Urbanization represents one of the most striking threats to biodiversity due to habitat loss and fragmentation, particularly in forest biomes. The role of the surrounding matrix in maintaining native diversity in urban areas remains poorly studied. We compared taxonomic and functional bird diversity and dissimilarity between urban settlements (USs) and natural habitats (NHs) in the forest landscape matrix (FM) and agriculture landscape matrix (AM) of the Atlantic Forest of South America, and explored the relationships of species traits between USs and NHs. Birds were surveyed in five USs and five NHs in the FM, and four USs and three NHs in the AM using point counts. We used generalized least squares models to test for differences in diversities and dissimilarities between sites from the two landscapes, and the similarity percentage and fourth-corner analysis to determine the bird species and traits that contributed most to the differences between habitat types. Taxonomic and functional diversities were higher and dissimilarities were lower for US in the FM than for those in the AM. Species with urban and generalist traits occurred in all USs, but assemblages also included forest species in the FM. Urban areas should be managed to maintain a forest component, provided that it is necessary for forest bird species to breed successfully.
Urbanization is expanding worldwide and represents one of the most striking threats to biodiversity due to habitat loss and fragmentation (Marzluff et al., 2001), particularly in forest biomes (Millennium Ecosystem Assessment, 2005). Tropical and subtropical forests, which are hotspots concentrating most of the world’s biodiversity, are under the threat of accelerated habitat change due to human activities (Myers et al., 2000). In this context, cities have typically experienced unplanned growth, making excessive use of natural resources (Fontana et al., 2011; MacGregor-Fors, 2008). Despite the contrasting environments between cities and native forests (Palacio et al., 2018), many studies have demonstrated that local planning of urban green areas facilitates the presence of forest birds both in tropical (Fontana et al., 2011) and non-tropical cities (Mason et al., 2007). From a conservation point of view, a special focus has been placed on their possibilities to breed successfully (Marzluff and Ewing, 2008). However, the role of the surrounding matrix in maintaining native diversity in urban areas remains poorly studied.
Land-use changes resulting from the replacement of original habitats lead to fragmented landscapes, where species abundance and occupancy in the different patches depend on the habitat types in the landscape matrix (Watling et al., 2011) and on the original elements embedded in it (Cadavid-Florez et al., 2020). Some species can easily move across a landscape matrix composed of anthropogenic habitats, while others are reluctant to do so because they are exclusively adapted to native habitats (Antongiovanni and Metzger, 2005). Thus, the preservation of vegetation structure in cities may favor native biodiversity, as it increases the degree of similarity between native and anthropogenic habitats (Filloy et al., 2010). In this regard, forest birds have shown a preference for moving through habitats that are similar to their native forests and avoid agricultural and deforested areas (Sieving et al., 1996).
Taking into account that landscapes with features similar to the natural environment contribute to the conservation of native fauna (Schmiegelow, 2008), forested areas in the landscape matrix allow native bird conservation and dispersal (Zurita and Bellocq, 2010). If we view cities as types of habitats that may be surrounded by different matrix types, the number of native birds reaching the city is expected to be larger when the surrounding landscape matrix type is similar to the natural habitat than when cities are immersed in an agricultural matrix. This emphasizes the importance of considering the composition of the surrounding landscape matrix instead of just relying on local management for the achievement of a sustainable urban planning.
The simultaneous study of multiple facets of biodiversity is useful to solve conservation problems (Naeem et al., 2012). Currently, taxonomic and functional approaches are used to assess the influence of environmental disturbances and the relationship between species diversity and ecosystem function (Cadotte et al., 2011; Petchey et al., 2004). Anthropogenic activities can exert a different influence on the taxonomic diversity and functional diversity of native communities. For example, Santillán et al. (2018) found higher richness and less functional diversity of bird species in fragmented compared to continuous forests. In particular, urban areas are well known to reduce bird species richness (Chace and Walsh, 2006) and functional diversity (Croci et al., 2008). Urban settlements represent environmental filters that hinder the establishment of specialist species showing traits such as diet specialization, long-distance migration and high sensitivity to human disturbance (Seress and Liker, 2015). This environmental filter is expected to be more evident in cities embedded in a landscape matrix differing from the natural habitat.
