |
| Dasyproctidae | ||||||
| Dasyprocta spp. | Cutia | 473 | 22 | 253 | 275 | 748 |
| TOTAL NUMBER OF RECORDS: | 1214 | 249 | 896 | 1145 | 2359 | |
| SAMPLING EFFORT1 | 812 | 133.36 | 133.36 | 133.36 | ||
| TOTAL NUMBER OF SPECIES: | 29 | 22 | 23 | 29 | 33 | |
| NUMBER OF EXCLUSIVE SPECIES | 4 | 2 | 2 | |||
Treatments: camera traps (CT); count of sightings (visual and acoustic sampling) (SV); active search for tracks and signs (TS); sum of the species detected in the count of sightings and in the active search for tracks and signs (SV + TS). 1CT: number of camera traps X number of sampling days; SV and TS: total kilometers walked.
Detection rate (DR) of the orders registered in the Terra do Meio Ecological Station separated by method.
| TAXON | Camera trap | Sightings/vocalizations | Tracks and signs | SG + TS |
|---|---|---|---|---|
| AVES | 0.399 | 1.102 | 0.967 | 2.070 |
| Galliformes | 0.133 | 0.750 | 0.450 | 1.200 |
| Gruiformes | 0.095 | 0.052 | 0 | 0.052 |
| Tinamiformes | 0.171 | 0.300 | 0.517 | 0.817 |
| MAMMALIA | 1.096 | 0.765 | 5.751 | 6.516 |
| Artiodactyla | 0.200 | 0.157 | 1.792 | 1.950 |
| Carnivora | 0.062 | 0.015 | 0.120 | 0.135 |
| Cingulata | 0.058 | 0.015 | 0.810 | 0.825 |
| Didelphimorphia | 0.005 | 0 | 0 | 0 |
| Perissodactyla | 0.069 | 0.015 | 0.427 | 0.442 |
| Pilosa | 0.006 | 0.007 | 0.007 | 0.015 |
| Primates | 0.014 | 0.360 | 0.030 | 0.390 |
| Rodentia | 0.683 | 0.195 | 2.564 | 2.759 |
Treatments: camera traps (CT); count of sightings (visual and acoustic sampling) (SV); active search for tracks and signs (TS); sum of the species detected in the count of sightings and in the active search for tracks and signs (SV + TS). DR: [number of records/sampling effort].
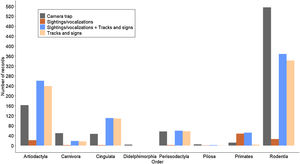
Based on the sightings/vocalisations, tracks and signs, and camera traps, we recorded 2359 observations of 33 species, 8 terrestrial game birds, and 25 medium and large terrestrial and arboreal mammals (Table 1). The most representative orders were Rodentia, Artiodactyla, Galliformes, and Tinamiformes, which accounted for approximately 80% of the data. Orders with fewer records were Carnivore, Primates, Pilosa, and Didelphimorphia, which together represented approximately 6% of the data.
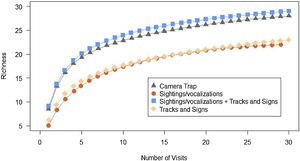
The rarefaction curve from the camera trap data and the sum of sightings/vocalisations with tracks and signs were closest to the asymptote (Fig. 2), recording 28 and 29 species, respectively, corresponding to 85% and 88% of the total species recorded. Separately, sightings/vocalisations and tracks and signs recorded 22 (67%) and 23 (70%) species, respectively.
Rarefaction curve from the sampling effort (in visits) of each method employed in obtaining the records. *For camera traps, a visit was considered a group of camera-trap by transect operating for seven consecutive days; and for sightings and tracks and signs one trip on a transect was considered a visit.
Terrestrial game birds represented 59% of sightings/vocalisations records with a detection rate of 1.102 (Table 2). For medium and large mammals seven orders were detected (DT = 0.765, Table 2), with Primates standing out, in which the method proved to be the most effective method of detection, with 48 records (DT = 0.36, Table 2) and two species detected exclusively by this method (Ateles marginatus and Plecturocebus vieirai). Only two carnivore records (Panthera onca) were recorded based on sightings/vocalisations during the three sampling years (Table 1 and Fig. 3).
