Hunting is one of the main threats to biodiversity due to its synergistic effect with other anthropogenic activities. Aversive hunting is a radical measure aimed at species considered threats to human health or goods. This practice has proved high impacts on wildlife. We quantified sighting and hunting of Beaded lizards and analyzed its relation with habitat disturbance through semi-structured interviews in seven localities. Sighting and hunting relation with habitat was analyzed individually using correlation tests and globally using discriminant function analysis. Hunting frequency is closed to 50% and justified on the potential direct threat of these species. Habitat structure explained sighting frequency but not hunting. Our study exposes that impact on Beaded lizards due to aversive hunting is high and that an education plan is required to decrease it; we also show the importance of habitat structure for the conservation of Beaded lizards.
Wildlife hunting is one of the main biodiversity threats due to its intensity and synergistic effects with activities like farming and ranching (Peres, 2001; Redford, 1992). Aversive hunting stands out as a radical measure aimed at species considered direct or indirect threats for humans. Several experiences have shown that aversive hunting can drastically affect ecosystem stability; besides in addition to habitat disturbance, can enhance negative effects leading to local extinctions (Woodroffe et al., 2005). A recent evaluation of the conservation status of reptiles has identified habitat disturbances and biological use as their main threats due to specialized habitat requirements and excessive poaching. Nonetheless impacts on most species populations remain unknown especially for those of rare occurrence, urging population level assessments of these activities (Böhm et al., 2013).
The importance of the ethnobiological studies for biodiversity conservation has increasingly been recognized recently (Albuquerque et al., 2013; Alves, 2012). In particular, the ethnozoology has showed great potential in solving problems of wildlife human conflict including those related with reptile folklore (Ceríaco, 2012; Alves et al., 2012; Mendonça et al., 2014). Ethnozoological research may be crucial in the evaluation of anthropization on wildlife and in the development of conservation strategies and management plans (Alves, 2012).
Helodermatids constitute an outstanding squamate group due to several morphological and ecological characteristics (Beck, 2005; Fry et al., 2006). Two groups have been recognized in the family; the Gila monsters (Heloderma suspectum), found in south-western United States and north-western Mexico, and the Beaded lizards (H. exasperatum, H. horridum, H. alvarezi and H. charlesbogerti) found in tropical dry forests of Mexico and Guatemala (Reiserer et al., 2013). Although population level studies are limited, it is recognized that habitat loss and fragmentation are their main threats because they are restricted to well-conserved environments and most of its distribution is found outside the protected areas (Beck, 2005; Domínguez-Vega et al., 2012). Moreover, Beaded lizards are included in the native folklore where they are considered extremely dangerous (Brown and Carmony, 1999), so aversive hunting is expected to be common although its intensity is unknown.
We analyzed sighting and hunting on Mexican Beaded lizards. Our objectives were to quantify sightings and hunting frequency and analyze the relationship between sightings and hunting frequency with habitat structure. We expected a spatial segregation between sighting and hunting events, with most sightings occur in areas with conserved vegetation and most hunting events occur in areas commonly used by people; e.g., roads, and human settlements.
