Understanding the origin and maintenance of microbial diversity patterns, and the relative importance of local and landscape processes for determining biodiversity is still challenging. We investigated the influence of environmental factors acting at local and landscape scales on several facets of phytoplankton diversity. We conducted standardized surveys in 45 artificial ponds in an agricultural landscape of the Brazilian Cerrado, measuring several local (i.e. limnological variables) and landscape characteristics, and phytoplankton abundance, species richness and functional diversity. Structural Equation Models were used to decompose the multiple relationships that local and landscape factors can have between each other and with phytoplankton diversity. Abundance was determined by pond connectivity and limnological variables (water conductivity, transparency, and ammonia), while species richness was positively related to abundance, but negatively affected by pond age. Further, species richness shows a direct negative relationship with functional evenness, so species-poor communities tended to be overdispersed in the functional space. This complex set of relationships highlights the importance of decomposing environmental, morphometric and spatial factors and considering multiple facets of biodiversity to understand community dynamics. These results provide valuable insights on how artificial pond configuration and management in farmstead strategies can allow maintaining high levels of phytoplankton diversity and other aquatic communities in tropical regions.
The distribution of biodiversity and the structure of ecological communities are the outcome of processes occurring at different scales (Hortal et al., 2010; 2012; Guisan and Rahbek, 2011). Indeed, understanding the mechanisms responsible for the maintenance of biodiversity depends on the ability to decompose the diversity variation that is associated with processes acting at different scales (Cottenie et al., 2003; Cottenie and De Meester, 2003; Legendre et al., 2009). Further, although many ecological studies focus on the drivers of species richness, the identities, abundance, phenotypes and phylogenetic relationships of these species are also critical determinants of the nature and strength of the relationships between diversity and a range of ecological functions (Edwards et al., 2013; Pavoine and Bonsall, 2011). In particular, functional diversity is a key aspect of biodiversity essential for understanding the processes behind community assembly and structure. Only species with certain traits can disperse, overcome environmental filters, establish and co-exist in a particular area and moment (Mittelbach and Schemske, 2015). Therefore, combining information from taxonomic and functional diversity allows ascertaining the processes dominating community assembly and structure, providing an effective way of assessing the effects of human interventions on biodiversity (Graco-Roza et al., 2021).
Microorganisms make out most of Earth’s diversity. Many can be found in freshwater systems, where diversity drivers acting at different scales are intertwined (Pajunen et al., 2017). Phytoplankton stand out as one of the most relevant primary producers in aquatic ecosystems. They are responsible for a great portion of global primary production and mediate the biogeochemical cycle of several elements, being the major source for silica, carbon, nitrogen and phosphorus uptake in some systems (Plus et al., 2015). Phytoplankton respond rapidly to environmental changes and disturbance gradients, being widely used as bioindicators for pollution (Wang et al., 2017). Their communities also respond to environmental gradients and spatial and historical processes (Stomp et al., 2011; Padisák et al., 2016; Ribeiro et al., 2018), as well as to biological interactions. These interactions include the control of phytoplankton biomass by macrophytes (through allelopathic effects that suppress phytoplankton; Vanderstukken et al., 2011) and by filter-feeding fish and zooplankton (through grazing and predation; Zhang et al., 2023) but also the positive contribution of the benthos to primary production and phytoplankton growth by resuspending sediments (Moncelon et al., 2022).
Importantly, phytoplankton communities are typically composed of species holding different functional traits, which are spatial and temporally distributed along a multi-dimensional setting (Edwards et al., 2013). The local environmental gradients within the water body (e.g. light irradiance, phosphate, nitrate) produce changes in the composition and functional structure of the community (Litchman and Klausmeier, 2008). Here, response traits may provide the link between environmental drivers and mechanisms of change, as they may respond to resource availability and other environmental conditions (Edwards et al., 2013). Importantly, as several phytoplankton traits (such as life form, presence of heterocysts or aerotopes, etc.) have direct effects on functional processes, the community changes associated to these responses also affect ecosystem functioning (Litchman et al., 2010; Thompson et al., 2015; Santos et al., 2015).
