While monitoring is essential for effective conservation, obtaining occurrence data is often challenging, time consuming and expensive. The Brazilian Atlantic Forest has a high number of threatened and endemic species that need effective and urgent conservation actions informed by sound monitoring data. Community (or citizen) science surveys can provide cost-effective data for large areas over extended time and these geocoded and time-stamped observations can deliver information on species of conservation interest. We provide a spatio-temporal analysis of Least Concern, Near Threatened and globally threatened Atlantic Forest endemic bird species from iNaturalist, eBird and WikiAves and analyze species according to their global trends. Together, these three datasets contained 838,880 unique observations of 218 species in 2000–2022, including 95 threatened and Near Threatened species. While the absolute number of observations of threatened and Near Threatened species increased annually, their proportion decreased compared to the total number of observations. Similarly, the proportion of observations of declining species decreased. Through time, the number of non-specialist birdwatchers could have increased, with the higher survey effort resulting in a higher proportion of common (i.e., more easily observed) species. However, this pattern can also reflect real trends, as most threatened and Near Threatened species were declining, leading to decreased detectability and relatively fewer observations, even with the same effort and skills. Decreasing and threatened species need special attention and targeted monitoring. In spite of the biases inherent in non-structured datasets and the difficulties of surveying rare species, community science can provide an effective warning system, and can improve monitoring of species at high risk of extinction.
Datasets of species occurrences provide the basis for studies of spatial distribution and assessments of population trends (Chapman, 2005; Soberón and Townsend Peterson, 2009), fundamental to inform conservation actions (Robinson et al., 2018). Unfortunately, individual researchers are constrained by logistics and funding, which prevent data collection at broad spatial scales and longer time frames. As rare or threatened species usually occur at low densities and require large sampling effort (Green and Young, 1993), they are often underrepresented in datasets compared to locally abundant or widely distributed species (Martikainen and Kouki, 2003). Unlike traditional research, community (or citizen) science (CS) has produced large amounts of data at large scales, occasionally also providing information on threatened species (Bonney, 2021; Lloyd et al., 2020; Wilson et al., 2020). The effort devoted by CS has reduced the Wallacean shortfall, i.e., the lack of knowledge with regard to species distribution (Deacon et al., 2023; Hortal et al., 2015).
Worldwide, many datasets collected by CS feed into the largest global biodiversity database, the Global Biodiversity Information Facility (GBIF; Bonney, 2021; Callaghan et al., 2021). While spatial, temporal, and taxonomic biases are inherent to most CS datasets and they often only record presences (Di Cecco et al., 2021; Szabo et al., 2012a,b), statistical methods are becoming available to handle most of these issues (Bird et al., 2014; Jiménez et al., 2019; Szabo et al., 2010) and after cleaning and adjustments, the data can inform conservation decision making (Newman et al., 2017).
Birds are the best-represented taxonomic group in global biodiversity databases (Troudet et al., 2017). For instance, in October 2023, GBIF included over 1.7 million bird records, representing approximately 6% of all biodiversity data (https://www.gbif.org/). To illustrate the volume of information available with regard to birds, in the same month, eBird had over 630,000 occurrence lists of 1800 species for Brazil (https://ebird.org/) and the Brazilian WikiAves hosted over 4.5 million photos and nearly 270,000 sound recordings of 1961 species (https://www.wikiaves.com.br/). At the forefront of producing unstructured data, iNaturalist brings together a global community of over 2.8 million observers. This platform, formerly managed by the California Academy of Sciences and National Geographic and now a non-profit organization, has been serving as a barometer of CS activity virtually around the globe and at the time of writing this article, contained 323,000 observations of 1702 bird species for Brazil (https://www.inaturalist.org/). However, we need to mention here that these platforms use somewhat different taxonomy that need to be reconciled before directly comparing species diversity and numbers.
As a result of this high interest in birds, in countries that have a relatively high number of birdwatchers and low avian species richness, the coverage of bird species on these CS platforms is nearly complete. For example, for the United States, almost 90% of extant bird species reported from the country are represented by at least one record on iNaturalist (Di Cecco et al., 2021). However, the species most frequently recorded on iNaturalist tend to be common in urbanized and other human-modified habitats, as well as large and easily observed (Di Cecco et al., 2021). Overall, CS has produced contrasting data with regard to the representativeness of threatened species observations (Sánchez-Clavijo et al., 2021). For instance, in Western Australia, volunteer surveyors were more interested in recording rare bird species compared to common species, practically oversampling rare and “interesting” or “birdwatching trophy” species (Tulloch and Szabo, 2012). On the other hand, in Colombia, during the Covid-19 pandemic the number of visits to less disturbed areas decreased, leading to a decreasing number of observations of species of conservation interest compared to Least Concern species (Sánchez-Clavijo et al., 2021). Nevertheless, the decreasing number of rare or threatened species observations in remote and protected areas can be a worrying indicator of species declines (Barnes et al., 2015). Therefore, these patterns need to be assessed, if possible, using an independent dataset collected using structured surveys, as they can indicate real declines (Szabo et al., 2011).
Following BirdLife International’s (2023) taxonomy, with 1816 species, Brazil has the third highest avian species diversity in the world, of which 257 species are endemic to the country. However, Brazil also ranks extremely high (second in the world) with regard to the number of species in danger of extinction (BirdLife International, 2023). The Atlantic Forest is the second largest tropical moist forest domain in South America after the Amazon and is considered a global biodiversity hotspot (Myers et al., 2000). This biome is home to over 800 bird species, 223 of which are endemic, a number that can further increase due to ongoing taxonomic revisions (Pizo and Tonetti, 2020). The three Endemic Bird Areas (Atlantic Forest lowlands and Atlantic Forest mountains and the Atlantic slope of Alagoas and Pernambuco) and 163 Important Bird and Biodiversity Areas in particular, hosts several endemic, restricted range and threatened taxa (Bencke et al., 2006). Most of these birds are threatened by habitat fragmentation and habitat loss, both ongoing and current (BirdLife International, 2023). In fact, over 92% of the original vegetation has been lost due to deforestation (Marques and Grelle, 2021). This level of habitat loss has led to a substantial extinction debt (Uezu and Metzger, 2016), which makes biodiversity surveys and the appropriate conservation actions particularly urgent (Szabo et al., 2011). The Atlantic Forest covers nearly 11,200,000 km2 of the Brazilian territory, spreading from sea level to above 3000 m and containing a mosaic of ombrophilous, deciduous and semideciduous forests, mangroves, dunes and high-altitude meadows (Ribeiro et al., 2011). The Atlantic Forest displays complex vertical stratification, offering a variety of substrates and microhabitats for its highly diversified biota (Morellato et al., 2000). Unfortunately, its remaining biodiversity continues to face challenges related not only to habitat fragmentation but also global climate change (de Lima et al., 2020; SOS Mata Atlântica/INPE, 2018).