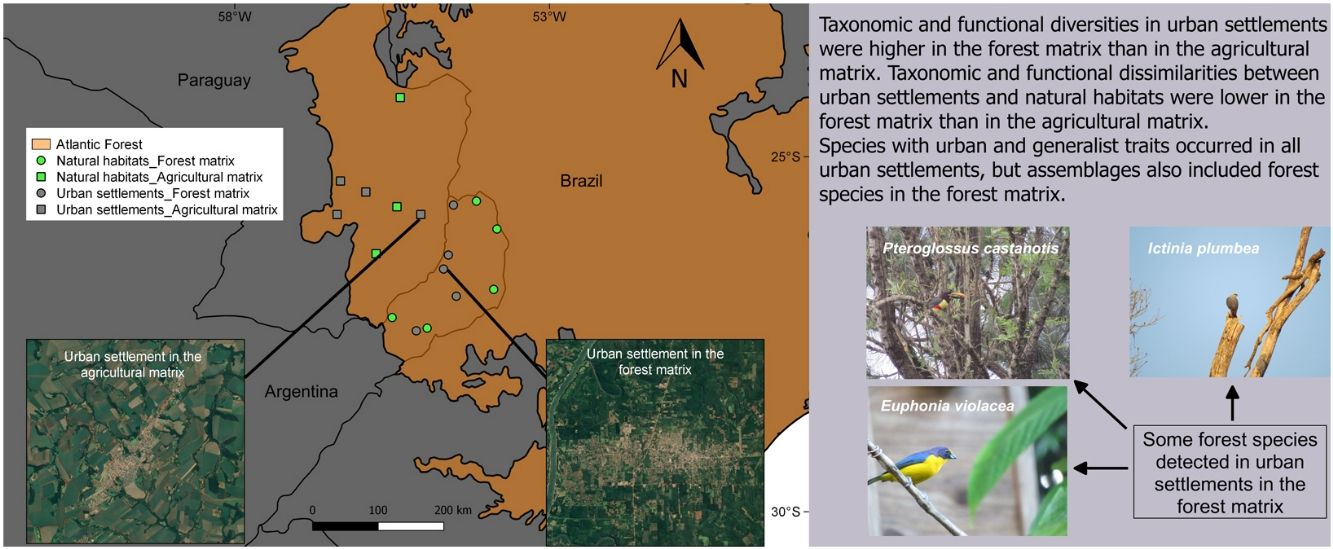
The Atlantic Forest in South America is one of the most endangered biodiversity hotspots (Myers et al., 2000) because urbanization (Baptista and Rudel, 2006) and agriculture (Galindo Leal and de Gusmão Câmara, 2003) produced large-scale forest replacement and fragmentation. In Argentina and Paraguay this ecoregion shows a contrasting landscape composition due to different human activities and regional political economies. Urban settlements in the Atlantic Forest of Argentina are embedded in a landscape matrix composed of natural reserves and tree plantations (hereafter referred to as forest landscape matrix; see Methods), while those of Paraguay are embedded in an agricultural landscape matrix.
Here, we ask if the taxonomic and functional diversity of bird assemblages in urban settlements vary with the landscape matrix, and if the structural similarity between the vegetation in the matrix and the original vegetation promotes the presence and abundance of native species in the bird assemblages. For this purpose, we compared the functional and taxonomic diversity between urban settlements and natural habitats in two subregions from the Atlantic Forest with different landscape matrices. Since tree plantations maintain a higher species richness of forest birds than does forest clearing for agriculture (Filloy et al., 2010; Vaccaro and Bellocq, 2019; Zurita and Bellocq, 2010), we hypothesize that a forest landscape matrix will promote native bird diversity to a greater extent than an agricultural landscape matrix. We predict that taxonomic and functional bird diversity, and bird dissimilarity relative to natural habitat, will be lower in urban settlements embedded in a forest landscape matrix than those in an agricultural landscape matrix. To test this, we compared taxonomic and functional bird diversity and dissimilarity between urban settlements and natural habitats in the Argentinian and Paraguayan portions of the Atlantic Forest with different landscape matrices. In addition, we explored the relationships between species traits and urban settlements and natural habitats to identify possible causes of habitat differences between the contrasted landscape matrices.