The generalized cosmopolitism of phytoplankton has led to consider changes in trophic conditions along the eutrophication–oligotrophication gradient as the main determinant of the distribution of this group (Pomati et al., 2012). However, while several studies indicate positive relationships between phytoplankton abundance and the amount of light and nutrients available (Lewis and Wurtsbaugh, 2008; Litchman et al., 2004; Ramdani et al., 2009), others show no evidence of the influence of these local environmental factors on phytoplankton communities (Beisner et al., 2006; Nabout et al., 2009). This may be related with the fact that, in freshwater systems, phytoplankton diversity is highly influenced by the spatial design of lakes and watersheds (Soininen et al., 2004). The relationship between taxon diversity and lake size differs widely among species (Scheffer et al., 2006), and it was weak (Søndergaard et al., 2005) or absent (De Marco et al., 2013) for phytoplankton in small ponds. In fact, several small waterbodies like ponds can support a more diverse microalgae community than a single large one (Bolgovics et al., 2019), calling for an important influence of landscape structure and spatial heterogeneity on phytoplankton diversity in freshwater systems. In a fragmented landscape, the limited connectivity among aquatic environments can lead to a decrease in the regional biodiversity able to colonize the ponds (Scheffer et al., 2006). In this context, the effect of processes acting at the landscape scale on the aquatic communities occurs indirectly via the influence that regional environmental conditions and/or the spatial structure of the landscape exert in the local physical and chemical conditions of waterbodies. Processes like riparian vegetation deforestation, hydrologic alterations or pollution cause changes in local physical and chemical conditions such as water temperature, erosion rates, light penetration, nutrient input and retention, flood magnitude and frequency, and/or heavy metal concentration, in turn affecting the population dynamics of freshwater organisms such as phytoplankton, zooplankton or benthos (Allan, 2004).
A consequence of the influence of landscape and local environmental conditions of water bodies on phytoplankton communities is that both factors can have significant effects on the functional diversity of their phytoplankton communities, which in turn may cause shifts in their functioning (Graco-Roza et al., 2021). For instance, phytoplankton body size has been related to both trophic status and lake isolation (Erdo¿an et al., 2021), although other studies have found that other traits, as well as functional diversity, are more associated to local factors than to landscape typology (Derot et al., 2020; Vadrucci et al., 2013). However, landscape management has a significant influence on waterbodies. Indeed, the increases in community biomass associated to high nutrient intake from agricultural practices often cause decreases in taxonomic and functional diversity (Da Silva et al., 2020; Pomati et al., 2012; Török et al., 2016). Further, the increase in phosphorus and nitrogen concentration in water bodies affected by the use of pesticides and fertilizers in the surrounding landscape cause decreases in phytoplankton functional evenness and functional richness, and increases in functional divergence, as eutrophication-tolerant species increase in importance (Wijewardene et al., 2021). Despite these evidences that certain trait combinations are filtered by both landscape and local factors with different degrees of importance, little is known about the scaling of such filtering in phytoplankton communities.
Here we evaluate the influence of several local and landscape factors on different aspects of phytoplankton diversity in agricultural ponds in the Brazilian Cerrado. We expected that local limnological variables such as pH, temperature, nutrients, and light would directly impact phytoplankton abundance. Specifically, increases in these variables should positively affect phytoplankton abundance, as they indicate favorable environmental conditions and resource availability (Padisák, 2004). Additionally, we hypothesized that pond characteristics (such as area, age, and perimeter) and landscape factors (such as presence of shadow, land use, and connectivity) would influence both local limnological variables and phytoplankton species richness, abundance, and functional diversity. Here, ponds with greater connectivity would show lower nutrient concentration as a result of the influence of the streams that provide such connectivity, which in this area are likely to have good water quality (Bichsel et al., 2016). Therefore, the connection between ponds and streams would indirectly and negatively affect the abundance of phytoplankton. In contrast, higher connectivity would have a direct and positive influence on species richness, as it facilitates colonization by new species (Oertli and Parris, 2019; but see Pęczuła and Szczurowska, 2013). Pond size could impact local limnological variables and indirectly affect phytoplankton abundance. Well-preserved ponds with greater shade and less intensive land use would have a positive influence on phytoplankton richness and functional diversity, while having the opposite effect on abundance. These preserved ponds would have lower productivity, resulting in lower dominance (Zhang et al., 2018). Furthermore, the age of the ponds would positively influence both phytoplankton richness and abundance (Tulsankar et al., 2020; Zimba et al., 2003). Lastly, we anticipated that variations in species richness and relative abundances of phytoplankton in local ponds would directly impact the functional diversity of phytoplankton within those ponds (Fig. 1).
Initial a priori Structural Equation Model, including the preliminary hypotheses on the relationships among variables. According to these hypotheses, land use (level of use and shadow), and pond morphology (area, age and isolation) should affect directly the limnological variables and species richness, and also indirectly all diversity metrics, including abundance and two different aspects of functional diversity; Functional Divergence (MTD) and Functional Evenness (FEve). See variable descriptions in Table 1.