In Brazil, CS-collected data have been used to study migratory patterns of birds (Cunha et al., 2022; Guaraldo et al., 2022; Lees and Martin, 2015; Lopes and Schunck, 2022; Schubert et al., 2019), as well as general species distribution (Santos et al., 2021; Zulian et al., 2021), habitat use (Barbosa et al., 2021; Devenish et al., 2021), feeding behavior (de Souza et al., 2022), novel nesting behavior (Alexandrino et al., 2022) and species interactions (Bosenbecker et al., 2023). However, there is no specific information on the spatio-temporal patterns of imperiled bird species in CS-collected data in Brazil, which can restrict decision making for species protection. Considering the importance of participatory monitoring in inform conservation actions, we aim to describe spatio-temporal patterns of globally threatened and Near Threatened Atlantic Forest endemic bird species based on observations collected by CS in the three most popular digital platforms hosting bird observations from Brazil. Based on the spatial data, we evaluate the representativeness of observations in urban, as well as protected areas. Using temporal data, we quantify the proportion of observations of threatened and Near Threatened (thereafter imperiled) species in relation to all species observed in 2000–2022. We also describe the monthly distribution of observations and observers. Finally, we discuss the representativeness of imperiled Atlantic Forest bird species in the dataset, providing recommendations and guidelines for future surveying efforts in partnership with the public to generate more robust datasets, in order to support conservation actions and to evaluate the effectiveness of past actions in the biome.
Materials and methodsBased on the list of Atlantic Forest endemic bird species in Vale et al. (2018), we compiled data from three CS platforms: (1) eBird (https://ebird.org/home), (2) WikiAves (https://www.wikiaves.com.br/index.php) and iNaturalist (https://www.inaturalist.org/). As eBird data are curated by experts, we used all observations, including species lists, while from the other two we used observations with audio and photo evidence. In order to use global threat status and trends, we adopted BirdLife International’s taxonomy (BirdLife International, 2023), obtaining trends from BirdLife Data Zone manually (http://datazone.birdlife.org/) and global threat status from IUCN (https://www.iucnredlist.org/; which in turn is based on the Red List assessment conducted by BirdLife) through the rredlist package (Chamberlain, 2020) of the program R version 4.0.5. (R Core Development Team, 2020). We used global threat status and trends from the assessment of 2020, as considering and recalculating the status and trends of these species at previous assessments (2000, 2004, 2008, 2012 and 2016) was outside the scope of this paper (see for instance Szabo et al. (2012a). Nevertheless, since 1988, only 93 species have been downlisted globally to a lower Red List category due to a genuine improvement in status, while and 436 species have been uplisted (BirdLife International, 2022). In addition, status is calculated for 10 years or three generations, whichever is the longer (IUCN Standards and Petitions Committee, 2022). Therefore, we can calculate 10 years for passerines and a 4-to-7-year generation length is realistic for the non-passerine endemic species (e.g., 4.1 years for Solitary Tinamou Tinamus solitarius and Long-trained Nightjar Macropsalis forcipata, 4.6 years for Rufous-capped Motmot Baryphthengus ruficapillus, 4.7 years for Red-breasted Toucan Ramphastos dicolorus 5.9 years for White-collared Kite Leptodon forbesi and 7.4 years for Black-fronted Piping-Guan Pipile jacutinga; Bird et al., 2020). Considering the evaluation period and the fact that habitat loss and fragmentation continue in the Atlantic Forest (Pizo and Tonetti, 2020; Schnell et al., 2013), we are confident that our methods are conservative.
We formally requested raw data for the area of interest from eBird and iNaturalist and received them in csv format. As bulk download request was not available for WikiAves, we used the instant data scraper app (https://webrobots.io/instantdata/), adhering to the “fair use” principle. We only considered research grade observations from iNaturalist, i.e., those that were identified at the species level and reached a 2/3 consensus among the identifiers on the suggested species.
The raw dataset contained 1,204,210 observations, with 58.8% of the observations in eBird, 39.8% in WikiAves and 1.4% in iNaturalist. Based on the species, date and location, we removed data points duplicated within and among the datasets and filtered observations to 2000–2022 using the distinct and filter functions in the dplyr R package (Wickham et al., 2022). Therefore, we excluded repeat observations of the same species from the same day and the same location, but treated observations of the same individual bird on different days as separate data points. We restricted the data to observations collected after January 1, 2000, because digital CS platforms became popular after this year (Mandeville et al., 2022), and to be able to use current threat and trend data, as discussed above. We also cleaned the data by deleting observations with missing coordinates and locations outside the limits of the Atlantic Forest using the clip tool in QGIS software version 3.16.9 (QGIS Development Team, 2021).
After removing duplicates and pre-2000 observations (approximately 30% of the data), we obtained a joint clean dataset of 838,880 observations of all Atlantic Forest endemic bird species for 2000–2022. Approximately 13.8% of these observations (115,582 data points) were of threatened and Near Threatened species. We used a fit test for Benford’s Law to evaluate the distribution of digits on the number of observations among species to check data quality of the joint dataset based on sampling heterogeneity (Szabo et al., 2023). For this test, we adopted the Benford function of the Benford analysis package (Cinelli, 2014), and following standard practice, we used only species with more than 100 observations (Nigrini, 2012). We tested the fit of the data to the unimodal gamma binomial distribution model, which is considered the best model of species abundance distributions for real communities with many rare species (Ugland et al., 2007) and used the fit abundance function in the gambin package to estimate statistical parameters and to test the fit using the maximum likelihood method (Matthews et al., 2014).
We identified 493,186 bird observations (about 40% of the raw data) that had exact geographic coordinates, i.e., we excluded the entire WikiAves dataset from this analysis, as locations are only provided at the scale of municipality level, as well as observations with incorrect or missing location information from the other two datasets. Using the subset with valid coordinates, we overlayed bird observations with urban and non-urban areas (IBGE, 2015), as well as protected areas (Centro de Estudos da Metrópole, 2021) using QGIS. We defined protected areas as conservation units that are strictly protected (IUCN Categories I–IV) and sustainable use (IUCN Categories V and VI). The remaining vegetation cover (about 70% of the vegetation cover) is protected by other area-based conservation measures, which may allow intervention and deforestation in specific situations (Rezende et al., 2018). We created distribution maps of observations of Atlantic Forest bird species using QGIS.
We compared observed and expected numbers of observations by region based on the area of each region using a χ2 goodness of fit through the chisq. test function in R. Considering that we had four regions (no relevant habitat in the north; Fig. 1), we adjusted the p-value for 0.0125, as a result of Bonferroni correction (0.05/4). We repeated the same test for observations inside and outside protected areas.