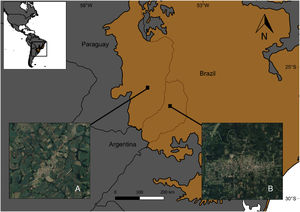
MethodsStudy area and selected sitesTo analyze the influence of the surrounding matrix on the response of forest birds to urban areas, these were surveyed in urban settlements and natural habitats in two different landscapes within the Atlantic Forest ecoregion, located in Argentina and Paraguay (Fig. 1). The Atlantic Forest originally covered 1.5 million km2 from the Brazilian coast to inland areas in the south and east of Paraguay and northeast of Argentina (Galindo Leal and de Gusmão Câmara, 2003). At present, almost 85% of its original vegetation cover has disappeared due to logging and colonization (FVSA and WWF, 2017; Rezende et al., 2018).
We selected a total of 17 study sites (Table S1) comprising five urban settlements and five natural habitats in the forest landscape matrix (Argentina), and four urban settlements and three natural habitats in the agricultural landscape matrix (Paraguay). Natural habitats were located in protected areas, while urban settlements were towns that had a population between 25,000 and 80,000 inhabitants and covered a mean area of 800 ha (700–1200 ha). In both countries, natural habitats and urban settlements were relatively similar in terms of structure and composition and level/density of urban development, respectively. The natural habitats belong to the Atlantic Forest biome and the towns share similar foundational and urban development processes dating back to the Spanish colonial period, when boundaries were not yet established (Maeder, 2010). The Paraguayan towns were founded before a massive deforestation driven by the agricultural expansion beginning in 1973 (Huang et al., 2007), while a reduction in the native forest of Misiones province, Argentina, began in the same year due to the expansion of forest plantations (Izquierdo et al., 2008).
Landscape matricesIn Argentina, Misiones province holds 45% of the original forest cover in protected areas and has extensive private lands dedicated to tree plantations for wood and pulp production (Di Bitetti et al., 2003; Galindo Leal and de Gusmão Câmara, 2003). It has the highest bird species richness of Argentina, hosting 55% of bird species. Moreover, this province encompasses the largest continuous portion of the Interior Atlantic Forest and includes diverse phytogeographic regions (Giraudo and Povedano, 2004). Misiones is covered by native forest in large public and small private protected areas, and commercial plantations of pine (Pinus spp.), araucaria (Araucaria angustifolia) and eucalyptus (Eucalyptus spp.) (Di Bitetti et al., 2003; Galindo Leal and de Gusmão Câmara, 2003). In the last years, the province experienced a dramatic increase in the urban population due to rural-urban migration (Izquierdo et al., 2011), highlighting the need for urban planning. Forest is the predominant habitat type in this region, thus comprising most of the landscape matrix around towns (Fig. 1B).
Paraguay has one of the highest levels of deforestation in Latin America due to agricultural development. Only 17% of the original cover of the Atlantic Forest is preserved in an extremely fragmented landscape, due to a massive forest loss that occurred between 1970 and 2000 (Galindo Leal and de Gusmão Câmara, 2003; Huang et al., 2007). The urban population increases because farmers benefit from some urban-based activities such as mechanization and expansion of commercial farming. Small towns serve as marketing centers for rural products, supporting agricultural production through the access to inputs and services (Zoomers and Kleinpenning, 1996). The fact that forest lands have long been viewed as unproductive (Cartes, 2003) in contrast to intensive agriculture, explains why most of the surface area is currently occupied by croplands and pastures, with 80% of the country's total soybean production being concentrated in this region (Di Bitetti et al., 2003; INBIO, 2008). Fig. 1A shows the typical agricultural landscape matrix around towns in eastern Paraguay.