The study area was located 25 km south from Goiânia (−48.8487, −16.6730, Goiás State, Brazil; Fig. 2), and surveys were conducted only on landscapes dominated by agriculture. The study area covers a total of 45 × 30 km2, and includes a buffer area of 10 km, and a core area of 35 × 20 km2 from where samples were taken. Water bodies within the core area were identified through satellite imagery, which was also used to calculate the size of ponds and their distance to the nearest neighboring pond. A total of 2,787 ponds were found in the core area, indicating a dense pond network within a small geographical extent. The focus of the research was solely on permanent ponds, with temporary ones being disregarded. Ponds were selected based on their size and isolation, and were grouped into five size classes, with maximum areas (in m2) of 190, 600, 1,900, 6,000, 10,000 (De Marco et al., 2013). Ponds with less than 100 meters of isolation were excluded from the study. Linearly distributed five isolation classes were created using the overall frequency distribution. To obtain a representative sample, we crossed pond size and isolation classifications, and ponds were randomly selected within each category of such joint classification. In total, 70 ponds were sampled for the study (De Marco et al., 2013).
All 70 ponds were sampled during the dry season, between June and September 2012. Phytoplankton samples were taken from the center of the pond at 30 cm depth. Each water sample was collected with 100 mL amber bottles and fixed with acetic lugol. A qualitative analysis was conducted using a plankton net with 20-µm and preserving this sample in Transeau (6 part of water: 3 parts of alcohol: 1 part of formaline 40%) at 1:1 proportion. The net sample was used to help in species identification and the bottle sample to estimate species density according to Uthermol (1958). All phytoplankton was identified to the species level, or the lowest taxonomic level possible. Ponds with fewer than 5 phytoplankton species were excluded, and therefore only 45 ponds were retained for further analyses (Fig. 2).
All physical and chemical water parameters were measured in situ (see Table 1). Limnological variables characterizing water environment (chlorophyll-a concentration, pH, dissolved oxygen, chloride, ammonium and water temperature) were measured at 30 cm depth using a 6820v2-1-C-S multi-parameter water quality logger. Chloride is an important driver of phytoplankton dynamics due to its role in osmoregulation, supporting photosynthesis, and pH regulation. It is also a common component of fertilizers, so agricultural activities impact phytoplankton abundance and composition (McClymont et al., 2023; Greco et al., 2023). Water transparency was measured using a Secchi disk. Phosphorus was not measured due to project limitations, which allowed to retrieve only parameters from the probe that could be obtained in situ. Land use variables were also obtained through visual inspection of the property during the sampling. These variables included percentage of shadow coverage in each pond (as a proxy of vegetation cover) and the presence or absence of fish-farming, agriculture and livestock uses in a 500 m radius around the pond. The latter three variables were summed to create one ordinal variable depicting the level of human land use: 0 (no human-associated land use); 1 (only one land use; e.g.: agriculture); 2 (two land uses, e.g.: fish-farming and livestock); and 3 (three land uses: agriculture, fish-farming and livestock). Pond age was obtained by interviewing the land owner. Ponds were classified according to the spatial configuration of their network, either isolated along ditches or springs (N = 13), or connected in dams (N = 32).
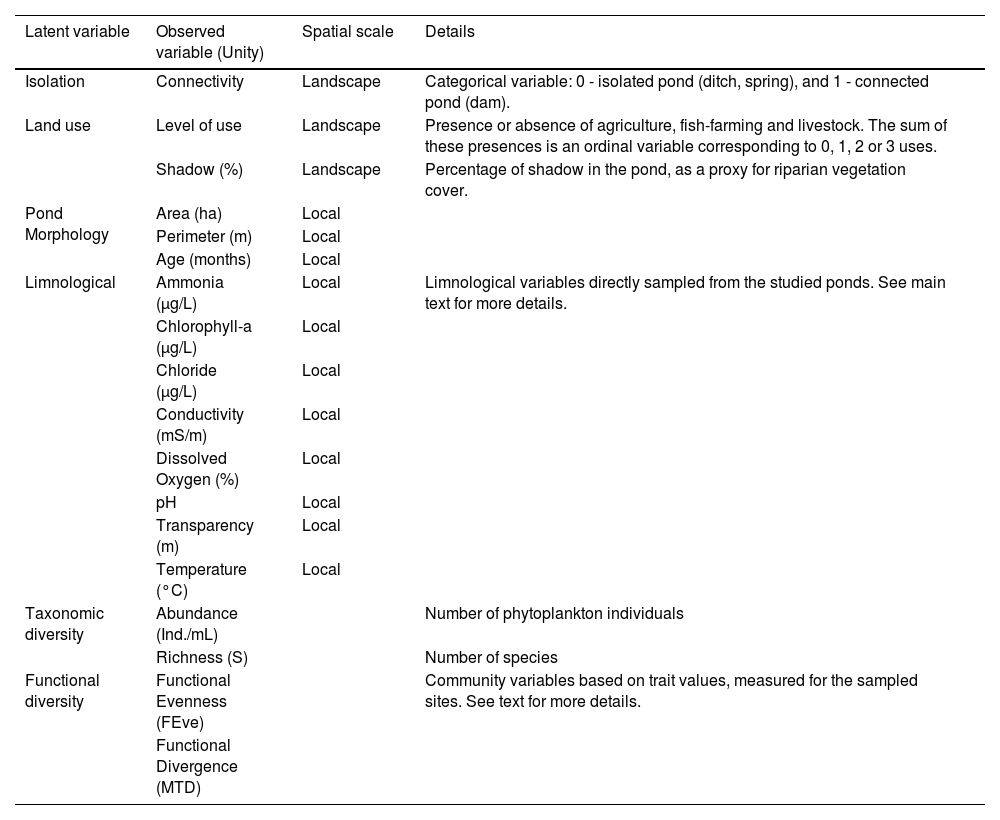
Details of the latent and observed variables used in the Structural Equation Modelling analyses.