Terrestrial game birds represented 27% of the camera trap records (DT = 0.399, Table 2), 14% of the tracks and signs records (DT = 0.967, Table 2), and the sum of sightings/vocalisations with tracks and signs represented 24% (DT = 2.07, Table 2). Two species (Crax fasciolata and Psophia dextralis) were not detected by the tracks and signs method. Eight orders of medium and large mammals were detected by the camera traps and seven were detected by tracks and signs (Table 1 and Fig. 3). The order Didelphimorphia, represented by a single species (Didelphis sp.), was only detected using the camera traps.
The camera trap and tracks and signs records stood out for Artiodactyla and Perissodactyla, with almost the same number of deer (Mazama spp.) and tapir (Tapirus terrestris) records. However, for Pecari tajacu and Tayassu pecari, the tracks and signs method yielded more than double the records obtained with the camera traps, showing the relative potential of the different methods for these species. Seven species were detected in the order Carnivora, but only one was detected using all three methods (Panthera onca). Although some species had only one record, the camera traps were more efficient for this order, with three exclusive species (Eira barbara, Puma yagouaroundi, and Speothos venaticus) and 27 records of the genus Leopardus compared to five records based on tracks and signs. Rodents accounted for the most records obtained using the camera traps (555) and based on tracks and signs (342). Dasyprocta records were particularly notable, accounting for 39% of the records from the camera traps and 28% from the tracks and signs method.
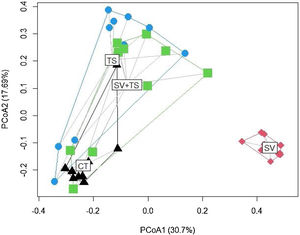
The PCoA ordination (Fig. A1 — Appendix A) showed clear differences between the sample clusters separated into two groups, which was further confirmed by the permutation tests (PERMANOVA; F(3) = 7.4109, p = 0.001) (Fig. A2 — Appendix A). However, there was an overlap between the camera trap (CT), tracks and signs (TS), and the sum of sightings/vocalisations with tracks and signs (SV + TS) samples, suggesting that many species were detected by all three methods. In particular, the overlap between the TS and SV + TS method was notable, which was confirmed by pairwise PERMANOVA (p = 0.263) (Fig. A2 — Appendix A). The PERMDISP analysis indicated that the dispersion of the samples did not significantly contribute to the detected differences (Fig. A3 — Appendix A).
Robust and systematic monitoring tools are essential to assess the effectiveness of conservation efforts. In developing countries, which often have extensive wilderness and high biodiversity levels with scarce resources, maintaining this effort is challenging (Chandler et al., 2017; Kühl et al., 2020; Stephenson, 2020). Despite previous studies using a combination of the methods used here (Benchimol and Peres, 2015; Lyra-Jorge et al., 2008; Mendes-Oliveira et al., 2017; Silveira et al., 2003; Srbek-Araujo and Chiarello, 2005), to our knowledge, this is the first attempt to provide a robust comparison of different methods through an adequate sampling effort with the specific aim of evaluating similarities and complementarity alongside local population involvement.
When evaluating the sightings/vocalisations and tracks and signs data together (given they can be applied concomitantly) in comparison to those of the camera traps, we verified that the same numbers of species could be broadly detected. However, our results further demonstrate that the methods used were selective and, therefore, their combined use is necessary to ensure comprehensive assessments. This corroborates other studies that used the same three methodologies for monitoring medium and large mammals and terrestrial game birds (Benchimol and Peres, 2015; Mendes-Oliveira et al., 2017; Peres and Cunha, 2011).
At the class level, there was wide variation in the detectability of the different methods. For terrestrial game birds, the camera traps and the sightings/vocalisations methods were the most efficient at capturing species composition, with the latter accounting for more than half of the records. Howevwe, more research is suggested with respect to the hunters’ knowledge about the species of terrestrial game birds. For example, the lack of detected or small number of records for species of the Cracidae family using the tracks and signs method can be explained by the difficulty in identifying the specific species based on the signs, and therefore, they generalised the species into the category “mutum/jacu”. These data were not included in the analyses because this category represents several genera.