Bird surveys and functional traitsTo obtain a comparative measure of bird diversity among sites, at each of them we established 10 observation points located at least 150 m apart to minimize double-counting bias (Bibby et al., 1998). In natural habitats, points were located in a homogenous forest area with a minimum of 20 ha. In urban settlements, they were set up every two blocks (200 m) at street intersections at the center of the town, and at least one block away from open green spaces. We used the point-count technique with a 50 m-fixed radius (Ralph et al., 1996). At each point, two trained observers simultaneously but independently recorded all birds seen or heard during 5 min, from sunrise to 4 h later; birds flying overhead were excluded. Owing to the regional scale of the study, we maximized the number of point counts rather than the time spent at each sampling point (Koper and Schmiegelow, 2006; Zurita and Bellocq, 2010). Observation points were visited once during the breeding season (September to November) on sunny days with calm winds. To ensure a reliable identification and to account for differences in visual detectability between habitat types (Zurita and Bellocq, 2010), we recorded all bird songs using a digital recorder (Zoom H4next Handy Recorder) during the 5-min bird count period. Finally, we analyzed the 170 recorded songs and verified their identity by comparing them with published recordings (Xeno-canto-Foundation, 2018).
We selected 11 functional traits (and their categories, see Table S2), including ecological traits (e.g., number of habitats used, sensitivity to human disturbance) and life-history traits (e.g., diet, clutch size and body mass). All traits were categorical and each category was binary, following the protocol for standardization of the trait matrix (e.g., Jaksic and Medel, 1990; Petchey and Gaston, 2002). We assigned 0 or 1 depending on whether the species presented each category of the trait. All trait categories were mutually exclusive (only one category of each trait was 1), except diet, foraging substrate, and nesting habitat (for more details see Vaccaro et al., 2019).
Data analysesWe assessed the taxonomic and functional diversity of bird assemblages for all study sites and compared the taxonomic and functional dissimilarity between urban settlements and natural habitats to analyze changes in the species and functional trait composition. To do so, we built two sets of two data matrices for each landscape matrix: (1) sites-by-species and (2) species-by-traits. In the sites-by-species matrices we included species abundance, which was recorded at the 10 observation points for each study site. The Shannon index was used to calculate taxonomic diversity for each study site with the “diversityresult” function in the “BiodiversityR” package of R (Kindt and Coe, 2005; R Core Team, 2018). Functional diversity was estimated using the multidimensional functional dispersion index (FDis) (Laliberté and Legendre, 2010). Taxonomic and functional dissimilarities between urban settlements and natural habitats for each landscape matrix were calculated with the Jaccard index and the functional Sorensen’s index (FSor), respectively (Baselga and Orme, 2012; Swenson et al., 2011). We obtained functional dissimilarity values by calculating 1-FSor. For each landscape matrix, we considered the pool of species recorded in natural habitats as representative of the natural forest assemblage, and calculated dissimilarities between each urban settlement and the species pooled from all natural habitats (Vaccaro et al., 2019).
We used generalized least squares models (GLS) to test for differences in taxonomic (Shannon) and functional (FDis) diversities between sites from the two landscapes. For each model, we considered two factors and their interaction: habitat type with two levels (natural habitat and urban settlement) and landscape matrix with two levels (forest landscape matrix and agricultural landscape matrix). For all combinations of habitat type and landscape matrix, the assumption of homoscedasticity among groups was checked with Levene’s Test and plots of standardized residuals vs fitted values. In case of heteroscedasticity, we used the varIdent function, which allows different variances per treatment. Models were fitted using the “gls” function in the “nlme” R package (Pinheiro et al., 2014). Tukey's post-hoc test was used to compare means between groups or mean factors when the interaction term was significant, with the “glht” function in the “multcomp” R package (Hothorn et al., 2008).
To test if bird dissimilarity in urban settlements relative to natural habitat depended on the surrounding matrix, we used GLS to compare values of taxonomic and functional dissimilarities between landscape matrices. In addition, we performed a similarity percentage analysis (SIMPER) to determine the bird species that most contributed to the differences between urban settlements and natural habitats in both landscape matrices (Clarke, 1993). Finally, we analyzed associations between functional traits and habitat types in each landscape matrix using a fourth-corner analysis (Brown et al., 2014), which tested for relationships between species traits and habitat types by comparing sites-by-species and species-by-traits matrices. To improve the visualization, we performed separate analyses for each of the four trait groups (Table S2). We used R software for all analyses (R Core Team, 2018) and differences were considered significant at P < 0.05.