(a) The distribution of endemic Atlantic Forest bird species observations from two popular community science platforms (eBird and iNaturalist) from 2000 to 2022; and (b) observations of threatened and Near Threatened species inside (red), and outside (blue) protected areas. The inset shows the five major regions of Brazil: N: north, NE: northeast, CW: central west, SE: southeast and S: south. Species threat status and taxonomy follows BirdLife International (2023).
We tested whether the numbers of observations of imperiled species and contributing observers on the platforms changed along years applying generalized linear models (glm) using the glm function in R. For this, we chose quasipoisson distribution, to avoid false positives due to overdispersion in the count data. We also tested whether the proportion of observations of imperiled species changed along the years in relation to the number of all species observations. Given the normal distribution of the data, here we applied glm of the Gaussian family. Similarly, we fitted two Gaussian glm to compare the proportion of observations of decreasing and stable species along the years (i.e., by dividing the number of observations of decreasing species by the sum of decreasing and stable for each year).
To understand whether the diversity and abundance of imperiled species correlate with the annual number of observers on the platform, after visually checking the linearity of the data, we applied Spearman’s correlation through the corr. test function of the psych package (Revelle, 2022). To test the seasonality in the number of observations of Atlantic Forest endemic birds, we used the Rayleigh test of uniformity based on the ρ-value using the circular package (Agostinelli and Lund, 2023) and visualized monthly and annual observation patterns using the ggplot2 package (Wickham, 2016).
ResultsGeneral data descriptionThe average number of observations per species was 3779, ranging from two sightings of the Alagoas Curassow (Mitu mitu) to 35,583 observations of the Ruby-crowned Tanager (Tachyphonus coronatus), which is a common, Least Concern species (Table 1). Eleven species had fewer than 100 observations, two of them classified as Least Concern. Among imperiled species, the ten most observed species accounted for 41% of the observations, and all of them were Near Threatened. Near Threatened species were represented in 9.4% of all (threatened + non-threatened species) observations, Vulnerable in 2.9%, Endangered in 1.3% and Critically Endangered species in 0.2% of observations.
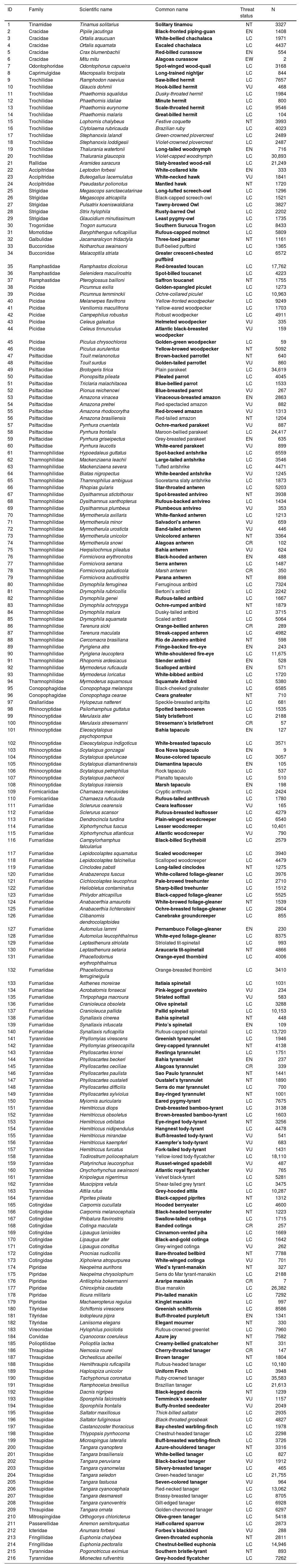
Endemic bird species of the Brazilian Atlantic Forest based on Vale et al. (2018) with global threat status (IUCN 2022) and the number of observations (N) on three digital community science platforms based on data from 2000–2022; LC — Least Concern, NT — Near Threatened, VU — Vulnerable, EN — Endangered, CR — Critically Endangered, EW — Extinct in the Wild, EX — Extinct. The common name of declining species (165 species) is marked in bold and with unknown trend (11 species) in italics (BirdLife International, 2023). Note: we use BirdLife International taxonomy and nomenclature.
| ID | Family | Scientific name | Common name | Threat status | N |
|---|---|---|---|---|---|
| 1 | Tinamidae | Tinamus solitarius | Solitary tinamou | NT | 3327 |
| 2 | Cracidae | Pipile jacutinga | Black-fronted piping-guan | EN | 1408 |
| 3 | Cracidae | Ortalis araucuan | White-bellied chachalaca | LC | 1971 |
| 4 | Cracidae | Ortalis squamata | Escaled chachalaca | LC | 4437 |
| 5 | Cracidae | Crax blumenbachii | Red-billed curassow | EN | 554 |
| 6 | Cracidae | Mitu mitu | Alagoas curassow | EW | 2 |
| 7 | Odontophoridae | Odontophorus capueira | Spot-winged wood-quail | LC | 3168 |
| 8 | Caprimulgidae | Macropsalis forcipata | Long-trained nightjar | LC | 844 |
| 9 | Trochilidae | Ramphodon naevius | Saw-billed hermit | LC | 7657 |
| 10 | Trochilidae | Glaucis dohrnii | Hook-billed hermit | VU | 468 |
| 11 | Trochilidae | Phaethornis squalidus | Dusky-throated hermit | LC | 1984 |
| 12 | Trochilidae | Phaethornis idaliae | Minute hermit | LC | 800 |
| 13 | Trochilidae | Phaethornis eurynome | Scale-throated hermit | LC | 9546 |
| 14 | Trochilidae | Phaethornis malaris | Great-billed hermit | LC | 104 |
| 15 | Trochilidae | Lophornis chalybeus | Festive coquette | NT | 3993 |
| 16 | Trochilidae | Clytolaema rubricauda | Brazilian ruby | LC | 4023 |
| 17 | Trochilidae | Stephanoxis lalandi | Green-crowned plovercrest | LC | 2489 |
| 18 | Trochilidae | Stephanoxis loddigesii | Violet-crowned plovercrest | LC | 2487 |
| 19 | Trochilidae | Thalurania watertonii | Long-tailed woodnymph | EN | 716 |
| 20 | Trochilidae | Thalurania glaucopis | Violet-capped woodnymph | LC | 30,893 |
| 21 | Rallidae | Aramides saracura | Slaty-breasted wood-rail | LC | 21,249 |
| 22 | Accipitridae | Leptodon forbesi | White-collared kite | EN | 333 |
| 23 | Accipitridae | Buteogallus lacernulatus | White-necked hawk | VU | 1841 |
| 24 | Accipitridae | Pseudastur polionotus | Mantled hawk | NT | 1720 |
| 25 | Strigidae | Megascops sanctaecatarinae | Long-tufted screech-owl | LC | 1296 |
| 26 | Strigidae | Megascops atricapilla | Black-capped screech-owl | LC | 1521 |
