Protected areas (PAs) are a widely recognized tool for biodiversity and ecosystem conservation. However, megadiverse countries struggle to manage, maintain, and expand PAs as they face mounting human pressures. The Brazilian Cerrado biome (a biodiversity hotspot) is experiencing increasing land-use changes paired with a loss of natural vegetation, and only 3.2% of its land area is under strict protections. The Brazilian Long-Term Ecological Research Program (LTER) was created in 1997 to monitor long-term changes in protected and non-protected areas in Brazilian biomes. The Environmental Protected Area of the Gama and Cabeça de Veado (AGCV) watersheds in Central Brazil's core distribution of the Cerrado (Brasília, Federal District), was one of the first sites to participate in the Brazilian LTER. The main goal of the AGCV-LTER site is to monitor long-term changes and ecological processes in aquatic and terrestrial ecosystems in PAs that are surrounded by landscapes facing extreme ecosystem shifts. Over 22 years, we investigated the effects of drivers such as fire, noise and light pollution, eutrophication, and biological invasions on aquatic (invertebrates and water quality) and terrestrial ecosystems (vegetation, vertebrates, and invertebrates). The results indicate that even within a PA, changes in the surrounding landscape affects biodiversity and ecosystem functions, revealing the essential nature of continuous monitoring for biodiversity conservation.
A key takeaway from the first global assessment of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) is the unprecedented decline in global biodiversity and increase in species extinction rates, with up to one million species facing extinction-many within decades due to human activities (IPBES, 2019). Protected areas (PAs) are recognized strategies for biodiversity conservation; however, 6 million km2 (32.8%) of protected land globally is facing intense human pressure (Jones et al., 2018). The effectiveness of PAs for biodiversity protection and sound conservation planning depends on management and protection actions as well as long-term monitoring of different drivers of change (Le Saout et al., 2013).
Megadiverse countries, such as Brazil, face challenges in maintaining, managing, and expanding PAs (Bacon et al., 2019; Vieira et al., 2019). A total of 18.6% of Brazil’s lands are currently protected (Brasil, 2021), but the distribution is highly unequal across the main biomes. When considering all categories of PA, 27.8% of the Amazon is protected compared with only 10% of the Atlantic Forest, 9.3% of the Caatinga, and 8.6% of the Cerrado (Brasil, 2021). However, knowledge of biodiversity within most PAs remains limited across all biomes (Oliveira et al., 2017; Vieira et al., 2019). This scarcity is even more worrying in two Brazilian biodiversity hotspots (Myers et al., 2000), the Atlantic Forest and Cerrado, each of which feature a high degree of endemism and extensive environmental alteration. While deforestation of the Atlantic Forest occurred predominantly in the 19th century, the Cerrado is still facing intense land-use changes, with approximately 45% of its native vegetation already converted for anthropogenic use and new deforestation fronts advancing to the northern portion of the biome (Souza et al., 2020).
The Cerrado accounts for 24% of Brazil’s lands, and is comprised of a structural gradient of woody cover from grasslands to savanna woodlands and forests (Ribeiro and Walter, 2008). Human occupation creates many environmental challenges for the Cerrado, including increasing pressure on water resources, carbon stock reductions, biodiversity and social diversity losses, changes in fire regimes, and fragmentation (Colli et al., 2020; Grande et al., 2020; Hoffmann and Jackson, 2000). Despite this intense human pressure, only 3.2% of the Cerrado is under strict protection (Françoso et al., 2015). Currently, Brazil has managed to protect 17% of the Amazon biome under the Aichi Biodiversity Target 11 (Brasil, 2021).
The large-scale land conversion of the Cerrado was stimulated by the transfer of the country’s capital to central Brazil, the construction of Brasília in the Federal District (FD) in the 1960s, and the expansion of the road and highway systems (Campolina, 2019). During the 1970s, state programs from the Brazilian government stimulated significant incentive for the occupation of this area, leading to deforestation and urban expansion (Grecchi et al., 2013; Jepson et al., 2010). As a result of these added pressures, fires are also increasingly frequent, with the Cerrado area accounting for approximately 53% of Brazil’s total burned area in 2017 (Projeto MapBiomas, 2021).
In the core area of the Cerrado, the Federal District urbanization is the greatest threat to native vegetation, followed by agriculture and cattle raising (Rodrigues et al., 2016). The Federal District is highly representative of the fauna and flora of the Cerrado, with approximately 236 species of woody flora (Bridgewater et al., 2004) and at least 1355 species of tetrapod’s (Mendonça et al., 2018). However, only 41.9% of the original vegetation cover remains (Souza et al., 2020), with remaining natural cover mainly distributed across 31 public PAs (Brasil, 2021), including the Environmental Protection Area of the Gama and Cabeça de Veado (AGCV) (Watershed of the Gama and Cabeça de Veado streams). This area is in the sustainable use category (equivalent to the IUCN protected area category V), where sustainable resource use occurs in conjunction with nature conservation, allowing human occupation of the PA. The primary purpose of this PA is to guarantee the ecological integrity of terrestrial and aquatic ecosystems and to protect the water sources of Brasilia.