In the case of mammals, direct sightings were the most efficient method for arboreal species, as can be seen in the richness of primates detected by this method (Fragoso et al., 2016, 2019; Mendes-Oliveira et al., 2017; Santos and Mendes-Oliveira, 2012). For species of the order Carnivora, the camera traps proved most efficient, especially for rarer species, such as Bush Dogs (S. venaticus) and Jaguarundis (P. yagouaroundi), which were only detected by this method. These species are not abundant, have nocturnal habits (Almeida et al., 2013; Jorge et al., 2013), and are rarely detected based on sightings or tracks and signs (Fragoso et al., 2016; Santos and Mendes-Oliveira, 2012). It remains to be determined whether the limited numbers of records allow for a comparison and evaluation of temporal trends for these species, which is typically the main long-term objective of most monitoring programmes.
Some genera proved consistently difficult to identify independent of the method used. This was the case for Crypturellus, Tinamus, Mazama, Leopardus, and Dasypus, as has also been pointed out in other studies (Munari et al., 2011; Santos and Mendes-Oliveira, 2012). However, species of the genera Crypturellus and Tinamus are easily identifiable by their vocalisations, which could be better used in monitoring programmes based on linear transections.
All of the adopted methods are affected by environmental conditions to some extent, and climatic and soil conditions might represent relevant limitations for the active searching of tracks, signs, and sightings (Silveira et al., 2003), which could affect species detectability. Camera traps can be installed in virtually any habitat, although in humid tropical forests, such as those sampled here, they can be damaged by humidity. In addition, temperature, light and the density of vegetation, among other factors, can also affect the performance (including detectability) of species. Indeed, Srbek-Araujo and Chiarello (2007), highlight the importance of performing constant maintenance to guarantee correct operation and accuracy. Thus, a lack of maintenance, especially in relation to humidity, can compromise the optical and/or electronic mechanisms of this type of monitoring equipment (Cutler and Swann, 1999). The same authors noted the existence of "chronic mechanical problems" with camera traps used across several studies, highlighting one of the disadvantages of this sampling method. In addition, alongside other factors, temperature, light, and vegetation density can affect the detectability of species using camera traps and, more broadly, a lack of appropriately trained personnel can be a significant challenge when identifying species based on camera trap images.
In comparative studies, scale issues have been a challenge (Danielsen et al., 2014, 2021). However, in our research, the data had different issues and characteristics; the camera traps were static, whereas the sightings/vocalisations and the tracks and signs were detected on pathways, which can remain for many days under favourable environmental conditions. Moreover, in both transect methods used here, each forest trail was surveyed only once per year. The possibility to register tracks and signs left by wildlife for up to seven days before (or even much earlier, depending on the objectives of the study) is inherent to the method. However the effort required for the distance (km) surveyed is the same.
Line transect surveys for direct and indirect observations carried out by local hunters proved to be efficient for detecting the composition of species in the region. Both approaches obtained a good number of records, equivalent to that of the camera traps, and better represented the diversity of primates. Including the experience of local populations in research activities, participatory research, and citizen science have many advantages (Constantino et al., 2014; Eaton et al., 2017; Liebenberg, 2008; Pocock et al., 2018). Indeed, our findings corroborate those reported by Danielsen et al. (2005) regarding the advantages of including local monitors in data collection alongside local experts and trained scientists. Silveira et al. (2003) and Peres and Cunha (2011) also point out the importance of field personnel with considerable experience for detecting the signs of local fauna. Local communities can play a significant role in monitoring programmes if sampling projects are carefully designed with appropriate investment in training. Moreover, integrating local communities into monitoring programmes brings new knowledge and income generation to the community, helping shape attitudes toward the management of environmentally sustainable natural resources among the participants. Danielsen et al. (2014) found that despite significant cultural and/or monitoring differences between countries, local participants and scientists produced similar results on the status and trends of species and natural resources. Accurate estimates of medium and large vertebrate densities have long been accessed using track surveys, especially in Africa (Ahlswede et al., 2019).
In our study, we found that for each detection event based on visualisation, a local resident detected approximately four indirect pieces of evidence suggesting the presence of an animal. Such data could not be obtained by biologists unless they had many years of experience with track and sign detection, which is not typically the case. Indeed, this ability is the result of a lifetime of observation and learning from accumulated knowledge transmitted between generations, often as a necessary aspect of survival in forest ecosystems (Liebenberg, 2003, 1990; J. C. B. Pezzuti et al., 2018). Therefore, building on the observations of Danielsen et al. (2005, 2021), by employing local experts and their skills, we obtained five-times more records than would have likely been obtained following the Minimum Protocol of the Monitora Program for terrestrial vertebrates. More importantly, local community involvement offers the opportunity to monitor several additional species based on indirect observations, including cryptic or elusive species (e.g., giant armadillos) as well as nocturnal groups (e.g., armadillos and pacas) without additional cost. Considering that the Monitora Program is now implemented in over 80 protected areas throughout all Brazilian biomes, with a high annual cost, we suggest that a strategy to involve local expert people in surveys would be highly beneficial.