| 27 | Strigidae | Pulsatrix koeniswaldiana | Tawny-browed Owl | LC | 3827 |
| 28 | Strigidae | Strix hylophila | Rusty-barred Owl | LC | 2202 |
| 29 | Strigidae | Glaucidium minutissimum | Least pygmy-owl | LC | 1735 |
| 30 | Trogonidae | Trogon surrucura | Southern Surucua Trogon | LC | 8433 |
| 31 | Momotidae | Baryphthengus ruficapillus | Rufous-capped motmot | LC | 5809 |
| 32 | Galbulidae | Jacamaralcyon tridactyla | Three-toed jacamar | NT | 1161 |
| 33 | Bucconidae | Notharchus swainsoni | Buff-belied puffbird | LC | 1365 |
| 34 | Bucconidae | Malacoptila striata | Greater crescent-chested puffbird | LC | 6572 |
| 35 | Ramphastidae | Ramphastos dicolorus | Red-breasted toucan | LC | 17,762 |
| 36 | Ramphastidae | Selenidera maculirostris | Spot-billed toucanet | LC | 4223 |
| 37 | Ramphastidae | Pteroglossus bailloni | Saffron toucanet | NT | 1755 |
| 38 | Picidae | Picumnus exilis | Golden-spangled piculet | LC | 1273 |
| 39 | Picidae | Picumnus temminckii | Ochre-collared piculet | LC | 10,963 |
| 40 | Picidae | Melanerpes flavifrons | Yellow-fronted woodpecker | LC | 9249 |
| 41 | Picidae | Veniliornis maculifrons | Yellow-eared woodpecker | LC | 1703 |
| 42 | Picidae | Campephilus robustus | Robust woodpecker | LC | 4911 |
| 43 | Picidae | Celeus galeatus | Helmeted woodpecker | VU | 335 |
| 44 | Picidae | Celeus tinnunculus | Atlantic black-breasted woodpecker | VU | 159 |
| 45 | Picidae | Piculus chrysochloros | Golden-green woodpecker | LC | 59 |
| 46 | Picidae | Piculus aurulentus | Yellow-browed woodpecker | NT | 5092 |
| 47 | Psittacidae | Touit melanonotus | Brown-backed parrotlet | NT | 640 |
| 48 | Psittacidae | Touit surdus | Golden-tailed parrotlet | VU | 860 |
| 49 | Psittacidae | Brotogeris tirica | Plain parakeet | LC | 34,619 |
| 50 | Psittacidae | Pionopsitta pileata | Pileated parrot | LC | 4045 |
| 51 | Psittacidae | Triclaria malachitacea | Blue-bellied parrot | LC | 1533 |
| 52 | Psittacidae | Pionus reichenowi | Blue-breasted parrot | VU | 267 |
| 53 | Psittacidae | Amazona vinacea | Vinaceous-breasted amazon | EN | 2863 |
| 54 | Psittacidae | Amazona pretrei | Red-spectacled amazon | VU | 882 |
| 55 | Psittacidae | Amazona rhodocorytha | Red-browed amazon | VU | 1313 |
| 56 | Psittacidae | Amazona brasiliensis | Red-tailed amazon | NT | 1204 |
| 57 | Psittacidae | Pyrrhura cruentata | Ochre-marked parakeet | VU | 887 |
| 58 | Psittacidae | Pyrrhura frontalis | Maroon-bellied parakeet | LC | 24,417 |
| 59 | Psittacidae | Pyrrhura griseipectus | Grey-breasted parakeet | EN | 635 |
| 60 | Psittacidae | Pyrrhura leucotis | White-eared parakeet | VU | 899 |
| 61 | Thamnophilidae | Hypoedaleus guttatus | Spot-backed antshrike | LC | 6559 |
| 62 | Thamnophilidae | Mackenziaena leachii | Large-tailed antshrike | LC | 3546 |
| 63 | Thamnophilidae | Mackenziaena severa | Tufted antshrike | LC | 4471 |
| 64 | Thamnophilidae | Biatas nigropectus | White-bearded antshrike | VU | 1245 |
| 65 | Thamnophilidae | Thamnophilus ambiguus | Sooretama slaty antshrike | LC | 1873 |
| 66 | Thamnophilidae | Rhopias gularis | Star-throated antwren | LC | 5203 |
| 67 | Thamnophilidae | Dysithamnus stictothorax | Spot-breasted antvireo | NT | 3938 |
| 68 | Thamnophilidae | Dysithamnus xanthopterus | Rufous-backed antvireo | LC | 1434 |
| 69 | Thamnophilidae | Dysithamnus plumbeus | Plumbeous antvireo | VU | 353 |
| 70 | Thamnophilidae | Myrmotherula axillaris | White-flanked antwren | LC | 1213 |
| 71 | Thamnophilidae | Myrmotherula minor | Salvadori’s antwren | VU | 659 |
| 72 | Thamnophilidae | Myrmotherula urosticta | Band-tailed antwren | VU | 446 |
| 73 | Thamnophilidae | Myrmotherula unicolor | Unicolored antwren | NT | 3364 |
| 74 | Thamnophilidae | Myrmotherula snowi | Alagoas antwren | CR | 102 |
| 75 | Thamnophilidae | Herpsilochmus pileatus | Bahia antwren | VU | 624 |
| 76 | Thamnophilidae | Formicivora erythronotos | Black-hooded antwren | EN | 488 |
| 77 | Thamnophilidae | Formicivora serrana | Serra antwren | LC | 1487 |
| 78 | Thamnophilidae | Formicivora paludicola | Marsh antwren | CR | 350 |
| 79 | Thamnophilidae | Formicivora acutirostris | Parana antwren | NT | 898 |
| 80 | Thamnophilidae | Drymophila ferruginea | Ferruginous antbird | LC | 7324 |
| 81 | Thamnophilidae | Drymophila rubricollis | Bertoni’s antbird | LC | 2242 |
| 82 | Thamnophilidae | Drymophila genei | Rufous-tailed antbird | LC | 1667 |
| 83 | Thamnophilidae | Drymophila ochropyga | Ochre-rumped antbird | NT | 1879 |
| 84 | Thamnophilidae | Drymophila malura | Dusky-tailed antbird | LC | 3715 |
| 85 | Thamnophilidae | Drymophila squamata | Scaled antbird | LC | 5064 |
| 86 | Thamnophilidae | Terenura sicki | Orange-bellied antwren | CR | 289 |
| 87 | Thamnophilidae | Terenura maculata | Streak-capped antwren | LC | 4982 |
| 88 | Thamnophilidae | Cercomacra brasiliana | Rio de Janeiro antbird | NT | 598 |
| 89 | Thamnophilidae | Pyriglena atra | Fringe-backed fire-eye | EN | 243 |
| 90 | Thamnophilidae | Pyriglena leucoptera | White-shouldered fire-eye | LC | 11,675 |
| 91 | Thamnophilidae | Rhopornis ardesiacus | Slender antbird | EN | 528 |
| 92 | Thamnophilidae | Myrmoderus ruficauda | Scalloped antbird | EN | 571 |