| Latent variable | Observed variable (Unity) | Spatial scale | Details |
|---|---|---|---|
| Isolation | Connectivity | Landscape | Categorical variable: 0 - isolated pond (ditch, spring), and 1 - connected pond (dam). |
| Land use | Level of use | Landscape | Presence or absence of agriculture, fish-farming and livestock. The sum of these presences is an ordinal variable corresponding to 0, 1, 2 or 3 uses. |
| Shadow (%) | Landscape | Percentage of shadow in the pond, as a proxy for riparian vegetation cover. | |
| Pond Morphology | Area (ha) | Local | |
| Perimeter (m) | Local | ||
| Age (months) | Local | ||
| Limnological | Ammonia (µg/L) | Local | Limnological variables directly sampled from the studied ponds. See main text for more details. |
| Chlorophyll-a (µg/L) | Local | ||
| Chloride (µg/L) | Local | ||
| Conductivity (mS/m) | Local | ||
| Dissolved Oxygen (%) | Local | ||
| pH | Local | ||
| Transparency (m) | Local | ||
| Temperature (°C) | Local | ||
| Taxonomic diversity | Abundance (Ind./mL) | Number of phytoplankton individuals | |
| Richness (S) | Number of species | ||
| Functional diversity | Functional Evenness (FEve) | Community variables based on trait values, measured for the sampled sites. See text for more details. | |
| Functional Divergence (MTD) |
For each site, we measured species richness (S) and total abundance (N), as well as other abundance-based metrics such as Simpson Diversity and Evenness. Functional diversity was calculated based on eight morphological, physiological and behavioral traits, which were either measured during the taxonomic identification or obtained from the literature. For each species, we calculated the maximum linear dimension (i.e., maximum length - MLD; Trait 1). Around 30 individuals of each species were measured to this end. Individuals were then classified according to their form (unicellular, cenobium, colonial or filamentous; Trait 2). We also considered the potential to produce toxins (Trait 3), aerotopes (Trait 4), flagella (Trait 5), mucilage (Trait 6), siliceous exoskeletal structures (Trait 7) and heterocysts (Trait 8). These traits are related to phytoplankton productivity through their effects on reproduction (MLD and Trait 2), resource acquisition (response to light and nutrients; except presence of toxin) and herbivory avoidance (all traits) (as in Santos et al., 2015). MLD was quantitative, while all the other phytoplankton functional variables were categorical.
Using these attributes, we calculated a trait distance matrix using Gower distance (Gower, 1971; Pavoine and Bonsall, 2009), using the function "gowdis" from the R package FD. This matrix was then used to calculate different facets of functional diversity: (i) functional richness was measured through FRich (convex hull volume; Villéger et al., 2008); (ii) functional evenness through FEve (Villéger et al., 2008); and (iii) functional divergence was calculated using unweighted mean trait distance (MTD), a metric originally used in community phylogenetic studies that corresponds to the mean pairwise distance in communities; (Webb, 2000), and (iv) MTD weighted by species density (MTDDens). All functional diversity metrics were calculated in R program (R Core Team, 2020) using the functions mpd from picante package (Kembel et al., 2010) for MTD, and dbFD from FD package (Laliberté et al., 2014) for Frich and FEve. We assessed the collinearity between diversity variables through Pearson correlations. Due to the high degree of correlation among these indices, for the analyses we selected only one index based on species presence (MTD) and another one based on species abundances (FEve).