ResultsWe recorded a total of 3104 individual birds belonging to 158 species (Table S3). In the forest matrix, we recorded 992 individuals of 114 species from natural habitats and 1208 individuals of 55 species from urban settlements. In the agricultural matrix, we found 276 individuals of 64 species from natural habitats and 628 individuals of 31 species from urban settlements.
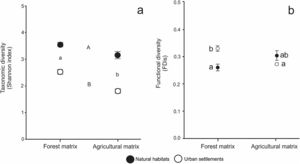
The results of the analysis of GLS for the influence of habitat type and landscape matrix on taxonomic diversity showed no significant interaction between factors (F1,13 = 2.449, P = 0.138) (Fig. 2A). Overall, taxonomic diversity was significantly higher in the forest matrix than in the agricultural matrix (Z = −3.215, P < 0.00131) and in natural habitats than in urban settlements (Z = −9.152, P < 2e−16).
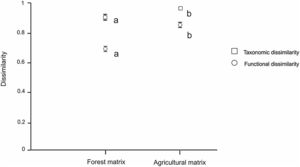
(a): Mean values of taxonomic diversity (estimated by Shannon index) in natural habitats (black circles) and urban settlements (white circles) in the forest and agricultural landscape matrices. Whiskers show standard errors. Different letters indicate significant differences (P < 0.05) between habitat types (capital letters) and between landscape matrices (low-case letters) determined by post-hoc Tukey's test. (b): Mean values of functional diversity (estimated by FDis index) in natural habitats (black circles) and urban settlements (white circles) in the forest and agricultural landscape matrices. Whiskers show standard errors. Different letters indicate significant differences (P < 0.05) between each type of habitat and each type of landscape matrix, determined by post-hoc Tukey’s test.
The analysis of GLS for functional diversity, accounting for heteroscedasticity, showed that the differences between urban settlements and natural habitats depended on the landscape matrix, as the interaction term was significant (F1,13 = 17.298, P = 0.001) (Fig. 2B). In the forest matrix, mean functional diversity was lower for natural habitats than for urban settlements (Z = 4.381, P < 0.001), while it did not differ significantly between these habitat types in the agricultural matrix (Z=-1.758, P = 0.275). Likewise, urban settlements showed higher functional diversity in the forest matrix than in the agricultural matrix (Z = −5.436, P < 0.001), while natural habitats showed non-significant lower functional diversity in the forest matrix than in the agricultural matrix (Z = 2.047, P = 0.157).
Both taxonomic and functional dissimilarities between urban settlements and natural habitats were significantly lower for the forest matrix than for the agricultural matrix (Fig. 3). Both matrices showed high mean taxonomic dissimilarities, but these were lower for the forest matrix than for the agricultural matrix (F1,7 = 31.736, P = 0.0008). Likewise, the mean functional dissimilarity between habitat types was lower for the forest matrix than for the agricultural matrix (F1,7 = 42.919, P = 0.0003).
Mean values of taxonomic and functional dissimilarities (estimated by the Jaccard and FSor indexes, respectively), between natural habitats and urban settlements in the forest and agricultural landscape matrices. Whiskers show standard errors. Different letters indicate significant differences (P < 0.05) between landscape matrices determined by GLS analysis.
SIMPER analysis showed that 45 species in the forest matrix and 29 in the agricultural matrix contributed the most to differentiate habitats within each landscape type (Tables S4 and S5). Results showed that taxonomic differentiation in both landscape matrices was due to forest species in natural habitats and generalist and urban-adapter species in urban settlements. Some examples of forest species were the Golden-crowned Warbler (Basileuterus culicivorus), Plain Antvireo (Dysithamnus mentalis), Surucua Trogon (Trogon surrucura), Boat-billed Flycatcher (Megarynchus pitangua), and the Rufous-winged Antwren (Herpsilochmus rufimarginatus). Some examples of generalist and urban-adapter species were the House Sparrow (Passer domesticus), Sayaca Tanager (Thraupis sayaca), Great Kiskadee (Pitangus sulphuratus), Rufous Hornero (Furnarius rufus), and Saffron Finch (Sicalis flaveola). Moreover, natural habitats and urban settlements in the forest matrix shared more species (25) than did natural habitats and urban settlements in the agricultural matrix (5), such as the Swallow-tailed Kite (Elanoides forficatus), Plumbeous Kite (Ictinia plumbea), Chestnut-eared Aracari (Pteroglossus castanotis), Scaly-headed Parrot (Pionus maximiliani), and Violaceous Euphonia (Euphonia violacea), among others (Table S3).