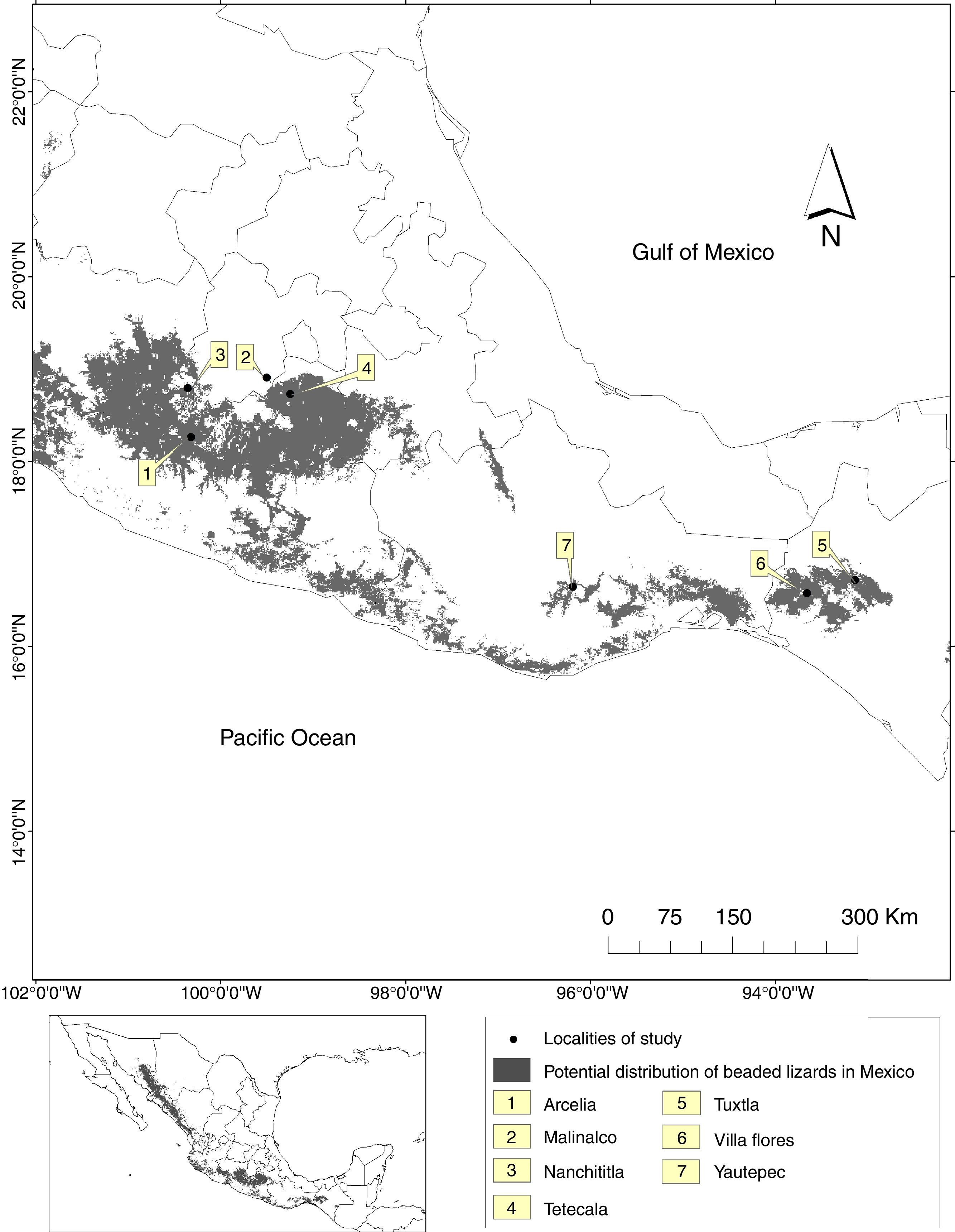
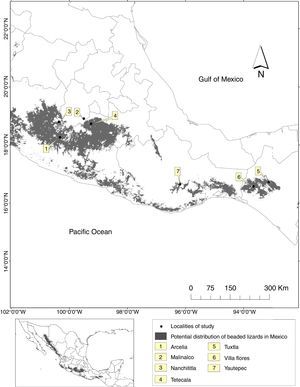
Material and methodsWe collected sighting and hunting records from 2010 to 2013 using a survey approach with a dimensional sampling (Robson, 2002). The study area included seven localities distributed through the central and southern distribution range of Beaded lizards in Mexico (Fig. 1).
We applied interviews directly on the field (where people work and where they see Helodermatids), some others at the homes of our interviewees and some more in places where people usually met; for example the center of some villages. The interviewed population included adults (men and women; older than 30 years) from rural communities. Besides, we selected people related to farming and/or ranching activities based on the premise that such activities may favor the probabilities of finding Helodermatids because they spend more time in areas coexisting with Beaded lizards. We used information from local, except for one person who was living in Malinalco 20 years before the interview (Table 1). We applied 136 semi-structured interviews, using predetermined questions that could change in order or wording to make them explicit to the interviewed. We ask the participants to restrict the information they provided to one-year period prior to the interview. Additionally, we applied the snowball sampling strategy to get more informants within each community (Robson, 2002).