We used structural equations models (SEM) to identify the direction and intensity of the effects of environmental factors at different scales on pond phytoplankton diversity. SEMs allow evaluating hypotheses that specify how variables are linked together in terms of direct and indirect causal effects (Shipley, 2009). This helps understanding direct and indirect interactions between variables, consequently allowing to detect the primary causes of variation in the dependent variables. All latent variables included in the SEM analyses are described in Table 1. Prior to the SEM analysis we performed a Principal Components Analysis (PCA) of the limnological variables (see Appendix 1) to summarise information and avoid having too many parameters in the model. The first two PCA axes represented almost 50% of the variation in limnological properties of the ponds (Fig. 3), so we used both of them as predictors in the SEM. Chlorophyll-a was not included in the PCA because this variable also represents phytoplankton biomass. Instead, we only used this metric to describe the trophic gradient in the ponds through the trophic status index (TSI), using Carlson’s (1977) TSI modified by Lamparelli (2004) for tropical lentic systems.
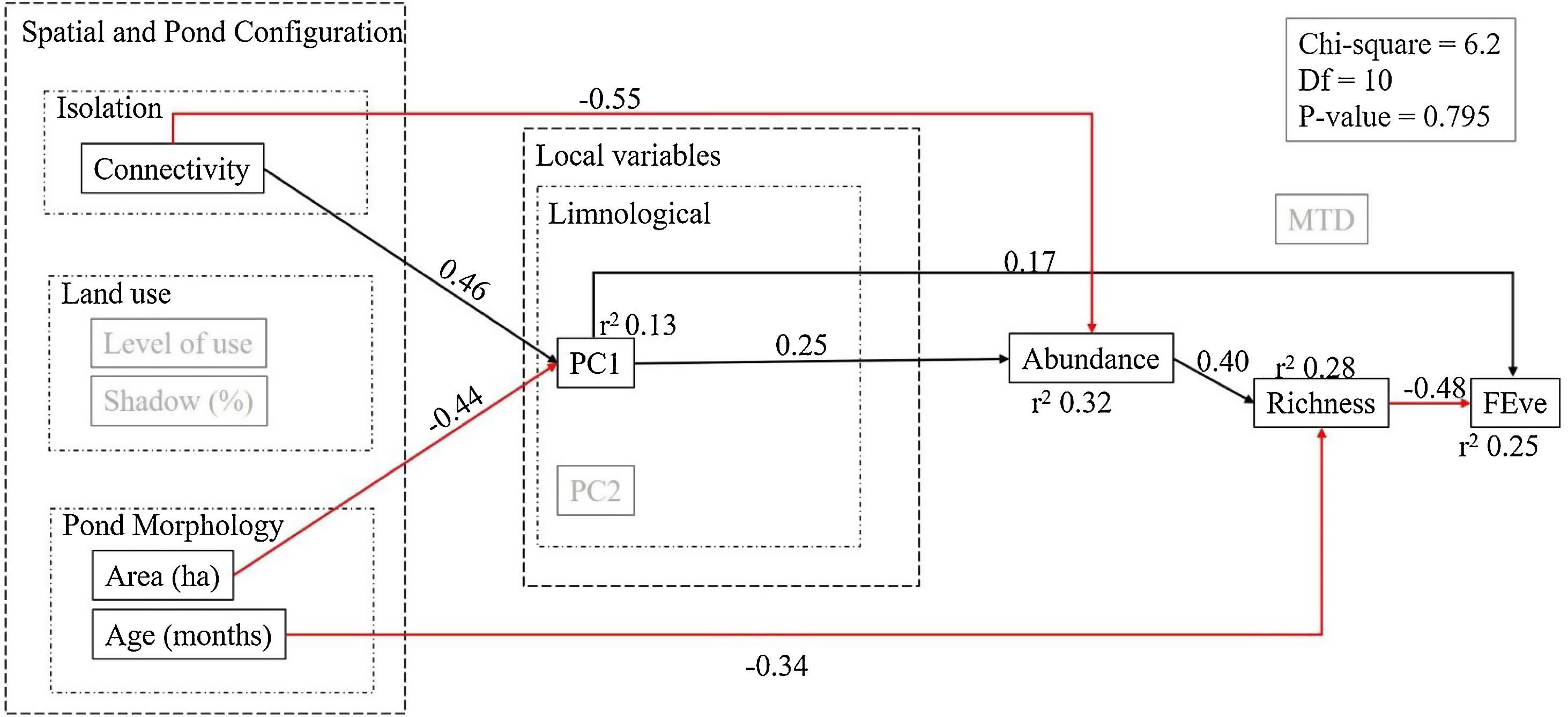
This initial model summarized our a priori hypotheses on the relationships between variables (Fig. 1). Isolation, land-use and pond morphology were considered to have a direct effect in the limnological variables. Limnological variables were in turn considered to have direct effects on abundance and functional diversity, while their effect on species richness would be indirect through their impact on abundance. Further, we also expected that landscape variables (land use and connectivity) had a direct effect on species richness. After defining the a priori model we evaluated whether it fitted well the data by checking its χ2 and p-values of the so-obtained structural model. This model fit was significant, implying the proposed initial model was not representing well the covariations in the observed data. To improve model fit, we checked the modification indices that indicate the amount of change in the χ2 of the model if a relationship is added (Grace and Bollen, 2006). We added the relationship with the highest modification index that linked connectivity and abundance, and the p-value of the model became non-significant indicating that the proposed model is compatible with the observed correlations in the data. To increase the parsimony of the model we eliminated non-significant relationships starting from the highest p-values. Each time we removed a variable we checked the fit indices of the model, and stopped removing variables when it diminished model fit. All SEM analyses were performed in AMOS (Arbuckle, 2014).