| 93 | Thamnophilidae | Myrmoderus loricatus | White-bibbed antbird | LC | 1720 |
| 94 | Thamnophilidae | Myrmoderus squamosus | Squamate Antbird | LC | 5380 |
| 95 | Conopophagidae | Conopophaga melanops | Black-cheeked gnateater | LC | 6585 |
| 96 | Conopophagidae | Conopophaga cearae | Ceara gnateater | NT | 710 |
| 97 | Grallariidae | Hylopezus nattereri | Speckle-breasted antpitta | LC | 681 |
| 98 | Rhinocryptidae | Psilorhamphus guttatus | Spotted bamboowren | LC | 1535 |
| 99 | Rhinocryptidae | Merulaxis ater | Slaty bristlefront | LC | 2188 |
| 100 | Rhinocryptidae | Merulaxis stresemanni | Stresemann’s bristlefront | CR | 57 |
| 101 | Rhinocryptidae | Eleoscytalopus psychopompus | Bahia tapaculo | EN | 127 |
| 102 | Rhinocryptidae | Eleoscytalopus indigoticus | White-breasted tapaculo | LC | 3571 |
| 103 | Rhinocryptidae | Scytalopus gonzagai | Boa Nova tapaculo | EN | 9 |
| 104 | Rhinocryptidae | Scytalopus speluncae | Mouse-colored tapaculo | LC | 3057 |
| 105 | Rhinocryptidae | Scytalopus diamantinensis | Diamantina tapaculo | EN | 105 |
| 106 | Rhinocryptidae | Scytalopus petrophilus | Rock tapaculo | LC | 537 |
| 107 | Rhinocryptidae | Scytalopus pachecoi | Planalto tapaculo | LC | 510 |
| 108 | Rhinocryptidae | Scytalopus iraiensis | Marsh tapaculo | EN | 198 |
| 109 | Formicariidae | Chamaeza meruloides | Cryptic antthrush | LC | 2424 |
| 110 | Formicariidae | Chamaeza ruficauda | Rufous-tailed antthrush | LC | 1780 |
| 111 | Furnariidae | Sclerurus cearensis | Ceara leaftosser | VU | 165 |
| 112 | Furnariidae | Sclerurus scansor | Rufous-breasted leaftosser | LC | 4279 |
| 113 | Furnariidae | Dendrocincla turdina | Plain-winged woodcreeper | LC | 6540 |
| 114 | Furnariidae | Xiphorhynchus fuscus | Lesser woodcreeper | LC | 10,401 |
| 115 | Furnariidae | Xiphorhynchus atlanticus | Atlantic woodcreeper | VU | 790 |
| 116 | Furnariidae | Campylorhamphus falcularius | Black-billed Scythebill | LC | 2579 |
| 117 | Furnariidae | Lepidocolaptes squamatus | Scaled woodcreeper | LC | 3940 |
| 118 | Furnariidae | Lepidocolaptes falcinellus | Scalloped woodcreeper | LC | 4479 |
| 119 | Furnariidae | Cinclodes pabsti | Long-tailed cinclodes | NT | 1275 |
| 120 | Furnariidae | Anabazenops fuscus | White-collared foliage-gleaner | LC | 3976 |
| 121 | Furnariidae | Cichlocolaptes leucophrus | Pale-browed treehunter | LC | 2710 |
| 122 | Furnariidae | Heliobletus contaminatus | Sharp-billed treehunter | LC | 1512 |
| 123 | Furnariidae | Philydor atricapillus | Black-capped foliage-gleaner | LC | 5525 |
| 124 | Furnariidae | Anabacerthia amaurotis | White-browed foliage-gleaner | NT | 1539 |
| 125 | Furnariidae | Anabacerthia lichtensteini | Ochre-breasted foliage-gleaner | LC | 2804 |
| 126 | Furnariidae | Clibanornis dendrocolaptoides | Canebrake groundcreeper | LC | 855 |
| 127 | Furnariidae | Automolus lammi | Pernambuco Foliage-gleaner | EN | 230 |
| 128 | Furnariidae | Automolus leucophthalmus | White-eyed foliage-gleaner | LC | 8375 |
| 129 | Furnariidae | Leptasthenura striolata | Striolated tit-spinetail | LC | 993 |
| 130 | Furnariidae | Leptasthenura setaria | Araucaria tit-spinetail | NT | 4866 |
| 131 | Furnariidae | Phacellodomus erythrophthalmus | Orange-eyed thornbird | LC | 4006 |
| 132 | Furnariidae | Phacellodomus ferrugineigula | Orange-breasted thornbird | LC | 3410 |
| 133 | Furnariidae | Asthenes moreirae | Itatiaia spinetail | LC | 1031 |
| 134 | Furnariidae | Acrobatornis fonsecai | Pink-legged graveteiro | VU | 234 |
| 135 | Furnariidae | Thripophaga macroura | Striated softtail | VU | 583 |
| 136 | Furnariidae | Cranioleuca obsoleta | Olive spinetail | LC | 3288 |
| 137 | Furnariidae | Cranioleuca pallida | Pallid spinetail | LC | 10,153 |
| 138 | Furnariidae | Synallaxis cinerea | Bahia spinetail | NT | 448 |
| 139 | Furnariidae | Synallaxis infuscata | Pinto’s spinetail | EN | 109 |
| 140 | Furnariidae | Synallaxis ruficapilla | Rufous-capped spinetail | LC | 13,720 |
| 141 | Tyrannidae | Phyllomyias virescens | Greenish tyrannulet | LC | 1946 |
| 142 | Tyrannidae | Phyllomyias griseocapilla | Grey-capped tyrannulet | NT | 4138 |
| 143 | Tyrannidae | Phylloscartes kronei | Restinga tyrannulet | LC | 1751 |
| 144 | Tyrannidae | Phylloscartes beckeri | Bahia tyrannulet | EN | 237 |
| 145 | Tyrannidae | Phylloscartes ceciliae | Alagoas tyrannulet | CR | 339 |
| 146 | Tyrannidae | Phylloscartes paulista | Sao Paulo tyrannulet | NT | 1441 |
| 147 | Tyrannidae | Phylloscartes oustaleti | Oustalet’s tyrannulet | NT | 1890 |
| 148 | Tyrannidae | Phylloscartes difficilis | Serra do mar tyrannulet | LC | 700 |
| 149 | Tyrannidae | Phylloscartes sylviolus | Bay-ringed tyrannulet | NT | 1001 |
| 150 | Tyrannidae | Myiornis auricularis | Eared pygmy-tyrant | LC | 7675 |
| 151 | Tyrannidae | Hemitriccus diops | Drab-breasted bamboo-tyrant | LC | 3138 |
| 152 | Tyrannidae | Hemitriccus obsoletus | Brown-breasted bamboo-tyrant | LC | 1603 |
| 153 | Tyrannidae | Hemitriccus orbitatus | Eye-ringed tody-tyrant | NT | 3256 |
| 154 | Tyrannidae | Hemitriccus nidipendulus | Hangnest tody-tyrant | LC | 4478 |
| 155 | Tyrannidae | Hemitriccus mirandae | Buff-breasted tody-tyrant | VU | 541 |
| 156 | Tyrannidae | Hemitriccus kaempferi | Kaempfer’s tody-tyrant | VU | 683 |