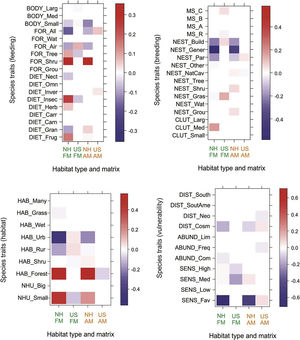
Overall, associations between functional traits and natural habitats and urban settlements were similar in both landscape matrices (Fig. 4). However, there were differences in the intensity of the associations; positive associations between natural habitats and traits such as birds foraging in shrubs or trees, with insectivorous and frugivorous diets, and using a few habitats mainly in the forest were more intense in the forest matrix than in the agricultural matrix. Moreover, we found negative associations between natural habitats and urban and generalist traits such as foraging on different substrates, nesting in buildings or other human-made structures, cosmopolitan distribution, and favored by human disturbance. Urban settlements were positively, but slightly associated with urban and generalist traits. In the forest matrix, urban settlements were positively associated with a variety of bird traits such as small body size, air as foraging substrate, buildings or other human-made structures and grass for nesting, and preference for urban and rural habitats. In the agricultural matrix, urban settlements were positively associated with a few traits, three of which were brood parasite, cosmopolitan distribution and benefit from human disturbance. Traits that were negatively associated with urban settlements in the forest matrix were large body size, trees as a foraging substrate, insectivorous diet, resident status, use of a small number of habitats, and high and medium sensitivity to human disturbance. In the agricultural matrix, urban settlements were negatively associated with natural cavities for nesting, forest as primary habitat, Neotropical distribution and frequent abundance.
Fourth-corner analysis for groups of functional traits and natural habitats (NH) and urban settlements (US) in the forest (FM) and agricultural (AM) landscape matrices. The stronger the signal, the darker the color, with positive associations in red and negative ones in blue. For acronyms of traits and groups of functional traits see Table S2.
Our results indicated that differences in the taxonomic and functional diversity of forest bird assemblages in urban settlements varied with the land-use in the surrounding landscape matrix, and that there were significant differences in the diversity of species pools between the studied landscape matrices. As expected, taxonomic and functional diversities were higher for urban settlements embedded in a landscape matrix mainly composed of natural habitat than in an agricultural matrix. Moreover, taxonomic and functional dissimilarities between urban settlements and natural habitats were lower in the forest matrix than in the agricultural matrix, suggesting that the forest matrix may act as a source of species and resources for cities (Dale, 2018). In agreement with other studies dealing with forests in general (Mason et al., 2007; Taylor et al., 2016) and the Atlantic Forest in particular (Fontana et al., 2011; Zurita and Bellocq, 2010), we found that tree cover in the landscape matrix plays a key role in the presence of forest birds within nearby cities. However, as long as birds do not achieve reproductive success ensuring colonization and population persistence, cities could act as sinks or ecological traps. This aspect should be taken into account for a better evaluation of the importance of the surrounding forest matrix in the preservation of forest birds in urban settlements.