ResultsThere was a large variation between ponds in terms of morphometry, limnological conditions (such as conductivity or chloride) and productivity (e.g., chlorophyll-a). Considering the values of chlorophyll-a concentration, the studied ponds covered a wide range of levels of productivity, some being ultraoligotrophic (14 ponds ≤1.17 µg/L), while others oligotrophic (17 ponds, chl-a: 1.17–3.24 µg/L), mesotrophic (9 ponds, chl-a: 3.24–11.03 µg/L), eutrophic (3 ponds, chl-a: 11.03–30.55 µg/L) or supereutrophic (1 pond, chl-a: 30.55–69.07 µg/L). The number of land uses around the ponds varied from only one to three, with 11 ponds presenting all three uses, 18 presenting both agriculture and livestock uses, five presenting agriculture and fish-farming land uses, two presenting livestock and fish-farming uses, four presenting only agriculture and five only livestock.
In total, 300 phytoplankton species were found across the 45 ponds sampled (Supplementary Table S1). The most representative classes in terms of number of species were Bacillariophyceae, Conjugatophyceae (desmids), Trebouxophyceae, Chrysophyceae, Dinophyceae and Chlorophyceae, while the most abundant classes were Cyanophyceae, Treuboxiophyceae and Conjugatophyceae (Supplementary Table S2). Regarding species characteristics, the majority of the individuals were unicellular (68%) and filamentous (28%); most of them were neither flagellate (91.52%) nor mucilaginous (71.58%), and did not present silica (96.14%), heterocites (99%) and aerotopes (74.92%). Although FEve showed a low variation between ponds, species evenness varied widely, from 0.086 to 0.942. Abundance showed a high variation among the ponds (SD 40028 individuals/mL; Supplementary Table S3).
Two axes were selected in the PCA analysis performed on the local limnological variables, which explained respectively 27.3% and 22.1% of the variability in these variables (Fig. 3). The first axis (PC1) was strongly associated with water transparency (23.58% of contribution), conductivity (31.33% of contribution) and ammonia (16.69% of contribution), while the second axis (PC2) was more associated to chloride (31.85 % of contribution), temperature (24.11 % of contribution), water transparency (21.11 % of contribution) and dissolved oxygen (16.96 % of contribution).
According to the fitted SEM, connectivity was the most important correlate of phytoplankton abundance, followed by PC1 (more influenced by transparency, conductivity and ammonium). These variables together explained 32% of phytoplankton abundance in the ponds, while connectivity and pond area explained 13% of PC1 (i.e. conductivity, water transparency and ammonia), and PC2 (more influenced by chloride, temperature, water transparency and dissolved oxygen) held no significant effect on any aspect of the phytoplankton communities. The direct influence of connectivity on abundance was negative (r = −0.55), evidencing that the most connected ponds (i.e. the dams) hosted lower phytoplankton abundance. The local variables also had direct effects on abundance. Further, pond age had an impact on richness and pond area affected local variables. The limnological variables represented by PC1 (i.e., conductivity, water transparency and ammonia) were positively related with connectivity and negatively with area, meaning that the largest ponds showed lower values of conductivity, ammonia and water transparency. Further, the direct effects of abundance and pond age explained together 28% of species richness variations. In turn, species richness and PC1 explained 25% of the variability in functional evenness (i.e., FEve). Strikingly, abundance was positively associated to phytoplankton richness, whereas the association between richness and FEve was negative (i.e., richer ponds showed highly packed functional trait spaces). Functional divergence was not associated to any predictor or diversity metric (Fig. 4).
Structural Equation Model (SEM) depicting the main determinants of community diversity in the studied system of artificial ponds. This SEM combination is the most congruent with the observed data, with no support for deleting further paths. Note that local variables in the a priori model depicted in Fig. 1 are here summarized as the two axes of a PCA (see Fig. 3). Black arrows represent positive significant relationships and red arrows represent negative significant relationships (p < 0.05).