| 157 | Tyrannidae | Hemitriccus furcatus | Fork-tailed tody-tyrant | VU | 1431 |
| 158 | Tyrannidae | Todirostrum poliocephalum | Yellow-lored tody-flycatcher | LC | 18,110 |
| 159 | Tyrannidae | Platyrinchus leucoryphus | Russet-winged spadebill | VU | 487 |
| 160 | Tyrannidae | Onychorhynchus swainsoni | Atlantic royal flycatcher | VU | 765 |
| 161 | Tyrannidae | Knipolegus nigerrimus | Velvet black-tyrant | LC | 5281 |
| 162 | Tyrannidae | Muscipipra vetula | Shear-tailed grey tyrant | LC | 3475 |
| 163 | Tyrannidae | Attila rufus | Grey-hooded attila | LC | 10,287 |
| 164 | Tyrannidae | Piprites pileata | Black-capped piprites | NT | 1312 |
| 165 | Cotingidae | Carpornis cucullata | Hooded berryeater | LC | 4600 |
| 166 | Cotingidae | Carpornis melanocephala | Black-headed berryeater | NT | 1223 |
| 167 | Cotingidae | Phibalura flavirostris | Swallow-tailed cotinga | LC | 1715 |
| 168 | Cotingidae | Cotinga maculata | Banded cotinga | CR | 257 |
| 169 | Cotingidae | Lipaugus lanioides | Cinnamon-vented piha | LC | 1669 |
| 170 | Cotingidae | Lipaugus ater | Black-and-gold cotinga | LC | 1642 |
| 171 | Cotingidae | Lipaugus conditus | Grey-winged cotinga | VU | 262 |
| 172 | Cotingidae | Procnias nudicollis | Bare-throated bellbird | NT | 7788 |
| 173 | Cotingidae | Xipholena atropurpurea | White-winged cotinga | VU | 701 |
| 174 | Pipridae | Neopelma aurifrons | Wied’s tyrant-manakin | NT | 327 |
| 175 | Pipridae | Neopelma chrysolophum | Serra do Mar tyrant-manakin | LC | 2188 |
| 176 | Pipridae | Antilophia bokermanni | Araripe manakin | CR | 7 |
| 177 | Pipridae | Chiroxiphia caudata | Blue manakin | LC | 26,382 |
| 178 | Pipridae | Ilicura militaris | Pin-tailed manakin | LC | 7292 |
| 179 | Pipridae | Machaeropterus regulus | Kinglet manakin | LC | 997 |
| 180 | Tityridae | Schiffornis virescens | Greenish schiffornis | LC | 8586 |
| 181 | Tityridae | Iodopleura pipra | Buff-throated purpletuft | EN | 1341 |
| 182 | Tityridae | Laniisoma elegans | Elegant mourner | NT | 330 |
| 183 | Vireonidae | Hylophilus poicilotis | Rufous-crowned greenlet | LC | 7960 |
| 184 | Corvidae | Cyanocorax coeruleus | Azure jay | NT | 7582 |
| 185 | Polioptilidae | Polioptila lactea | Creamy-bellied gnatcatcher | NT | 331 |
| 186 | Thraupidae | Nemosia rourei | Cherry-throated tanager | CR | 147 |
| 187 | Thraupidae | Orchesticus abeillei | Brown tanager | NT | 1804 |
| 188 | Thraupidae | Hemithraupis ruficapilla | Rufous-headed tanager | LC | 10,180 |
| 189 | Thraupidae | Haplospiza unicolor | Uniform Finch | LC | 3948 |
| 190 | Thraupidae | Tachyphonus coronatus | Ruby-crowned tanager | LC | 35,583 |
| 191 | Thraupidae | Ramphocelus bresilius | Brazilian tanager | LC | 21,613 |
| 192 | Thraupidae | Dacnis nigripes | Black-legged dacnis | NT | 1239 |
| 193 | Thraupidae | Sporophila falcirostris | Temminck’s seedeater | VU | 1157 |
| 194 | Thraupidae | Sporophila frontalis | Buffy-fronted seedeater | VU | 2049 |
| 195 | Thraupidae | Saltator maxillosus | Thick-billed saltator | LC | 2935 |
| 196 | Thraupidae | Saltator fuliginosus | Black-throated grosbeak | LC | 4827 |
| 197 | Thraupidae | Castanozoster thoracicus | Bay-chested warbling-finch | LC | 1978 |
| 198 | Thraupidae | Thlypopsis pyrrhocoma | Chestnut-headed tanager | LC | 2298 |
| 199 | Thraupidae | Microspingus lateralis | Buff-breasted warbling-finch | LC | 3726 |
| 200 | Thraupidae | Tangara cyanoptera | Azure-shouldered tanager | NT | 3316 |
| 201 | Thraupidae | Tangara brasiliensis | White-bellied tanager | LC | 827 |
| 202 | Thraupidae | Tangara peruviana | Black-backed tanager | VU | 1912 |
| 203 | Thraupidae | Tangara cyanomelas | Silvery-breasted tanager | LC | 465 |
| 204 | Thraupidae | Tangara seledon | Green-headed tanager | LC | 21,755 |
| 205 | Thraupidae | Tangara fastuosa | Seven-colored tanager | VU | 964 |
| 206 | Thraupidae | Tangara cyanocephala | Red-necked tanager | LC | 13,062 |
| 207 | Thraupidae | Tangara desmaresti | Brassy-breasted tanager | LC | 8705 |
| 208 | Thraupidae | Tangara cyanoventris | Gilt-edged tanager | LC | 6928 |
| 209 | Thraupidae | Tangara ornata | Golden-chevroned tanager | LC | 6297 |
| 210 | Mitrospingidae | Orthogonys chloricterus | Olive-green tanager | LC | 5418 |
| 211 | Passerellidae | Arremon semitorquatus | Half-collared sparrow | LC | 2873 |
| 212 | Icteridae | Anumara forbesi | Forbes’s blackbird | VU | 288 |
| 213 | Fringillidae | Euphonia chalybea | Green-throated euphonia | NT | 2811 |
| 214 | Fringillidae | Euphonia pectoralis | Chestnut-bellied euphonia | LC | 14,946 |
| 215 | Tyrannidae | Pogonotriccus eximius | Southern bristle-tyrant | NT | 893 |
| 216 | Tyrannidae | Mionectes rufiventris | Grey-hooded flycatcher | LC | 7262 |
The joint dataset had satisfactory heterogeneity, as it achieved marginally acceptable conformity to Benford’s Law with regard to digit distribution of the number of observations per species (Mean Absolute Deviation (MAD) = 0.01468667; Distortion Factor: −1.158198; Mantissa Arc Test: L2 = 0.0002087; df = 2; p = 0.9567; n = 212). Even though over 44% of the species were imperiled (presumably rare), the data did not fit the gamma binomial model (α = 29.789; X2 = 439.851; df = 13; p = 0.0000).