To monitor long-term ecological processes across different Brazilian biomes (protected and non-protected areas), the Brazilian Long-term Ecological Research Program (LTER) was created in 1997 and funded by the Brazilian Federal Agency National Council for Scientific and Technological Development (CNPq). The Brazilian LTER program is a member of the International Network for Long Term Ecological Research (ILTER) and has 41 LTER sites in different regions and ecosystems throughout the country (Brito et al., 2020), including the AGCV site, since 1999.
Here, we present a compilation of the main results obtained at the AGCV-LTER site over 22 years (1998–2020), while highlighting the importance of long-term data in the context of extreme changes to PA surrounding landscapes as well as discussing perspectives and challenges for the future.
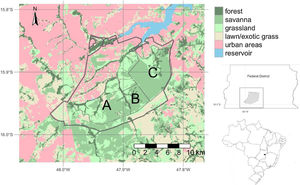
Brasília LTER site descriptionThe Environmental Protection Area Bacias do Gama e Cabeça de Veado (AGCV) was created in 1986, covering 25,000 hectares and representing approximately 4% of the territory of the Brasília-FD (Fig. 1). The AGCV also has approximately 30,000 inhabitants located within a 10 km radius. In this region, direct anthropic use, urban zones, and rural areas, such as residences and farms with specific activities, are allowed (Felfili and Santos, 2003; Unesco Brasil, 2003). In addition, the AGCV has multiple PAs with unique management regimes. These include one strictly protected area: the Brasília Botanical Garden Ecological Station (JBB) with 4500 ha (equivalent to IUCN category Ia); it also includes two sustainable use areas: Capetinga-Taquara and Cerradão areas of Relevant Ecological Interest (equivalent to IUCN category IV). Furthermore, the areas under special management regimes include the Água Limpa Farm (FAL) belonging to the University of Brasília and the Ecological Reserve of the Brazilian Institute of Geography and Statistics (IBGE). All three areas (JBB, FAL, and IBGE) constitute the Brasília LTER site and are referred to as AGCV here.
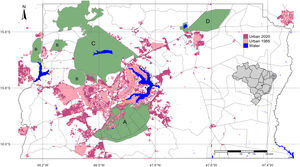
Environmental Protection Area Bacias do Gama e Cabeça de Veado (AGCV; thick line) located in Brasília, Brazil in the core of the Cerrado biome. (A) Água Limpa Farm (FAL), (B) Ecological Reserve of the Brazilian Institute of Geography and Statistics (IBGE), (C) Brasília Botanical Garden Ecological Station (JBB). Landuse map source: Mapbiomas v6 (Souza et al., 2020).
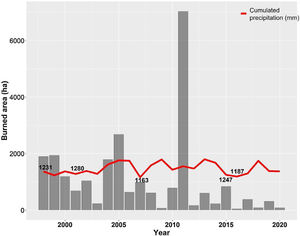
The Cerrado climate is marked by strong seasonality, with a dry season from May to September and a rainy season from October to April. The annual mean precipitation is approximately 1500 mm (da Silva et al., 2008), with approximately 90% of precipitation occurring during the rainy season. The average rainfall in Brasilia between 2001–2020 was 1466.4 mm, and the driest years were 1999, 2001, 2007, 2015, and 2016 (Fig. 2).
Annual burned area (ha) of the Environmental Protection Area Bacias do Gama e Cabeça de Veado (AGCV) and cumulated precipitation (mm) in Brasilia city from 1998 to 2020, highlighting the five driest years. Precipitation data are soured from National Institute of Meteorology (INMET): https://bdmep.inmet.gov.br. Burned area data are from Projeto MapBiomas Fogo (2021; collection 1).
Latosolos (Brazilian Soil Taxonomy, Embrapa), which are deep and highly weathered soils, are the dominant soils throughout the AGCV. In areas with higher topography, shallow Cambissolos (Brazilian Soil Taxonomy, Embrapa) occur, while lithologic soils are common in rocky outcroppings in the southwestern regions. The average altitude in the AGCV is 1100 m (Aguiar and Antonini, 2008) with four distinct topographic types: flattened levels, slopes, alluvial plains, and murundus fields (carthmound fields) (Unesco Brasil, 2003).
Vegetation and faunaThe AGCV features all of the common Cerrado plant physiognomies, ranging from grasslands and savannas to forests (campo limpo, campo sujo, cerrado sensu stricto, cerradão, gallery forests, and veredas [linear physiognomies that occur on hydromorphic soil usually along with narrow water courses]) (Oliveira-Filho and Ratter, 2002), with cerrado s.s. (savanna) being the dominant plant physiognomy. The vascular flora is representative of Central Brazil, containing 30% of the species and 78% of the plant families found in the Cerrado biome (Unesco Brasil, 2003).