Fragoso et al. (2016) highlighted the efficiency (i.e., faster data accumulation) and importance of sampling indirect observations in daytime censuses. For example, for carnivores and ungulates (species of extreme importance as game), even with large sampling efforts, it is not possible to increase detectability based on sightings (Fragoso et al., 2016, 2019). Thus, methods based on tracks and signs more reliably detect the presence of animals and are promising as reliable indices or even direct measures of abundance (Fragoso et al., 2016, 2019). Therefore, it is essential that the counting of sightings is carried out together with active searching for tracks and signs (Fragoso et al., 2019). Our study indicates that for both preserved and human-impacted areas, systematic data collection based on active searching for tracks and other traces carried out by specialised local experts can be effective for monitoring populations of carnivorous species, complementing or even replacing camera traps.
The teams of biologists and local experts spent considerably more time and attention than required to only remove the previously installed cameras. Considering that the transects were long in terms of distance, there was a perception of fatigue and decreased focus in the latter part of the transect, which might have affected the data collection. In addition to fatigue, some local experts showed difficulty in maintaining their focus on data collection repeatedly, as they already had their own notion of ecological patterns, and did not seem to understand the reason for the quantitative effort to explore these patterns. As an alternative, it would be ideal to include shorter stops of 10 min for every 1 -hour of sampling or longer stops 20–30 min every 2 hours. Furthermore, for the implementation of citizen science-based monitoring, there would be a need for constant encouragement, both educational and financial, for them to appreciate the importance of their work.
Bridging traditional (indigenous and local) knowledge with scientific knowledge is essential for the generation of legitimate, credible, and usable knowledge to move towards sustainability (Austin et al., 2019; Tengö et al., 2021, 2017, 2014). The Multiple Evidence Base Approach (MEB) developed by Tengö et al. (2014, 2017), which aims to bring this pool of knowledge into global science policy processes, reinforces the importance of the results obtained in our work. Specifically, this type of approach allows for the accurate and efficient identification of gaps in the knowledge base and opportunities for collaborative research engagements and has been used in several projects (Ali, 2016; Braga-Pereira et al., 2022; Keeping et al., 2018; Mburu, 2016; Pezzuti et al., 2018).
Our results demonstrate that the adopted monitoring methodologies were selective in detecting the target species and, therefore, their combined use is necessary to ensure robust sampling. The methodologies carried out by local hunters proved essential for obtaining information on the composition of medium and large mammals and terrestrial game birds. Furthermore, for the Terra do Meio Ecological Station, the high maintenance costs and the need to have a researcher present on site are a challenge when monitoring continuity in this region. Considering that the protocol applied in the region currently incurs high researcher costs, and the inclusion of data collected by local hunters proved essential for a more complete sampling, we suggest that monitoring programmes in the region can be improved by assigning more responsibilities to local experts, including the installation and removal of cameras and the recording of direct sightings as well as indirect observations. By adopting such an approach, spending per monitoring campaign could be reduced and more complete sampling could be achieved. Whereas the initial costs of implementation might be higher because of the necessary training and evaluation processes, in the long term, monitoring with the participation of local experts tends to be cheaper than to monitoring exclusively by researchers (Danielsen et al., 2005). In addition, the involvement of local communities in monitoring can increase local engagement in management responses as well as the speed of decision-making in the face of environmental threats (Danielsen et al., 2014, 2021) and will provide relevant information for the monitoring of ecosystems (de Sherbinin et al., 2021; Neupane et al., 2020; Pocock et al., 2018; Schmeller et al., 2017). So, our study demonstrates the crucially important role of citizen science and local expert participation in the success and longevity of ecological monitoring programmes, offering significant opportunities for cost saving and improving data quality.
The expeditions of this project were funded by Chico Mendes Institute for Biodiversity Conservation (ICMBio) and the Programa ARPA - Áreas Protegidas da Amazônia (Fundo Brasileiro para a Biodiversidade - FUNBIO). MPM thanks to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the master's scholarship. JCBP thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We would like to thank Editage (www.editage.com) for English language editing.