Spatial distributionMost of the observations of Atlantic Forest bird species were collected in the southern and south-eastern regions of the Atlantic Forest (Fig. 1a), while observations in the northeast were sparse. The number of observations was not proportionally distributed as expected based on the size of each region (χ2 = 385,324, df = 3, p < 10−16). Similarly, imperiled species were not evenly distributed (Fig. 1b). Even though most observations (74.5%) were located outside protected areas, it was lower than expected based on the area of non-protected areas (91.7% of all Atlantic Forest) and this difference was significant (χ2 = 382,532, df = 1, p < 2.2 × 10−16).
Temporal distributionEven though the observations of imperiled species increased annually (estimate = 0.1579, t = 10.86, p = 4.52 × 10−10, n = 23), their proportion in relation to all species observations (imperiled/all species records) decreased (estimate = −0.18379, t = −5.348, p = 2.65 × 10−5, n = 23), remaining below 15% (average proportion between 2000 and 2022) in the last five years (Fig. 2a and b). After 2017, this proportion was below 13.8%, and in 2022 the proportion of imperiled species reached its lowest (12%) in the 21 years of our study. This pattern was maintained in spite of the increase in the number of birdwatchers on the platforms in the later years (estimate = 0.1469, t = 9.942, p = 2.15 × 10−9, n = 23; Fig. 2c). Species richness and the number of observations of Atlantic Forest bird species recorded annually on the platform was strongly correlated with the number of observers (Table 2). We also found that while the proportion of observations of declining species was decreasing through the years (estimate = −0.34384, t = − 7.173, p = 4.52 × 10−16, N = 23), the proportion of stable species observations was increasing (estimate = 0.34384, t = 7.173, p = 4.52 × 10−7, n = 23).
The number (a) and the proportion (b) of observations of threatened and Near Threatened bird species in the Brazilian Atlantic Forest compiled by community scientists and (c) number of observers that submitted observations of birds from the Brazilian Atlantic Forest in 2000–2022; the dashed line in b is the trend line form a smooth linear model.
Matrix with Spearman rank correlation coefficients (n = 23) for the correlations between threatened and Near Threatened species richness, number of observations of endemic species and the number of observers each year in the interval between 2000 and 2022. All correlations were p < 0.01, but exact p-values could not be calculated.
| Richness | Number of observers each year | Number of observations of endemic species | |
|---|---|---|---|
| Richness | – | 0.84 | 0.87 |
| Number of observers each year | – | – | 0.95 |
| Number of observations of endemic species | – | – | – |
Bird species were observed more in October and November, with smaller peaks in observation in January, May and July (Fig. 3). This pattern on the number of observations was considered synchronic (ρ = 0.0369, and p < 0.01; n = 838,880).
DiscussionWe saw a general increase in the number of bird observers and bird observation in 2000–2022, reflecting an increasing interest in citizen science in Brazil. The marginally acceptable conformity to Benford’s Law of the joint dataset suggests that the number of observations had satisfactory heterogeneity, which is evidence for the proportional real abundance of each species. In spite of this, the data did not fit the gamma binomial distribution model, potentially because of the relatively high number of observations of imperiled (and therefore presumably rare) species. In total, 79 species had 2000–10,000 observations, while only eight species, all Least Concern, had over 20,000 observations (Table 1). Given this pattern, our results reveal that imperiled Atlantic Forest bird species with decreasing populations are relatively often recorded by CS.
Five species, including the Near Threatened Bare-throated Bellbird (Procnias nudicollis) and Azure Jay (Cyanocorax coeruleus) had over 4000 observations, both of them being relatively large, attractive birds that are easy to detect when present and vocalizing and are sought after by birdwatchers. These characteristics are similar to those preferred by birdwatchers in South Australia (Szabo et al., 2011). We assume that on all three platforms, many observers are experienced birdwatchers who focus on finding desired species to complete their personal list of observed birds, similar to a type of observer identified in Western Australia by Tulloch and Szabo (2012). Nevertheless, populations of Critically Endangered species, which are by definition extremely rare, are restricted to small remnant patches of native vegetation. These remote habitats are usually beyond the reach of most observers using these CS platforms. Two Critically Endangered species, Stresemann’s Bristlefront (Merulaxis stresemanni) and Alagoas Antwren (Myrmotherula snowi), were represented by a total of 57 and 102 observations, respectively, on the three platforms in 2000–2022. In fact, Stresemann’s Bristlefront is only known to persist in one forest patch (Lees and Pimm, 2015). Similar to the results of Barbosa et al. (2021), most observations were concentrated in areas of high human population density, i.e., in southern and south-eastern Brazil. In 2020 and 2021, restrictions imposed by the pandemic also limited the movement of bird observers to areas closer to urban centers (Sánchez-Clavijo et al., 2021) and the number of observations in protected areas declined globally compared to pre-pandemic levels (Qiao et al., 2023). This convenience sampling can inflate the number of observations of Least Concern and urban-tolerant species compared to those restricted to particular habitats, usually found within protected areas. Our results confirm this pattern and demonstrate that the probability of observing a threatened or Near Threatened species is much higher (98.5%) outside urban areas. Unfortunately, strictly protected areas only cover 9% of the Atlantic Forest (Rezende et al., 2018), which could explain the relatively high number of observations of imperiled bird outside protected areas. This pattern does not confirm with bird observations made by CS in Australia (Barnes et al., 2015). In addition, many threatened and Near Threatened species use habitats outside protected areas for feeding and reproduction, which raises an important question about prioritizing areas for monitoring and conservation.
Even more worrying is the proportional decline of imperiled species observations in 2000–2022 compared to all bird observations in the Atlantic Forest. We assume that the number of generalist observers (i.e., non-bird specialist in the case of iNaturalist or non-threatened species specialist on the other two platforms) has grown throughout the years, leading to a relatively large increase in the number of observations of common species and simultaneously decreasing the relative number of observations of rare species, some of which are threatened or Near Threatened. This hypothesis is supported by the correlations between species richness, the number of observations of imperiled species and the number of observers that are active on the platforms each year. However, real population declines also result in lower reporting rates, which is a cause for concern, particularly in the case of globally threatened species. These kinds of declines are more pronounced when we consider the increase in the number of observers on all three CS platforms. In fact, 67% of threatened and Near Threatened bird species observed on the platform show declining population trends and a further 5% have unknown trends (BirdLife International, 2023).