The fauna is also quite diverse and are representative of those found within Brasilia-FD. The AGCV harbors 71.5% of the 137 species of mammals that occur in Brasília-DF, including rare and threatened or vulnerable species (according to the Brazil Red Book of Threatened Species of Fauna; ICMBIO-MMA, 2016), such as the puma (Puma concolor), the maned wolf (Chrysocyon brachyurus), ocelot (Leopardus pardalis), and Dekeyser's nectar bat (Lonchophylla dekeyseri) (Unesco Brasil, 2003).
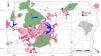
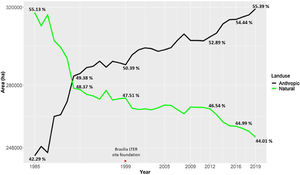
Urbanization and fireThe city of Brasília, which was planned in the late 1950s to hold 500,000 inhabitants, now has more than three million residents (IBGE, 2019). Urban growth has occurred in an accelerated and uncontrolled way, stimulated by an increased migratory flow toward Brasília (Costa and Lee, 2019). In 1985, the anthropic areas increased in Brasília-FD, followed by a corresponding decrease in natural vegetation (Fig. 3). Between 1985 and 2020, urban areas within the Federal District increased by 143.6% and by 105.5% within the AGCV, making it the most impacted protected area by urbanization (Fig. 4).
Changes in Brasília-FD land use considering urban and natural areas. Data from Projeto MapBiomas (2021; Souza et al., 2020).
Urbanized areas in and around the Environmental Protection Area Bacias do Gama e Cabeça de Veado (area A) in 1985 and 2020. Other PAs are: Brasilia National Forest (B), Brasília National Park (C), Águas Emendadas Ecological Station (D). Land use data from Projeto MapBiomas (2021; collection 6).
Further, several neighborhoods have been consolidated around the AGCV over the past few years, which has intensified urbanization and traffic and consequently increased the isolation of the individual PAs making them more vulnerable to external impacts. The invasion of domestic animals, people, new garbage dumps, and increasing noise and light pollution frequently impact the region.
In addition, increasing urbanization has made the AGCV more vulnerable to fire. In 2005 and 2011, extensive fires consumed approximately 2680 ha and 7032 ha within the AGCV, respectively (Fig. 2). After the 2011 fire, preventative actions were taken through the Plan for Prevention and Fighting of Forest Fires, coordinated by the Brasília Secretary of Environment (https://www.ibram.df.gov.br/prevencao-apa-gama-e-cabeca-de-veado-recebem-aceiros-negros/), including the construction of mechanical firebreaks and black firebreaks. These firebreaks remove fuel material (e.g., dry vegetation), create safety strips in the vegetation, and prevent fires from reaching or spreading.
LTER site goalsThe main goal of the Brasília LTER site is to monitor long-term biotic community changes in aquatic and terrestrial ecosystems in the context of intensifying pressures on PA lands. ACGV has serious land use and occupation problems due to a lack of rigorous land-use planning (Unesco Brasil, 2003). Thus, LTER studies evaluated the impact of fire, noise, light pollution, climate variability, and eutrophication on community and ecosystem dynamics within protected areas.
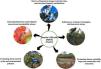
The studies have five main goals: (1) to evaluate the drivers of long-term changes in biodiversity, ecosystem integrity, and ecosystem functions; (2) to define new methods, technologies, and innovative science support services; (3) to evaluate the impact of climate variability on ecosystem processes; (4) to evaluate altered nutrient cycles and environmental pollution; and (5) to advance long-term socioecological research and sustainability science.
In aquatic ecosystems, we monitored water quality in six low-order streams and sampled micro-and macroinvertebrates on varying time scales (seasonal, interannual, and short term). In terrestrial ecosystems, we monitored birds, small mammals, butterflies, and drosophilid communities. We also conducted manipulative experiments to understand the impacts of different fire regimes on ecosystem function and of eutrophication on natural ecosystem nutrient cycles.
Here we describe the main results corresponding with our five goals and highlight the relevance of long-term monitoring for evaluating how changing human pressures impact the effectiveness of PAs for biodiversity conservation.
Evaluating the drivers of long-term changes in biodiversity, ecosystem integrity, and ecosystem functionsAquatic ecosystem monitoringWe monitored physical and chemical variables of both water and sediments in six low-order streams and two shallow lakes in the AGCV for seven years during the dry (August–September), rainy (February–March), and transition seasons (May and November; Fonseca et al., 2014). We used a Surber sampler placed on the streambed to collect macroinvertebrates by washing and rubbing the substrate through the sampler and a plankton net to sample microinvertebrates (mesh size of 80 μm). We also performed manipulative experiments to understand the influence of nutrients and sunlight on aquatic ecosystem function (Kisaka et al., 2021).
Streams and lakes were slightly acidic (pH < 6.0) with relatively low electrical conductivity (EC < 10.00 µS/cm), dissolved oxygen saturation (%OD) above 70%, and low nutrient concentrations (Appendix 1). Climatic seasonality also influenced aquatic systems, with higher temperature, turbidity, pH, EC, and % OD measured during the rainy months (Fonseca and de Mendonça-Galvão, 2014). While most streams were generally in pristine condition, for some streams we noted local signs of incipient water quality degradation. For example, both a stream with a recently established nearby rural area (‘Onça’) and a stream with margins facing urban influences (‘Gama’ stream) had higher nutrient concentrations, pH, and electrical conductivities compared to pristine streams. However, no increasing trends were observed (Appendix 1).