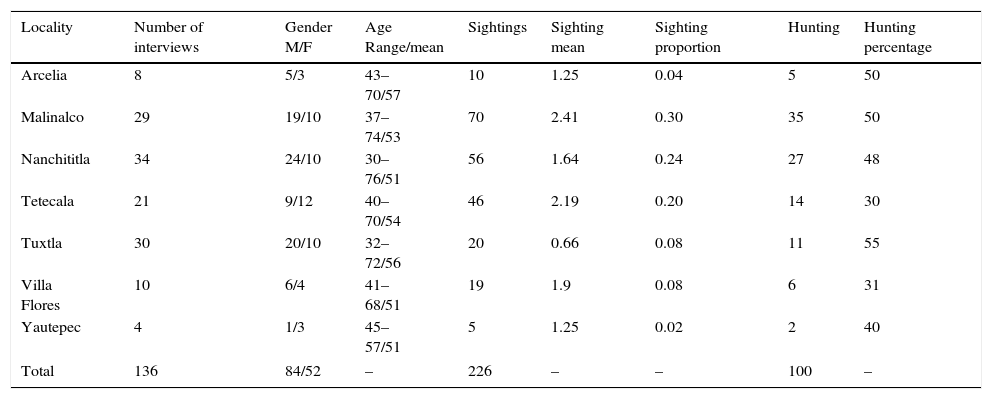
Sighting and hunting frequency of Beaded lizards in seven localities of their distribution in Mexico, and the main characteristics of the interviewed population. M, number of interviewed males; F, number of interviewed females.
| Locality | Number of interviews | Gender M/F | Age Range/mean | Sightings | Sighting mean | Sighting proportion | Hunting | Hunting percentage |
|---|---|---|---|---|---|---|---|---|
| Arcelia | 8 | 5/3 | 43–70/57 | 10 | 1.25 | 0.04 | 5 | 50 |
| Malinalco | 29 | 19/10 | 37–74/53 | 70 | 2.41 | 0.30 | 35 | 50 |
| Nanchititla | 34 | 24/10 | 30–76/51 | 56 | 1.64 | 0.24 | 27 | 48 |
| Tetecala | 21 | 9/12 | 40–70/54 | 46 | 2.19 | 0.20 | 14 | 30 |
| Tuxtla | 30 | 20/10 | 32–72/56 | 20 | 0.66 | 0.08 | 11 | 55 |
| Villa Flores | 10 | 6/4 | 41–68/51 | 19 | 1.9 | 0.08 | 6 | 31 |
| Yautepec | 4 | 1/3 | 45–57/51 | 5 | 1.25 | 0.02 | 2 | 40 |
| Total | 136 | 84/52 | – | 226 | – | – | 100 | – |
Our questionnaire was designed to gather sighting and hunting frequency, landscape characteristics of the sighting locations, and the hunting reasons when applied. We verified the correct identification of the species presenting photographs of Beaded lizards and other lizard species commonly known with the same local name (“escorpión”) or that might be confounded with Helodermatids (Barisia spp., Coleonyx spp., Ctenosaura spp., and Gerrhonotus spp.). The location of sighting and hunting events was determined using a Global Position System device when possible (i.e. when the interviewed took us to the sighting place); otherwise, we ask for landscape references (e.g. human settlements, roads, rivers and streams) to locate sites an accuracy of 0.5km2 using “Google earth 7.1”. The landscape characteristics were determined using fixed classes of vegetation (tropical dry forest, temperate forest and xerophyte vegetation), topography (streams, hill base, middle portion of hills and hill top), habitat disturbance (native vegetation dominated, native vegetation-human modified, human modified dominated), and rockiness (presence or absence).
Before each interview, we exposed the aims, and methods of our study to each potential informant and we ask them for their willingness to participate on it. We also declared to the participants that they will have no economic benefits for their information.
We analyzed the relationship between sighting and hunting frequency using a Spearman's simple correlation. To analyze the relationships between habitat fragmentation (Fahrig, 2003) with sighting or hunting events, we used the recorded sights. The records were mapped over a digital layer of vegetation types and land uses for Mexico (INEGI, 2010). Then we defined sampling units to capture the landscape associated to those records. For the multiple-sighting reports we generated a minimum convex polygon using referenced sightings (i.e. we link the outlying records). For the localities where only one sighting event was recorded, we used the mean home range reported for H. horridum to define a buffer zone around the sighting record. For each polygon, habitat structure was estimated using the “Patch analyst” extension for ArcGIS 9.3 to obtain fragmentation indicators (crops distance, mean patch edge, mean patch size, number of patches). Furthermore, we included two digital layers as habitat perturbation indicators: human population density and a road distance. Then we extracted the habitat characteristics for each sighting record.
A database for the studied localities was constructed using the habitat structure variables and then we performed correlation tests using sighting and hunting frequency as dependent variables against structural and perturbation indicators. Environmental differences between locations with different sightings or huntings intensity were classified with a discriminant function analysis using sighting and hunting frequencies as grouping variables (five groups in each analysis) and the habitat structure variables as dependent. Finally, we performed a correspondence analysis to identify differences in environmental variables (landscape structure) determining differences between sighting and hunting.
ResultsWe acquired 226 sightings of Beaded lizards; the interviewees efficiently identified these species (95%). As it was expected, the aversion to Beaded lizards is high; hunting frequency was close to 50%. Sighting frequency showed inter-locality variation; Malinalco stands out, with the highest mean of sighting frequency per person (2.41); whilst Tuxtla Gutierrez, presented the lowest frequency (0.66; Table 1). Spearman's test showed a positive and significant relationship between sightings and huntings (rs=0.52; p<0.05), highlighting the generalized aversion to Helodermatids in the study area.
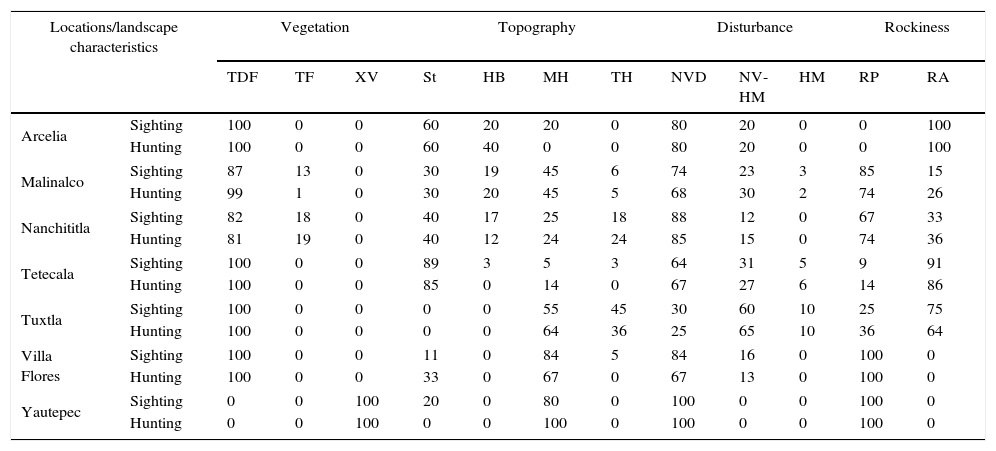
According to the interviewees, the sighting locations are characterized by tropical dry forest cover; even in those where temperate forest are abundant (Malinalco and Nanchititla). Most of the records corresponded to the middle portion of the hills and streams; instead, the top portion of the hills were found as the less frequent areas of sighting events. No general pattern in sighting events were found in relation to rockiness. Potential threat of these animals to people was the only justification for hunting, evidencing the lack of knowledge regarding these species biology (Table 2).
Environmental characteristics of the sighting sites presented as the percentage of sighting and hunting events. TDF, tropical dry forest; TF, temperate forest; XV, xerophyte vegetation; St, streams; HB, hill base; MH, middle portion of the hill; TH, top of the hill; NVD, native vegetation dominated; NV-HM, native vegetation-human modified; HD, human modified dominated; RP, rocks presence; RA, rocks absence.
| Locations/landscape characteristics | Vegetation | Topography | Disturbance | Rockiness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDF | TF | XV | St | HB | MH | TH | NVD | NV-HM | HM | RP | RA | ||
| Arcelia | Sighting | 100 | 0 | 0 | 60 | 20 | 20 | 0 | 80 | 20 | 0 | 0 | 100 |
| Hunting | 100 | 0 | 0 | 60 | 40 | 0 | 0 | 80 | 20 | 0 | 0 | 100 | |
| Malinalco | Sighting | 87 | 13 | 0 | 30 | 19 | 45 | 6 | 74 | 23 | 3 | 85 | 15 |
| Hunting | 99 | 1 | 0 | 30 | 20 | 45 | 5 | 68 | 30 | 2 | 74 | 26 | |
| Nanchititla | Sighting | 82 | 18 | 0 | 40 | 17 | 25 | 18 | 88 | 12 | 0 | 67 | 33 |
| Hunting | 81 | 19 | 0 | 40 | 12 | 24 | 24 | 85 | 15 | 0 | 74 | 36 | |
| Tetecala | Sighting | 100 | 0 | 0 | 89 | 3 | 5 | 3 | 64 | 31 | 5 | 9 | 91 |
| Hunting | 100 | 0 | 0 | 85 | 0 | 14 | 0 | 67 | 27 | 6 | 14 | 86 | |
| Tuxtla | Sighting | 100 | 0 | 0 | 0 | 0 | 55 | 45 | 30 | 60 | 10 | 25 | 75 |
| Hunting | 100 | 0 | 0 | 0 | 0 | 64 | 36 | 25 | 65 | 10 | 36 | 64 | |
| Villa Flores | Sighting | 100 | 0 | 0 | 11 | 0 | 84 | 5 | 84 | 16 | 0 | 100 | 0 |
| Hunting | 100 | 0 | 0 | 33 | 0 | 67 | 0 | 67 | 13 | 0 | 100 | 0 | |
| Yautepec | Sighting | 0 | 0 | 100 | 20 | 0 | 80 | 0 | 100 | 0 | 0 | 100 | 0 |
| Hunting | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 100 | 0 | 0 | 100 | 0 | |
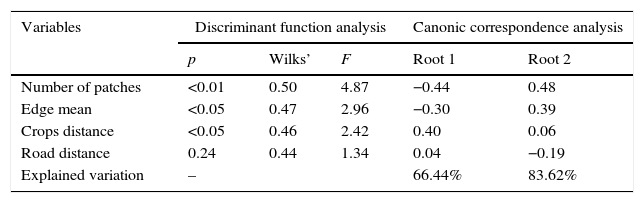
We find no significant relationships between sighting or hunting frequency and individual habitat variables, using the Spearman's test (correlation indexes from −0.021 to 0.28; p>0.05) indicating that sighting and hunting may be explained by the combination of several habitat characteristics. Discriminant function analysis showed significant environmental differences between locations with different intensity of sighting events (Table 3). The number of vegetation patches and the mean edge of those patches were the most important characteristics related to environmental differences. Sighting frequency was negatively related to those variables; thus Beaded lizards are expected to be more common in homogeneous environments (Table 3). Discriminant function analysis for hunting frequency did not find significant differences among locations, supporting the hypothesis that hunting occurs wherever Helodermatids are found.
Environmental differences between locations (discriminant analysis) and important characteristics that define these differences (correspondence analysis).
| Variables | Discriminant function analysis | Canonic correspondence analysis | |||
|---|---|---|---|---|---|
| p | Wilks’ | F | Root 1 | Root 2 | |
| Number of patches | <0.01 | 0.50 | 4.87 | −0.44 | 0.48 |
| Edge mean | <0.05 | 0.47 | 2.96 | −0.30 | 0.39 |
| Crops distance | <0.05 | 0.46 | 2.42 | 0.40 | 0.06 |
| Road distance | 0.24 | 0.44 | 1.34 | 0.04 | −0.19 |
| Explained variation | – | 66.44% | 83.62% | ||
Sighting frequency of Beaded lizards confirmed that these species are rarely found even by people sharing their habitat. According to our interviews, sightings occur in areas of tropical dry forests dominated by native vegetation; moreover the landscape analysis added that homogeneous environments favor the frequency of these events. The relation between Beaded lizards and tropical dry forests has been proved earlier using different approaches (Beck, 2005; Domínguez-Vega et al., 2012). Conservation status of the vegetation has been suggested as a determinant factor for the presence of these reptiles due to their feeding habits, activity patterns and restricted displacement capabilities (Beck and Lowe, 1991). Nonetheless the lack of empirical evidence has limited proving this idea. The information we provide, supports this hypothesis and suggest that Beaded lizards can be valuable as habitat quality indicators.
The discriminant analysis of hunting frequency found no differences among locations, supporting that hunting is determined by cultural rather than environmental factors. Interviewed people pointed out potential threats to humans as the only justification for hunting; exposing the importance of folklore in the negative impacts on these species. Helodermatids can truly cause serious injuries to humans; nonetheless its disposition to attack and its capabilities to do so, seem to be limited. In fact, reports of bites are scarce and restricted almost exclusively to captive breeders or people attempting to handle them in field (Beck, 2005).
Although we depicted the frecuency of hunting on Beaded lizards, more detailed research is needed to determine how this activity is affecting important parameters of its populations and ultimately its persistance. Moreover, it is necessary to investigate how the synergy between intrinsic characteristics of these vertebrates (e.g. low reproductive potential, and niche especializations; Ariano-Sánchez and Salazar, 2013; Golberg and Beck, 2001; Gonzalez-Ruiz et al., 1996) and habitat perturbations may enhance negative effects on Beaded lizards.
In realtion to habitat preferences our results supports the observations on habitat selection by Beck and Lowe (1991). The areas surrounding streams and hill bases presented the highest frequency of sightings. Besides, the interviewees suggest that Beaded lizards avoid the top portion of hills. This behavior may be related to refugees and food availability and suitability. Beck and Lowe (1991) proposed that near-stream areas provide suitable refuges but prey availability should be tested.
Field studies of Beaded lizards in Mexico are scarce (Balderas-Valdivia and Ramirez-Bautista, 2005; Beck and Lowe, 1991; Gienger et al., 2005), an important portion of biological knowledge regarding this group has been collected from haphazard encounters although it is a widely distributed group. Our survey gathered 226 records that correspond to three years (2010–2013). Museums and scientific literature contain approximately 150 well referenced records for the last 23 years (Pers. Obs); based on this remarcable difference in presence records, we propose that field work directed to Beaded lizards must include native people as means to improve scientific knowledge and change the attitudes about these species.
Our results have clear implications for conservation of Beaded lizards in Mexico; it is true that Beaded lizards might have real potential to cause serious injuries to humans, but the beliefs of people exaggerate its capabilities, and are the real culprits of hunting. In this sense, it is clear that an education plan is mandatory in order to reduce the negative impacts. This strategy has proven to be successful; a recent study showed that environmental education actions based on direct contact with wildlife are able to modify public attitudes toward biodiversity, even when the subject group is the least appreciated vertebrates (Sousa et al., 2016).
Beck (2005) suggests two conservation strategies applied with Gila monsters that may be applied with the Beaded lizards too: (1) sharing the habitat and (2) relocation. According to our results, sharing the habitat could benefit Beaded lizards greatly. In the first place this strategy implies a radical change in people's perception to recognize the real capabilities and limitations of Beaded lizards to hurt people. In the second place, sharing the habitat also implies that habitat management should be modified to favor the persistence of Beaded lizards. Our results show that Beaded lizards are associated with conserved vegetation areas. Nonetheless, information about more specific requirements (e.g. habitat connectivity, food and shelters availability) is necessary to define appropriate habitat sharing.
The relocation should be taken with caution (Beck, 2005; Sullivan et al., 2004). This strategy has at least two drawbacks; it is ineffective when the individuals are moved below certain distance (1000m, for Gila monsters; unknown distance for Beaded lizards), and it may dramatically increase the mortality. Thus, the relocation should be applied only when no other option is available.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful to our interviewees for their support, we also thank to Edgar Reyes, Jesús Rodríguez and Víctor Muñoz for their help in field work. Christopher Gienger revised the first English translation and two reviewers provide valuable comments. The first author thanks to CONACYT for its support through the scholarship 265954.