Two endemic species listed as Critically Endangered that are present on the list of Vale et al. (2018) and were absent from the CS dataset: Purple-winged Ground-Dove (Claravis geoffroyi; Columbidae) last seen in 2007 and Kinglet Calyptura (Calyptura cristata; Tyrannidae) seen only once in 1996 within the last century — both had a high probability of being globally extinct (Butchart et al., 2018; Lees et al., 2021; Lees and Pimm, 2015). The third missing species, Pernambuco Pygmy-Owl (Glaucidium mooreorum; Strigidae) was last seen in 2004 and is listed as Critically Endangered (Possibly Extinct) by BirdLife International (2023). On the other hand, there were a few records of two Furnaridae species in the CS datasets, one observation of the Cryptic Treehunter (Cichlocolaptes mazarbarnetti) in 2001 and 26 observations of the Alagoas Foliage-gleaner (Philydor novaesi), which was last seen in 2011, and is also possibly extinct (Butchart et al., 2018; Lees and Pimm, 2015). The joint dataset also contained two observations of the Alagoas curassow, a species listed as Extinct in the Wild by IUCN, possibly of individuals reintroduced in 2019 (Francisco et al., 2021).
The negative trend in the proportion of observations of threatened and Near Threatened species is worrying and needs to be confirmed by focused analysis of targeted studies. Most observations of imperiled species occurred before the beginning of the austral summer (Jones and Carvalho, 2002). Arthropod abundance in the Atlantic Forest is highest during the wet season, in December, while fruit availability is highest in April–June (Develey and Peres, 2000), which in turn increases bird activity and thereby detection rates. For many forest birds, the breeding season ends in December–January (Sick, 1997), when the number of individuals is augmented by fledglings, and this can explain a small peak of observations in January. The Atlantic Forest also receives a handful of Neotropical migrants during this period (Moreira-Lima, 2013) and some austral migrants (Lees and Martin, 2015). Another explanation is that the general public is also on holiday during this time, so they have more time to observe birds. The other peak in sightings of imperiled species occurred in May and July, possibly explained by the weather, as conditions are more favorable for observers (i.e., dryer and cooler) to visit more remote areas of the Atlantic Forest. Observers in unstructured surveys are known to have a preference for weekends, holidays and fair weather (Knape et al., 2022).
The interannual variability in climatic conditions can also affect the number of birds present and CS effort, as El Niño years bring extreme conditions that manifest in longer periods of drought in some regions and higher temperature with torrential rainfalls in others (Costa et al., 2021). The 2015–16 El Niño event resulted in extended drought in the Atlantic Forest of the northeast (Gateau-Rey et al., 2018), possibly affecting the number of birds observed in this region as well as CS effort. Measures to combat the Covid-19 pandemic may have affected the activities of observers, which can explain the lower number of observations of imperiled species in 2020 and 2021 compared to the previous two years. Even though the pandemics has brought some positive changes to nature conservation (Forti et al., 2020), most of its effects were negative (Gibbons et al., 2022). However, the relatively high number of observers in 2020–21 (Fig. 2c) demonstrates the resilience of community scientists that engaged in birdwatching even during these difficult times. Even if the number of surveys remained the same during these two years, the spatial patterns could have changed. In Colombia, observers submitted more data from modified than from natural landscapes as a result of lockdowns and other travel restrictions (Sánchez-Clavijo et al., 2021). Thus, hard-to-reach sites, such as remote protected areas, where there is a higher probability of observing certain rare species, have presumably received fewer CS surveys. This could explain the high number of records we identified outside protected areas, since many protected areas limited the number of visitors and, therefore tourist activities related to birdwatching were negatively affected in 2020–22 (Spenceley et al., 2021). Nevertheless, protected areas represent only 9% of the remaining natural vegetation of the Atlantic Forest in Brazil, with over 90% being on private land (Rezende et al., 2018).
Based on our results, more effort should be directed to remote areas of the Atlantic Forest to improve species coverage and thereby provide more data to inform biodiversity conservation. Expeditions to specific locations targeting species of conservation concern should be encouraged by CS platforms, protocols should be provided to minimize the risks presented to birds by tourist activities. We recommend targeted surveys for imperiled species to fill knowledge gaps for decision making. The central corridor of the Atlantic Forest is one of the areas that potentially needs a larger sampling effort to increase its representation on the platforms, as this alone could help to create a more robust dataset of Atlantic Forest biodiversity.
ConclusionsConsidering the increasing pressures for landscape modification and the consequences of climate change on the Atlantic Forest (de Lima et al., 2020; SOS Mata Atlântica/INPE, 2018), there is an ever-increasing need to understand the biodiversity of this threatened biome. There are also ongoing restoration (Rezende et al., 2018) and refaunation (Galetti et al., 2017) efforts and further opportunities to be taken advantage of in the future in this global biodiversity hotspot. Community science can offer an ideal tool to monitor and document (hopefully positive) changes in the number and abundance of threatened species and to fully understand changes in functional diversity and species-specific responses to active restoration (Melo et al., 2020; Uezu and Metzger, 2016).
Developing a reliable monitoring system is a crucial challenge in the conservation of Atlantic Forest biodiversity, as conservation prioritization depends on the availability of such data. While CS data have influenced conservation decisions in other parts of the world (Fontaine et al., 2022; Fraisl et al., 2020), the lack of understanding of biases often prevents the use of this approach to derive reliable population trends (Bayraktarov et al., 2019). Here we provided the first exploration of CS data for a large number of Atlantic Forest endemic bird species considering two decades and multiple data sources. Even considering the biases inherent in CS data and the difficulties of surveying, it has proved to be an exceptional tool to reduce the Wallacean shortfall (La Sorte and Somveille, 2019; Lees and Martin, 2015), and to bring a new perspective to monitor species facing extinction and their responses to management actions.
Conflict of interestThe authors declare no conflicts of interest.
Availability of dataThe raw data for this study are available at https://zenodo.org/records/10044588.
We appreciate the work of thousands of community scientists who share species occurrence data on digital platforms and therefore made this study possible. We thank Anderson Sandro and Gabriel Bonfa, two community scientists from iNaturalist who gave us permission to use their photographs in the graphical abstract. Arthur Queiros (grant number 127626/2022-0) and Talita Oliveira, (grant number 135165/2022-9) were financed by the Institutional Scientific Initiation Scholarship Program (Programa Institucional de Bolsas de Iniciação Cientifica; PIBIC), provided by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES), while Juan Lima received financial support (grant number 88887.687023/2022-00) from the Postgraduate Program in Ecology and Conservation (Programa de Pos Graduação em Ecologia e Conservação) supported by CAPES. We are grateful to Darius Pukenis Tubelis, two anonymous reviewers and the handling editor for their constructive comments on previous versions of this manuscript.