Both lotic and lentic systems in the AGCV had a high diversity of microinvertebrates (12 cladoceran species)-about 21% of the FD total richness in a unique lake (Sousa et al., 2018)-and macroinvertebrates (50 families and 28 genera of insects; unpublished data). Ephemeroptera, Plecoptera, and Trichoptera were the most common and frequent taxa in the community. Importantly, individual streams have unique community structures, with low similarity between streams. We also recorded several new occurrences of aquatic insect species for the FD: Macrelmis sp., Heterelmis sp., Cylloepus sp., Gyrelmis sp., and Xenelmis sp. (Elmidae, Coleoptera). Additionally, we recorded low population densities of the endemic species Macrobrachium candango (Crustacea), with no clear population trends over time.
Fire affects small mammals in forest formationsThe AGCV was sampled continuously for non-volant small mammals (rodents and marsupials) between 2008 and 2018. We sampled several plant physiognomies during this period, including typical savannas, savanna woodlands, and gallery forests. There was a total trapping effort of 223,698 trap-nights, which resulted in 4707 captured individuals. This massive effort in different physiognomies and distinct layers of the forests (including understory and canopy) allowed us to record 20 rodents and eight marsupials, representing 25% of the entire small mammal richness recorded in the Cerrado domain (Mendonça et al., 2018). Through these efforts, a previously undescribed species of a strictly arboreal spiny rat was captured and described for the first time (Phyllomys centralis, Echimyidae; Machado et al., 2018). Moreover, this continuous and long-term sampling effort advanced scientific knowledge of the theoretical and applied aspects of small mammal ecology and general biology, specifically related to long-term responses to fires, which are a significant disturbance in the Cerrado (Miranda et al., 2009). We found that small mammals of forested formations are not as resilient to fire disturbances as species from a typical savanna environment (Mendonça et al., 2015). In addition, fire in forests drastically altered habitat characteristics and favored the invasion of generalist rodents from open Cerrado habitats at the expense of more specialized forest species (Camargo et al., 2018). Thus, forest burning contributes to the biotic homogenization of mammalian fauna in the Cerrado. In addition, fire occurrence has potential effects on the mid- and long-term regeneration of forest trees. We observed that even three years after fire, the role of mammals as seed predators and potential dispersers is still affected, with a sharp reduction in seed removal of six native tree species in gallery forests. This indicates that the dispersal services provided by mammals (mainly the agouti Dasyprota azarae) for large-seeded species may be jeopardized by forest burning (Cazetta and Vieira, 2021). Such indirect effects of fire on ecosystem function could only be assessed through such a long-term research project.
Arrival and establishment of exotic fly speciesDue to the high levels of habitat conversion and degradation, the Cerrado has been invaded by non-native species, some of which are particularly invasive (Zenni et al., 2018). Drosophilid fly assemblages have been monitored in the AGCV since 1998, revealing 80 neotropical and 11 non-neotropical species. Three of these exotic species were established in the Neotropical Region during this monitoring period. Zaprionus indianus, an Afrotropical species, was first detected in South America in 1998 (Vilela, 1999) and in our collections in 1999 (Tidon et al., 2003). This species is known as the fig fly because of its catastrophic impact on fig plantations, but is widely a generalist species and has been recorded breeding on 41 plant species (representing 25 families) in the Neotropical Region (Valadão et al., 2019). Drosophila nasuta, a species of Indo-Pacific origin, was first collected in the AGCV in December 2013 (one specimen). Two years later, its abundance reached 20% of drosophilids in this area (Leão et al., 2017). In 2015, D. nasuta was recorded in the Atlantic Forest of southeastern Brazil (Vilela and Goñi, 2015), and is also widespread in the northeast region of the country which is dominated by semiarid vegetation (Montes et al., 2021). The third newly arrived species, Drosophila suzukii, is known as the spotted wing Drosophila. It originated in southeast Asia but has gained pest status in Europe and North America as it damages many fruit crops. D. suzukii was recorded in southern Brazil in February 2013 (Deprá et al., 2014) and ten months later was collected in the AGCV (Paula et al., 2014). The early detection of these non-native species provided a unique opportunity to study their population dynamics in newly invaded communities (Döge et al., 2015; Mata and Tidon, 2013; Roque et al., 2013) and emphasizes the need to monitor cultivated areas in the region.
Monitoring fruit-feeding butterfliesFrom 2017 to 2019, we sampled fruit-feeding butterflies (Lepidoptera, Nymphalidae) along a gradient of Cerrado physiognomies of the Brasilia Botanical Garden (JBB) ranging from woodland forests to typical savannas. We used a standardized protocol applying Van Someren-Rydon traps (Lucci Freitas et al., 2014), as this sampling scheme is low-cost and easy to sustain for long periods. We sampled twice a year: at the end of the rainy season (February–March) and at its onset (September–October). Four sampling stations, each containing four traps baited with bananas fermented in sugarcane juice were checked daily for eight consecutive days in each season. We photographed all captured butterflies with open wings, dorsally and frontally, and marked and released them immediately. We uploaded pictures and metadata containing the date, time, geolocation, and observations in an iNaturalist project (https://www.inaturalist.org/projects/monitoramento-de-borboletas-no-jbb).
We sampled 2561 butterflies with 768 traps*days of sampling effort for an average of 3.3 butterflies/trap/day. The butterfly assembly numbered 38 species, with three additional potential species added after a detailed revision of specimens from the genus Yphthimoides and the tribe Euptychiina. This number represents over a quarter of the 132 species registered at the Federal District (Emery et al., 2006) and is unlikely to increase significantly with additional effort as sampling locations are fixed under this sampling scheme. However, one of the most common species sampled at JBB—Nhambikuara cerradensis (Freitas et al., 2018)—was described after we initiated sampling, prompting us to revise the whole dataset to identify previously captured individuals. This event was an important test of the quality of our data registry choice, which allowed repeated verification of all observations.
We sought to define the proportion at which each species relates to an intact or disturbed environment, as this will allow us to establish a standard for monitoring changes in the butterfly community over time. Some species appear as possible indicators: Callicore sorana, Siderone galanthis, Eunica bechina, and Opsiphanes invirae. Additionally, the proportion of the two most abundant species (Hamadryas februa and Hamadryas feronia) seemed to change according to the degree of disturbance of the Cerrado areas.
Defining new methods, technologies, and innovative scienceRapid community and ecosystem assessments are desirable when monitoring resources are limited. Areas facing intense human pressures, such as reserves or native fragments embedded in urban areas, need to be evaluated to understand how biodiversity responds to isolation, disturbances, invasion by exotic species, human intrusion, and sound and light pollution, among other impacts (Parrish et al., 2003). Depending on the trends of a given species' population, occurrence, or activity, decisions on how to interfere must be made in an adaptive management approach (Hockings, 2003). Here, we evaluated the use of bioacoustics to monitor birds and bats associated with the two main ecosystems in AGVC: gallery forests and cerrados.s.
The basic idea is to use a low-cost, long-term, easy-to-collect, and easy-to-process method to obtain data about community characteristics through time to inform protected area management decisions. Biodiversity survey costs can vary greatly depending on the number of people involved, the number of studied taxa, the survey location, and the type of equipment used. Salaries, for instance, can represent up to 40% of the survey and species identification costs, while equipment, accommodations, and transport comprise the rest of a typical project budget (Gardner et al., 2008). To reduce costs, we tested the use of automatic recording units (ARUs) to register the sound profiles of the AGCV ecosystems. ARUs are advantageous over other methods because they can be conducted by anyone with minimal training, and can provide data that is comparable to a researcher's perception in loco (Alquezar and Machado, 2015).
Our approach is based on soundscape principles, that is, collecting sounds emanating from a particular landscape (Farina et al., 2014; Pijanowski et al., 2011). Such sounds are distributed on elements that form landscapes, including native and anthropogenic areas. According to the acoustic adaptation hypothesis, where an acoustic signal is associated with a habitat's structure and properties (Morton, 1975), different ecosystems will have different acoustic profiles. Such profiles may change accordingly with changes in ecosystem structure. We hypothesized that the acoustic profiles of the gallery forest and the Cerrado would be different from each other and associated with species diversity in each ecosystem. To test the effectiveness of bioacoustics in monitoring animal communities, we deployed ARUs at 30 sampling points in the AGCV, with 15 located in the gallery forest and 15 in the cerrados.s.
We used different protocols to record diurnal (dominated by bird vocalizations) and nocturnal animals (dominated by bat and insect vocalizations). Diurnal recordings started 30 min before sunrise and ended 2.5 h later. The recorders (SongMeter II from Wildlife Acoustics) were set to record for 10 min in 10 min intervals. All files had a sampling rate of 48 kHz, 16-bit depth, stereo mode, and were stored in an uncompressed wave format. Nocturnal recordings started 30 min before sunset and ended 12 h later. The recorders (SongMeter II Ultrasonic from Wildlife Acoustics) were set to record for one min at four min intervals. All files had a sampling rate of 364 kHz, 16-bit depth, monophonic, and were stored in an uncompressed wave format. The recorders stayed at the sampling points for two days, and the field campaign was repeated at three-month intervals.
Based on the recordings, we calculated the most common acoustic indices available in R packages, including seewaves (Suer et al., 2008) and soundecology (Villanueva-Rivera et al., 2018). The initial acoustic monitoring results are promising, and we confirmed that indices such as the Acoustic Diversity Index and Normalized Difference of Soundscape Index are correlated to the species richness and the level of landscape noise, respectively (Machado et al., 2017). We also confirmed that fire disturbances can non-linearly affect the acoustic indices calculated for birds, with low values observed in areas with low or high fire frequency and higher values observed in areas with intermediate fire frequency. For bats, high activity records, measured by the number of passes (a sequence of vocalizations from each flighting over the recorder), are associated with recently burned areas.
In summary, these results suggest a high potential theoretical and practical bioacoustics applications for monitoring the Cerrado's vocal species biodiversity.
Evaluating climate variability impacts on communities and ecosystemsAquatic ecosystemsIn general, depth and width of all streams tended to decrease during the dry season, as a result of low water levels in aquifers. However, we observed more significant reductions in Onça stream volume. The headwater of Onça stream is the only one located in a small rural area, with agricultural activities. Moreover, Roncador lake has completely dry up during the extreme drought in 2017. From August to November 2017 (dry season), the volume of Roncador lake fell and recovery slowly just in 2018. However, from August to November 2018, there was a new period of below-average rainfall, resulting in a reduction in Roncador water level; however, a complete dry out was not observed again.
Climate variability with more extreme drought events is projected to continue to increase for the central part of Brazil in the future (South American Moon Region) (IPCC, 2021). Climate variability and changes in vegetation structure may interact to alter aquatic ecosystems. The low-order streams studied have a low resistance to disturbances, such as increased light incidence and nutrients, and showed significant differences in chlorophyll-a concentration (Clo-a) and ash-free dry matter (AFDM). More shaded streams maintained low Clo-a values, even in areas with higher nutrient concentrations. The density of macroinvertebrate scrapers also increased significantly with sunlight exposure and nutrient enrichment, as a response to biofilm increase (Kisaka et al., 2021). This is an essential indication of the low resilience of small pristine streams to the loss of the riparian zone and nutrient intake from fertilizers. Changes in riparian gallery forest cover and an increase in water nutrients can affect the characteristics and functioning of food webs in these streams.
Small mammals, parasitism, and droughtWe took advantage of this long-term research project to evaluate the potential effects of several sources of variation on the population dynamics and health condition of small free-living mammals, including possible harm caused by the occurrence of parasites. To this end, we provided food supplements to small mammals in their natural environments and monitored their responses for seven years. We observed that food supplementation does not directly affect the density of the most abundant rodent (long-tailed climbing mouse Rhipidomys macrurus) or the health (evaluated by changes in hemoglobin concentration) of the marsupial Gracilinanus agilis. However, climatic variables (as measured by maximum daily temperature and humidity) but not seasonality or food supplementation negatively affects hemoglobin concentration in animals parasitized by warble bot flies (Cuterebra apicalis) (Zangrandi et al., 2019). Thus, dehydration appears to interact with parasite-induced anemia, causing the worst health conditions during the driest days. We concluded that bot fly parasitism might become a significant threat to small populations considering projected increases in the frequency of drought conditions due to climate variability. Moreover, climate variability may also affect small mammal communities. We detected a trend of declining open-area species density (mainly in wet grasslands) over time, which requires further investigation in future studies.
Evaluation of altered nutrient cycles and environmental pollutionTropical soils are generally poor and acidic, yet can harbor a high diversity of plants species adapted to such conditions. However, global environmental changes such as N deposition and land-use changes are altering nutrient availability in natural ecosystems with negative impacts on biodiversity (Penuelas et al., 2020). To better understand the relationships between nutrient availability and Cerrado ecosystem functioning (from plant ecophysiology to community composition), we initiated a long-term experiment in 1998 in the AGCV LTER site with five different nutrient treatments (N, P, N plus P, liming, and control) applied in a typical savanna area over eight years (from 1998 to 2006) (Kozovits et al., 2007). We documented responses over 22 years, ranging from changes in stomatal conductance (and hence drought resistance) to the root architecture of woody plants (Bucci et al., 2006; Bustamante et al., 2012). Long-term monitoring of the impacts was particularly relevant for understanding biological invasion by exotic grasses (e.g., Melinis minutiflora P. Beauv, an African C4 grass) (Bustamante et al., 2012). Biological invasion and nutrient enrichment resulted in changes in plant and microbial biodiversity and ecosystem processes such as decomposition. The long-lasting effects of nutrient additions were also associated with the water relations in this seasonally dry ecosystem, indicating how nutrients can interact with responses to climate variability.
Advancing long-term socioecological research and sustainability sciencePopulations of the marsupial Gracilianus agilis occurring in forests close to soybean plantations provide a relevant ecosystem service as a predator of the principal soybean pest, the brown stink bug Euschistus heros. We determined that the ecosystem service provided by this marsupial may reach up to US$ 31 ha−1 year−1 of native forest. Considering the amount of native vegetation within farmlands in the Cerrado; this interaction might represent savings of tens of millions of dollars per year (Camargo et al. submitted). These results highlight the importance of natural vegetation conservation near crops for the maintenance of agricultural pest control.
DiscussionThe tropics contain most of the Earth's biodiversity; however, their ecosystems are critically endangered by factors such as climate variability, land-use changes, and deforestation (Barlow et al., 2018). PAs are a key element in the conservation of tropical ecosystems; however, established PAs are becoming increasingly isolated and facing both new and old stressors, such as intense human pressure along their boundaries (Jones et al., 2018) and the strong political forces against the creation and expansion of public PAs. PAs are also among the most sensitive fields because they are often small and isolated fragments enclosed by degraded or developed landscapes containing rare or unique species and communities with narrow environmental tolerances. Therefore, long-term ecological studies within these areas are critical for highlighting their significance and understanding how the transformation of adjacent areas influences isolated and protected ecosystems.
The Brasilia LTER has been investigating the effects of fire, eutrophication, noise, light pollution, and climate variability on the community dynamics of an environmental protection area of the Cerrado for more than 20 years (Table 1). Today, the AGCV is located within a highly urbanized matrix affected by various factors such as isolation, species invasions, intrusion of people, light and noise pollution, fires, and garbage dumping. Despite the intense land use change, local aquatic ecosystems have remained generally healthy; yet, initial signs of degradation have now been detected. Fire in forested formations contributes to biotic homogenization of non-volant mammals and flies and impacts the acoustic indices of birds and bats. Furthermore, extreme droughts affect the physical-chemical conditions of streams and their associated fauna, as well as parasite-host interactions in small mammals. In addition, long-term changes in the functioning of Cerrado ecosystems (e.g., eutrophication) can facilitate the establishment of invasive species such as grasses and drosophilids.
Summary of the main works developed at LTER site “Environmental Protection Area Bacias do Gama e Cabeça de Veado – AGCV” located in Brasília, Brazil during 22 years (1998–2020).
| Main goals | Results | Conclusions | References |
|---|---|---|---|
| 1 | Good values of physical and chemical variables of water and sediments on streams | Good water quality of streams—In general, most streams have been in their pristine conditions | Fonseca et al., 2014; Fonseca and de Mendonça-Galvão, 2014; Kisaka et al., 2021 |
| Sampling aquatic invertebrates-high diversity of invertebrates | 12 cladoceran species (about 21% of the FD total richness in a unique lake), and 50 families and 28 genera belonging to Insect; one endemic species recorded (Macrobrachium candango, Crustacea, Decapoda) | Sousa et al., 2018 | |
| Sampling for non-volant small mammals—4707 individuals captured | 20 rodents and eight marsupials and 1 new rodent species described, representing 25% of the entire small mammal richness recorded in the Cerrado domain | Mendonça et al., 2018; Machado et al., 2018 | |
| Small mammals' theoretical and applied aspects of this group's ecology and general biology | Forest burning contributes to the biotic homogenization of mammalian fauna in the Cerrado and reduces seed removal and potential dispersal of native tree species | Mendonça et al., 2015; Camargo et al., 2018; Cazetta and Vieira, 2021 | |
| Monitoring of drosophilid fly assemblages—80 neotropical and 11 non-neotropical species | High diversity of drosophilids and early detection of four non-neotropical species | Tidon et al., 2003; Tidon, 2006; Roque et al., 2013; Paula et al., 2014 | |
| Monitoring fruit-feeding butterflies | 2561 butterflies—38 species | Non published data | |
| 2 | Evaluated bioacoustics' use to monitor birds and bats | Acoustic Diversity Index and Normalized Difference of Soundscape Index are correlated to the species richness and the level of landscape noise | Machado et al., 2017 |
| Acoustic indices values varies accordingly to Connell's Intermediate Disturbance Hypotheses | Cambraia 2019 | ||
| 3 | Disturbances on streams-reduction in water level, increase in water nutrients concentration and sunlight exposure | Changes in riparian gallery forests cover, and an increase in water nutrients can affect the characteristics and the functioning of the food web | Kisaka et al., 2021 |
| Food supplementation does not cause a direct effect on the density of the most abundant rodent, or in the health of the marsupial Gracilinanus agilis | Bot fly parasitism might become a significant drawback on small populations considering the occurrence of more days under drought conditions due to climate variability | Zangrandi et al., 2019 | |
| 4 | Changes in the plant and microbial biodiversity and ecosystem processes due to biological invasion and nutrient enrichment | Long-lasting effects of nutrient additions were also associated with the water relations, indicating how nutrients can interact with responses to climate variability | Bucci et al., 2006; Kozovits et al., 2007; Bustamante et al., 2012; Silveira et al., 2021a, b |
| 5 | Populations of small mammal Gracilianus agilis occurring in forests close to soybean plantations provide a relevant ecosystem service as a predator of the principal soybean pest, the brown stink bug Euschistus heros | Results highlight the relevance of natural vegetation conservation near crops for the maintenance of agricultural pest control | Camargo et al. submitted |
The Southern Hemisphere harbors a megadiversity of species but is also heavily impacted by direct drivers, such as habitat loss, and by indirect drivers such as socioeconomic conditions. Further, ecological and conservation science is strongly biased toward studies in the Northern Hemisphere, where most biodiversity and ecosystem function (BEF) experiments are performed (Clarke et al., 2017). Long-term ecological research of international importance in the Global South can help fill this gap and bring science and nature closer to the public.
The Brasília LTER site also organizes scientific education activities, mainly for children and teenagers. In 2018, the Brasília LTER site promoted the “Cerrado Week” at the Botanical Garden of Brasília dedicated to presenting the Cerrado Biome to over 3000 students from public schools in the Federal District through activities such as exhibitions, games, field excursions, and workshops with social inclusion (leaflets in Braille; Fig. 5). Despite their proximity to the city, most students and teachers visited the PA for the first time, demonstrating the importance of such events. As a follow-up, in 2019, the Brasília LTER site participated in the “National Week of Science and Technology” with an exhibition and educational games about the Cerrado’s fauna and flora.
Brazil is currently experiencing a weakening of environmental laws and reductions in science funding for biodiversity conservation, resulting in increases in deforestation rates, reduced PAs, and increasing threats to biodiversity (Barbosa et al., 2021; Hallal, 2021, Vale et al., 2021). In comparing governance scenarios, abandoning deforestation control policies would result in an annual loss of 18,000 km² in the Cerrado (Rochedo et al., 2018).
The results of this work demonstrate that long-term data are necessary to detect trends in biodiversity and ecosystem function and to support effective conservation of Brazilian biodiversity. Therefore, it is imperative to continue such long-term programs. Additionally, the development of field projects, research synthesis, and new methodologies provide training opportunities in interdisciplinary research and supports public environmental policies that promote both community well-being and the species biodiversity maintained in PAs throughout Brazil.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq [grant numbers (PELD/AGCV #441518/2020-6) (personal research grant to EMV #307303/2017-9) (personal research grant to RT #309973/2017-1) (personal research grant to RBM #304221/2019-8) (personal research grant to LMSA #304989/2019-3) (#151196/2021-4 Post-doctoral Fellowship to NM)]. Fundação de Apoio a Pesquisa do Distrito Federal- FAPDF (#00193-00001257/2018-60). Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- CAPES (#88887.136269/2017-00 Post-doctoral Fellowship to NM). We are grateful to the Botanical Garden of Brasília (JBB) and Ecological Reserve of the Brazilian Institute of Geography and Statistics (IBGE) for allowing this research.
| Variables | CV (N = 49) | CP (N = 49) | BO (N = 42) | GA (N = 42) | ON (N = 49) | MO (N = 49) |
|---|---|---|---|---|---|---|
| Water temperature (ºC) | 21.18 ± 0.88 | 20.21 ± 0.17 | 20.14 ± 2.40 | 21.06 ± 1.47 | 19.59 ± 1.87 | 20.56 ± 1.57 |
| Water velocity (m s−1) | 0.22 ± 0.15 | 0.18 ± 0.19 | 0.23 ± 0.17 | 0.31 ± 0.21 | 0.17 ± 0.14 | 0.14 ± 0.08 |
| Electric conductivity (µS cm−1) | 6.43 ± 1.57 | 5.36 ± 1.59 | 6.73 ± 2.89 | 9.50 ± 3.23 | 18.33 ± 3.92 | 5.32 ± 0.48 |
| Dissolved oxygen (mg L−1) | 6.51 ± 0.76 | 6.22 ± 1.42 | 7.15 ± 1,51 | 6.23 ± 1.36 | 6.57 ± 1.47 | 5.85 ± 0.89 |
| Oxygen (% saturation) | 75.07 ± 8.10 | 67.33 ± 14.44 | 74.57 ± 11.63 | 71.52 ± 11.97 | 70.43 ± 14.87 | 62.88 ± 8.86 |
| pH | 5.65 ± 0.68 | 6.12 ± 0.98 | 6.35 ± 0.97 | 6.23 ± 0.88 | 6.21 ± 0.92 | 5.32 ± 0.48 |
| Nitrate (N-NO3−) (µg L-1) | 47.20 ± 27.09 | 45.64 ± 25.94 | 29.23 ± 17.62 | * | 53.75 ± 11.85 | 59.85 ± 30.14 |
| Total phosphorus (µg L−1) | 8.19 ± 10.13 | 5.37 ± 4.91 | 8.00 ± 17.88 | 2.50 ± 4.70 | 4.78 ± 3.75 | 13.01 ± 8.69 |
| Turbidity (NTU) | 1.54 ± 1.32 | 2.74 ± 1.44 | 3.36 ± 4.05 | 2.92 ± 2.27 | 4.56 ± 3.12 | 2.55 ± 1.95 |
| Total solids (mg L−1) | 20.08 ± 22.06 | 13.12 ± 14.48 | 13.38 ± 17.79 | 9.82 ± 2.27 | 20.88 ± 23.74 | 48.27 ± 137.01 |
| Alkalinity (mg L−1) | 7.72 ± 4.62 | 6.51 ± 4.88 | 7.93 ± 4.81 | 7.52 ± 4.79 | 13.18 ± 8.59 | 6.40 ± 4.84 |