Results indicated differences in the taxonomic diversity of birds from both urban settlements and natural habitats, regardless of the landscape matrix. However, the fact that in both habitat types taxonomic diversity was lower for the agricultural matrix than for the forest matrix suggests that the landscape matrix may influence the species pool at the landscape scale. Natural habitats in the agricultural matrix represent highly isolated ecological “islands” due to the high rates of forest loss in the surrounding areas (Huang et al., 2007). More isolated remnants embedded in an agricultural matrix that hinders dispersal are likely to affect the persistence of native species because they would depend on the abundance of habitat available (Saunders et al., 1991). On this basis, species in native forests within an agricultural matrix exhibit poor conservation status compared to those within a forest matrix (Giraudo and Povedano, 2004). In contrast to the forest matrix, the agricultural matrix typically lacks the “stepping stones” (corridors or smallest patches) that connect bird communities between natural habitats (Taylor et al., 2016). In our study, urban settlements in the agricultural matrix were surrounded by open habitats and a few forest patches, which may explain why they showed lower taxonomic diversity than did urban settlements in the forest matrix.
Urban influence on functional diversity of bird assemblages differed between landscape matrices, which seemed to play a key role in the variety of traits exhibited by birds in urban settlements. So far, empirical evidence shows that highly urbanized environments lead to a decreased functional diversity compared to natural habitats (Ortega-Álvarez and MacGregor-Fors, 2009; Sol et al., 2020). However, towns with abundant vegetation offer a variety of small-scale habitats, resulting in a higher functional diversity than that in semi-natural habitats (Oliveira Hagen et al., 2017). In fact, we found that functional diversity was higher for urban settlements in the forest matrix than for natural habitats and urban settlements in the agricultural matrix and, surprisingly, higher than for natural habitats in the forest matrix. Cities embedded in the forest matrix not only provide a variety of microhabitats, but also could favor the colonization of generalist forest birds because they are close to forested areas and hold elements of the natural forest such as native trees (Filloy et al., 2019). Nevertheless, some bird species occurring in cities (e.g., flycatchers) may experience low population growth rates due to an increase in predation risk or brood parasitism (Rodewald et al., 2013). On the other hand, urban settlements showed slightly lower functional diversity than did natural habitats in the agricultural matrix, possibly because forest-adapted species are unable to move across the agricultural matrix. In highly fragmented, anthropogenic landscapes, specialist bird species are more affected than generalists because of low matrix permeability and the presence of unsuitable patch types (Khimoun et al., 2016). Gillies and Clair (2010) reported that both generalist and specialist birds from a tropical forest required small patches of trees as stepping stones to return to their original territory. This supports our results suggesting that the agricultural landscape matrix hindered the movement of forest generalists into urban settlements and probably reduced the availability of source populations from which individuals may disperse. In addition, as a consequence of the tradeoffs between adjacent ecosystems, cities may differ in local characteristics due to the landscape matrix, which may contribute to drive functional diversity of bird assemblages, and ecosystem functions and services (Hagen et al., 2017). According to Gounand et al. (2018), urban settlements may function as meta-ecosystems subsidized by resources and species from the surrounding habitat. In regard to urban settlements embedded in the forest matrix, the surrounding habitat provides them with resources and species having functional traits related to the forest. This is not the case for urban settlements in the agricultural matrix, as they are surrounded by agricultural habitat.
The lower taxonomic and functional dissimilarities between habitat types in the forest matrix compared to the agricultural matrix indicate that they shared some species and traits. The forest landscape matrix represents a less powerful filter than open habitats in the agricultural landscape matrix (Filloy et al., 2010; Vaccaro and Bellocq, 2019; Zurita and Bellocq, 2010), thus allowing the presence of species with forest traits in urban settlements embedded in a forest landscape matrix. In the present study, urban settlements and natural habitats in the forest matrix showed insectivorous and frugivorous species foraging on and nesting in trees such as the Streaked Flycatcher (Myiodynastes maculatus), Plumbeous Kite and Violaceous Euphonia. Instead, only a few birds with these traits were found in urban settlements in the agricultural matrix. These results are in line with those obtained in the meta-analysis by Sekercioglu (2012), suggesting that forest replacement by agricultural areas may lead to the loss of specialized bird species, such as those feeding on invertebrates (e.g., insectivores). It is also possible that the high functional and taxonomic dissimilarity between urban settlements and natural habitats in the agricultural matrix resulted from the extensive use of pesticides in monocultures that dominate the agricultural landscape matrix in the Atlantic Forest (Clay, 2013), causing the decline in insect-prey abundance in cities. Then, the dissimilarity between urban and native bird communities is expected to be lower in a landscape matrix that allows the dispersal of generalists and some specialized forest birds, as long as urban characteristics favor their occurrence. Moreover, cities in the forest matrix may be acting as sinks for forest species in a source-sink dynamics at the meta-community level (Leibold et al., 2004), explaining the presence of forest species even under the adverse conditions of an urban environment.