Our results show that the characteristics of both ponds and landscape are important direct and indirect drivers of the diversity of phytoplankton communities in artificial tropical ponds. Overall, the diversity and structure of pond communities were mediated by abundance; the richest ponds showed less functional evenness, and therefore higher functional redundancy. In turn, abundance depended on both pond connectivity and several local limnological variables. Joint effects of pond and landscape factors on phytoplankton communities have been already found for coastal and terrestrial lakes (Spatharis et al., 2019; Loewen et al., 2020), tropical floodplains (Moresco et al., 2020) or pond microcosm experiments (Pereira et al., 2018). Strikingly, our structural model presented higher explanatory power and is simpler (i.e., includes less variables) than most of these former works.
One of the most important results of this work is that abundance mediates species richness and, through it, functional evenness. Such preeminence of abundance on phytoplankton biodiversity dynamics is related with water quality gradients (Lavoie et al., 2014). Indeed, the high abundances found in several of the ponds studied point to the existence of more eutrophic environments. The species that become dominant in more eutrophic lakes often share similar traits (Reynolds, 1998). Therefore, eutrophic shallow lakes are typically dominated by one or few species from the same functional group (Borics et al., 2012), showing lower functional diversity and, in particular, lower trait evenness and a high degree of redundancy (as species are not sparsely distributed in the trait space). For example, most of the microalgae in the ponds we studied were unicellular or filamentous, so they show a high-rate surface/volume, which is an important trait in the efficiency of resource use (Reynolds, 2006), in their case determined by cell size and shape.
The level of use had no direct influence in the community metrics, however, several of the ponds studied are fish-farming (18 ponds). Fish can be an important factor in shaping the food web within these small ecosystems (Søndergaard et al., 2005). Therefore, the positive effect of phytoplankton abundance on species richness may be also related with the characteristics of the farm ponds themselves such as the use type. Phytoplankton diversity in small lakes and ponds can depends indirectly on surface area through its effects on fish and the structure of aquatic vegetation (Scheffer et al., 2006). Furthermore, previous research has shown that variations in zooplankton on shallow lakes were also correlated to factors such as fish abundance, macroinvertebrates and turbidity (Cottenie et al., 2001).
Abundance also mediated the effect of connectivity on phytoplankton communities. The connected ponds showed lower levels of abundance, which in turn limits species richness; also, these poorer communities were functionally even, composed by species with differing traits and little redundancy. Phytoplankton communities in lentic environments respond to factors acting at different scales, such as spatial structure, limnological conditions and watershed characteristics (Loewen et al., 2020). Indeed, the relevance of the spatial configuration of the landscape on phytoplankton structure depends on the study scale (Heino et al., 2015a, b), type of aquatic system (Heino et al., 2015c), eutrophication level, connectivity, environmental gradients or even hydrological season (Soininen, 2014; Izaguirre et al., 2016; Brasil et al., 2020). Dispersal limitations can decrease local diversity, so the biodiversity of pond communities is strongly linked to the connection and density of other ponds in the landscape (Oertli and Parris, 2019). However, it is possible that, in these studies, connectivity actually represents the influence of lotic systems. In our particular case, the connected ponds are dams, which may present constant or intermittent diluter and disturber effects from the river or stream that limit phytoplankton development. This contrasts with isolated ponds, which present more stable conditions, having less residence time than ditches or springs, thus favoring the buildup of more diverse communities through longer assembly processes. Indeed, similar effects have been observed on lake phytoplankton, where river connections (with low trophic level) can decrease phosphorus, conductivity and transparency, consequently diminishing richness and abundance (Pȩczuła and Szczurowska, 2013).
Light (water transparency), nutrients and conductivity were positively associated to abundance and Functional Evenness, reflecting the access and availability of resources to phytoplankton (Padisák, 2004). Factors like area, depth, residence time, water temperature, transparency, pH, conductivity, nitrogen and phosphorus determine phytoplankton abundance and functional structure on tropical reservoirs and lakes (Lewis et al., 2020; Santos et al., 2016). Phytoplankton biomass is commonly related with resource variation (Korhonen et al., 2011), and another study in temperate streams using SEMs found a positive effect of both water temperature and nutrients on phytoplankton biomass (Pajunen et al., 2017). In our study, ponds with higher conductivity and lower pH had poorer communities, a result that has been related with an increase in dominance and the consequent decrease in richness and functional evenness in progressively more acidic and eutrophic water bodies (Lau et al., 2017; Wang et al., 2017). However, the relationship between phytoplankton functional evenness and nutrients that we reported in the tropical farm ponds is the opposite to the one reported for temperate farm ponds (Wijewardene et al., 2021), where increasing functional evenness of microalgae has been related with increasing conductivity of rock pools in the warmer months (Aarnio and Soininen, 2021). The limited number of phytoplankton ecology studies in Cerrado farm ponds (but see, Bichsel et al., 2016; Carneiro et al., 2019; De Marco et al., 2013; Fonseca et al., 2018) prevents from determining whether these differences with temperate systems are characteristic of this biome, or merely restricted to the studied landscape.
Lake area was also an important determinant of phytoplankton diversity in our study system, as the largest ponds showed negative values of PC1, representing conductivity, ammonia and light, i.e. variables that can reflect water quality (Amengual-Morro et al., 2012). Another study from this same area showed that large ponds hold more stable phytoplankton communities because they can buffer the effect of heavy rains, while smaller ponds often harbor high levels of chlorophyll-a concentration (De Marco et al., 2013). Here is worth noticing that although land use variables did not affect pond communities in a direct way, landscape management can indirectly influence both nutrients and conductivity (Søndergaard et al., 2005). In any case, these results highlight the importance of phytoplankton as an indicator of water quality and anthropogenic impact (Shi et al., 2015), even when the effects of other aspects of land intensification are not evident.
Despite the typical complexity of ecological communities, changes in functional trait distributions along environmental gradients are often predictable (Götzenberger et al., 2012; Edwards et al., 2013). Here, the relationships between abundance and connectivity, pond size (area) and, indirectly, limnological variables, provided a mechanism to relate functional evenness with pond characteristics. Eutrophication would lead to ponds with higher abundances and richer communities that are formed by functionally highly redundant species. Evenness is known to decrease in the most diverse communities, which paradoxically can show reduced compositional turnover in response to environmental changes. It follows that even, but less abundant, communities maintain higher functional diversity and ecosystem functioning (Isbell et al., 2015). Here we must note that the traits used here (e.g.: MDL, aerotopes, siliceous structures; body form; mucilage) accounted for responses to both environmental gradients and interspecific interactions, since PC1 and Richness influenced directly the Functional Evenness (see also Litchman and Klausmeier, 2008; Santos et al., 2015).
Our results pointed to pond connectivity being the main determinant of phytoplankton abundance, species richness and functional diversity, together with other variables mediated by human activities that are associated to resource availability, such as light, nutrients and conductivity. This highlights the importance of human actions in the structure and functioning of artificial pond ecosystems, as the size and connectivity of man-made ponds reflect the needs and actions of landowners (Clifford and Heffernan, 2018). These anthropogenic effects operate through the regulation of phytoplankton abundance, which in turn mediates species richness, and through it, functional evenness. This effect should be taken into account for the construction and management of farm ponds in this region devoted to livestock herding, as the balance between eutrophication and functional evenness –and thus functioning– is related with the design of the ponds themselves. Therefore, conserving small farm ponds is crucial in order to preserve aquatic biodiversity in general, as these systems are also home to a range of organisms, such as fish, macrophytes, benthos, and zooplankton, which are intricately interconnected with phytoplankton, and their population dynamics are likely affected by comparable local and regional factors. Besides that, the complex relationships we unveiled highlight the importance of considering multiple facets of biodiversity for understanding community dynamics. Analyzing the interaction of factors that influence the dynamics of communities can contribute to the advance of limnological research in understanding the relationships between various stressors and determinants of freshwater communities.
FundingUniversidade Estadual de Goiás; Ramón Y Cajal program; Spanish Ministerio de Ciencia e Innovación; Universidad de Alcalá; CNPq; INCT in Ecology, Evolution and Biodiversity Conservation MCTIC/CNPq/FAPEG.
Author contributionsF.M.C., A.M.C.S. and J.H. designed the study, with N.G.M. and P.D.M.J.; F.M.C. and P.D.M.J. retrieved and processed the data; F.M.C., A.M.C.S. and N.G.M. analysed the data; F.M.C. wrote the paper, with A.M.C.S. and J.H.; all authors discussed the results, contributed substantially to revisions and approved the final version of the manuscript.
Data availability statementData are available from the authors upon reasonable request.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
F.M.C. was supported by UEG (Pro-Projetos/05/2021). A.M.C.S. was supported by a Spanish Ramón y Cajal fellowship RYC2020-029407-I, funded by MICIN/AEI/10.13039/501100011033 and by “ESF Investing in your future”, and was also supported by a travel grant from Universidad de Alcalá (‘Ayudas de movilidad de personal docente y personal investigador’, 2018 call). P.D.M.J. research is funded by CNPq (grant 308694/2015-5). J.H. was supported by the projects ‘Predicting diversity variations across scales through process-based models linking community ecology and biogeography’ (CNPq PVE 314523/2014-6) and SCENIC (PID2019-106840GB-C21/AEI/10.13039/501100011033, funded by Spanish AEI). This paper is a contribution of the INCT in Ecology, Evolution and Biodiversity Conservation funded by MCTIC/CNPq/FAPEG (grant 465610/2014-5).