Although the species and functional traits that most contributed to differentiate habitat types were, in general, similar in both landscape matrices, species with urban and generalist traits made a greater contribution in the agricultural matrix. In both landscape matrices, the latter were associated with urban settlements, while specialists and species vulnerable to anthropogenic changes were associated with natural habitats. Urban-adapted species have a combination of traits involving diet, preferred nesting sites and migratory status, among others, that enables them to exploit urban resources (Kark et al., 2007), and urban bird assemblages are typically composed of generalist species (Croci et al., 2008). We found generalist and urban species such as the House Sparrow, the Sayaca Tanager and the Kiskadee Flycatcher in both landscape matrices, but they mainly occurred in the urban settlements of the agricultural matrix. Species with urban and generalist traits occurred in both urban settlements of each landscape matrix, but a “dilution” effect of these traits may have occurred in the forest matrix because bird assemblages also included forest species, such as the Swallow-tailed Kite or the Scaly-headed Parrot, among others. Conversely, the agricultural landscape matrix may sustain or even enhance the selective pressure of urban settlements on native birds, as suggested by the observed contribution of urban and generalist species and traits to these habitats.
ConclusionsResults indicated that the functional and taxonomic composition of bird assemblages varied with the surrounding habitat, and that a landscape matrix mainly composed of native habitat may mitigate the urban impact on native communities. This is in agreement with previous studies (Antongiovanni and Metzger, 2005; Jokimäki and Huhta, 1996; Zurita and Bellocq, 2010) emphasizing the importance of the landscape matrix for the occurrence of native birds, and reveal that management decisions should not be restricted to the improvement of urban habitats for forest birds. However, beyond community composition, reproduction and survival must be maintained, restored and monitored to ensure bird conservation (Marzluff and Ewing, 2008). Moreover, the landscape matrix could also be determinant of the native species pool. Although urban settlements embedded in a forest landscape matrix harbored relatively fewer species compared to native forests, they presented a variety of forest traits, as evidenced by the occurrence of both urban and forest generalist species. Urban development shapes the functional and species composition of bird assemblages because it favors traits that better match the new environment, acting as a driver of species replacement (Loreau et al., 2001; Sol et al., 2020). In this regard, we found that species and traits turnover was more evident in the agricultural than in the forest landscape matrix. Based on these considerations, we suggest that management measurements should be aimed at improving not only the presence of native diversity in the cities but also the permeability of the landscape matrix. For example, trees in an agricultural landscape proved to increase landscape connectivity for forest birds (Cadavid-Florez et al., 2020). Our approach of simultaneous analysis of taxonomic and functional diversity provided useful and comprehensive information on the role of the surrounding matrix structure in maintaining native diversity in urban areas, including landscapes of the Atlantic Forest and other fragmented tropical forests in South America.
Conflict of interestThe authors declare no conflict of interest.
We thank D. Friedrich, A. de Miguel, J. Vrdoljak and G. Nicosia for field assistance, J. Klavins for species identification and field assistance, and the two anonymous reviewers for their valuable comments which greatly improved the manuscript. Logistical support and legal permissions were provided by Administración Parques Nacionales (RNE San Antonio), Fundación Temaikén (Reserva Natural Osununú), Reserva Privada Surucuá, Centro de Investigaciones Antonia Ramos and E. Avigliano, Ministerio de Ecología de Recursos Naturales Renovables de la provincia de Misiones and F. J. Castía (Parque Provincial Esmeralda), Pomera Maderas S.A., Campos Morombí, PAYCO S.A., Fundación Moisés Bertoni and GUYRA Paraguay. We thank Silvia Pietrokovsky for language editing. The research was funded by the Agencia Nacional de Promoción Científica y Tecnológica of Argentina, the Universidad de Buenos Aires, and the Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina).