Despite the growing concern and literature on biological invasions, few studies have adopted an explicit landscape perspective to understand occurrence patterns of invasive species. We investigated how landscape composition influences the occurrence of a widespread invasive bird species, the Monk Parakeet Myiopsitta monachus, across landscapes of the Brazilian Atlantic Forest. We determined occurrence patterns and habitat composition in 24 sites, half of which harboring established populations of the species. Landscape composition had clear effects on species occurrence. Probability of occurrence decreased as the amounts of anthropogenic and agricultural areas in the landscape increased. Landscape composition was mostly strongly related to occurrence patterns when measured at the largest spatial scale considered (search radius=3km). Our results show that landscape composition may affect occurrence patterns of invasive species across landscapes. They also suggest that invasive species are not necessarily favored by habitat disturbance at the landscape level.
Habitat loss and biological invasions are regarded as the major drivers of global biodiversity loss (Klink and Moreira, 2002; Clavero and Garcia-Berthou, 2005). Although there is a large literature assessing the effects of habitat loss on biodiversity (Fahrig, 2003; Jackson and Fahrig, 2013), as well as a large and growing literature on biological invasions (Blackburn and Duncan, 2009; Strubbe et al., 2013), relatively few studies have addressed explicitly possible interactions between these two variables, in particular how the amount of different habitats in landscapes affects the occurrence of invasive species (With, 2002; Bruno et al., 2004). This occurs, at least in part, because studies on biological invasions have mostly focused at local rather than landscape scales, rarely adopting an explicit landscape ecology approach to investigate the factors regulating the occurrence of invasive alien species across different areas (Gardiner et al., 2009; Decker et al., 2012). For this reason, relatively little is known about whether and how landscape composition, in terms of the quantity and quality of different landcover types, regulates the occurrence of invasive species in landscapes (Pyšek et al., 2010; Thomas and Moloney, 2013).
A central challenge in the study of biological invasions is to predict which characteristics of the invasive alien species, the local environment and the introduction process itself affect the potential of species to establish successfully in new areas (Cassey, 2002; Lockwood et al., 2007). The number of introduction attempts, the number of locations at which the introduction occurs, and the number of individuals introduced are widely recognized as important determinants of invasion success (Duncan et al., 2001). In addition, species with generalist habits are generally more successful at establishing in new, altered habitats (Cassey, 2002). Finally, many studies have suggested that the abiotic and biotic characteristics of invaded habitats, including landscape structure and composition, could affect the establishment and spread of invasive species (Song et al., 2005; Lockwood et al., 2007).
A well-known hypothesis in the literature on biological invasions is that disturbed or altered natural habitats are more susceptible to invasion (review in Lockwood et al., 2007). According to this hypothesis, intensive and frequent landcover changes would increase the vulnerability of local habitats to invasive species (McKinney, 2006), because they would provide “windows of opportunity” for the establishment of invasive species (Vilà and Ibáñez, 2011). This hypothesis could explain why invasive alien species are especially frequent and abundant in habitats historically affected by strong and repeated landcover changes (Domènech et al., 2005). However, the view that disturbed habitats are more susceptible to invasion has been questioned by many studies (Minchinton, 2002; Gerhardt and Collinge, 2003).
An example of model organism to understand whether and how landscape composition, in particular the amount of native and disturbed habitats, affects the occurrence of invasive species is the Monk Parakeet (Myiopsitta monachus; Psittacidae). This species is native from South America, more specifically Bolivia, Paraguay, Uruguay, Argentina and southern Brazil (Sick, 2001). It has established naturalized breeding populations in several parts of the world (United States, Puerto Rico, Bahamas, West Indies, England, Belgium, Italy, Spain, Israel, and Chile), through accidental or purposeful introductions resulting from illegal trade (Roll et al., 2008). Monk parakeets have expanded their range across Latin America in response to human-induced landcover changes, occupying especially areas where original grasslands have been converted for crops or tree planting, which can increase the availability of food and shelter for the species (see Bucher and Aramburú, 2014). In Brazil, this species naturally occurs in the south and southwest region of Rio Grande do Sul and Mato Grosso do Sul states, but it has established breeding populations in other seven states, as a result of captive releases (Viana et al., 2016). In both native and non-native areas of occurrence of M. monachus, the species has caused considerable damage to agriculture, related to its feeding activities (Bucher and Aramburú, 2014) and electric power distribution, related to its nesting activities (Avery et al., 2008). Considering the successful establishment of M. monachus across in a wide geographical area, understanding the factors affecting its occurrence may provide important insights for understanding and predicting biological invasions.
Here we studied M. monachus to determine how landscape composition influences the occurrence of invasive species across different landscapes. To do so, we quantify landscape composition within 24 landscapes located outside the native distribution range of the species, one half harboring established populations of M. monachus, and the other half unoccupied by the species, and determine whether the occurrence of this invasive species is affected by the amount of native and altered (disturbed) habitats. Our main hypothesis is that landscape composition affects the occurrence patterns of M. monachus across landscapes. More specifically, we hypothesize that the occurrence of this invasive species is favored by increases in the area covered by altered habitats in the landscape (anthropogenic and cropland areas), and decreases in the area covered by native habitats.
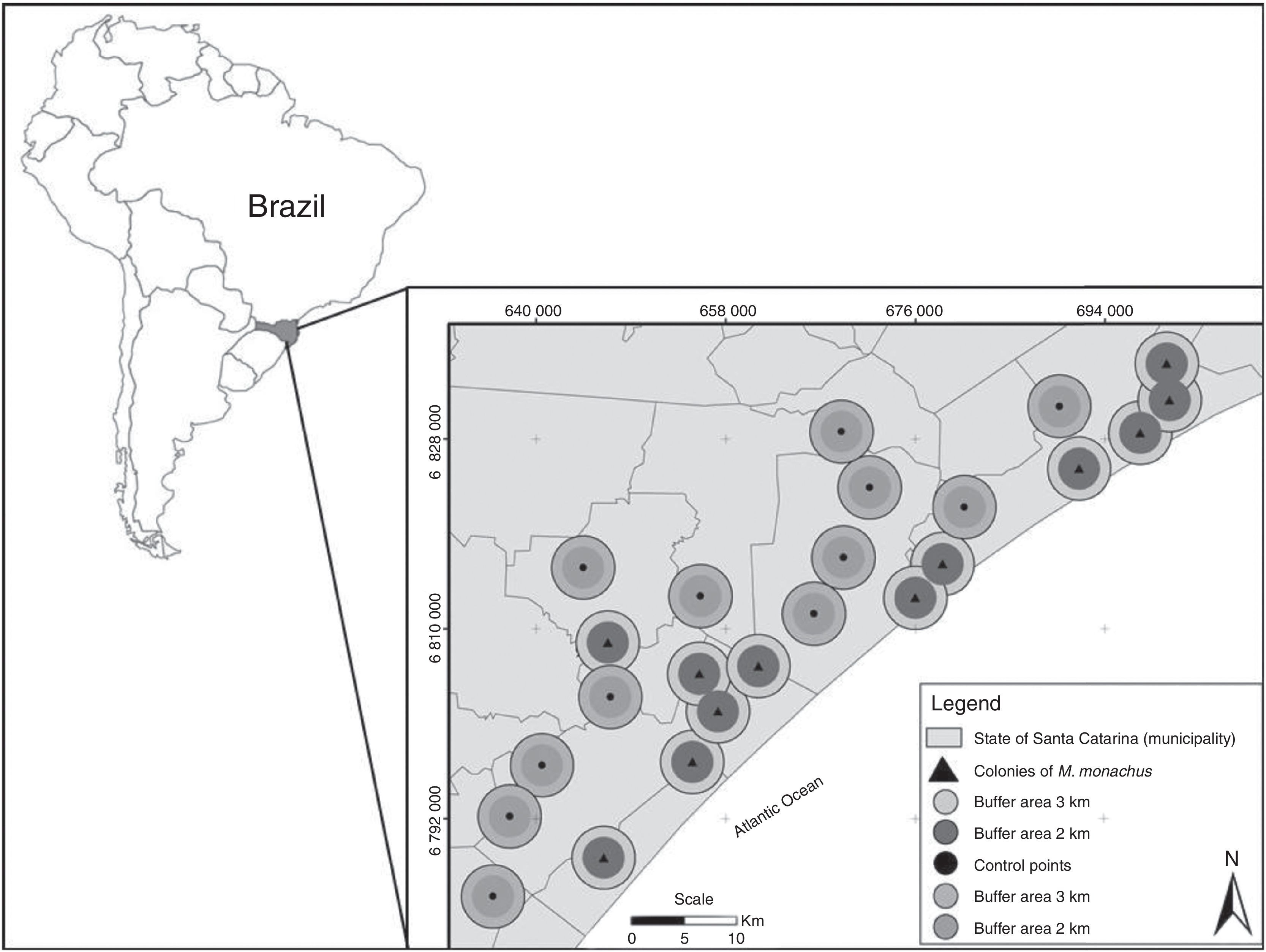
Material and methodsStudy site and field protocolsStudied landscapes were located within the domains of the Atlantic Forest biodiversity hotspot, at the Atlantic coast of Santa Catarina state (28°51′S, 49°20′W) in southern Brazil (Fig. 1). The main native vegetation types in the study area are pioneer vegetation under marine influence (sandbank) and dense ombrophilous forest. The sandbanks are threatened due to real estate speculation. Ombrophilous forests are fragmented and embedded within a matrix composed mainly of open areas (including horticultural crops, fallow fields, and pastures), rural buildings and Eucalyptus spp. and Pinus spp. commercial plantations (Viana et al., 2015).
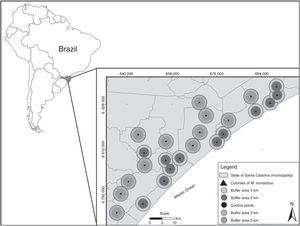
Location of the 24 studied landscapes (circles) in southern Brazil. Landscapes were centered at twelve sites in which Myiopsitta monachus established colonies (triangles) and twelve “control” sites in which the species was absent (points). Two buffer areas (with radius of 2 or 3km) were used to measure landscape composition surrounding each site.
The first record of the monk parakeet in Santa Catarina State dates from 2002 in the capital Florianópolis, located ca. 300km from the limit of the species’ natural distribution area (Amorim and Piacentini, 2006; BirdLife International and NatureServe, 2011) and ca. 150km from our study area. At our study area, the species’ was firstly detected in 2006 in two different places (IR. Viana, unpublished data). Since then, the species has gradually expanded its range across different parts of the study area, but remaining absent from some particular locations (Viana et al., 2016).
To investigate occurrence patterns, we adopted a systematic rather than random strategy to distribute sampling areas, considering the sparse distribution of the species across the study region. Twenty-four sites were selected for analysis across the study area, 12 with established colonies of M. monachus, and 12 in which the species is confirmed absent. The sites with established colonies were identified through an extensive field survey across the study area, and were spaced at least 4km apart (up to 87km; Fig. 1), to maximize spatial independence in terms of landscape composition. At all these sites, nesting sites were always in Eucalyptus trees located in relatively open areas (Viana et al., 2016). We then selected other 12 “control” sites in which the species does not occur, that had similar local conditions as the 12 sites where the species occurs. These local conditions were: (i) presence of human houses in the vicinities of sampling areas, located at a distance similar to the average distance between the 12 colonies and human buildings, as the Monk Parakeet apparently prefers building nests near human houses (Burger and Gochfeld, 2005); (ii) presence of suitable sites for nesting, represented by sparse Eucalyptus trees near human buildings (Burger and Gochfeld, 2005); and (iii) presence of native vegetation, cropland and anthropogenic areas in the surroundings (see Table S1). By controlling the variation in local conditions across the 24 sites, we were able to better assess the potential influence of the composition of the surrounding landscape on the occurrence patterns of M. monachus.
To confirm that the species was absent from the 12 control sites, we interviewed local residents, since the species is charismatic and of easy detection, and also conducted audio/visual samplings, from January 2011 to July 2011. Sampling occurred at 10 randomly distributed points spread over a circular buffer area of 1km radius, centered at each of the 12 previously chosen sites, for two consecutive days. Each point was sampled by the first author for 30min, totaling 10h of sampling per site. We consider that a single visit to each site provided enough information to determine occurrence patterns of M. monachus, as our focus was on established colonies of multiple individuals, rather than one single, foraging individual. Colonies are easily detected by researchers and by local residents, considering that M. monachus is a charismatic species that is promptly detected by their vocalizations (Sick, 2001).
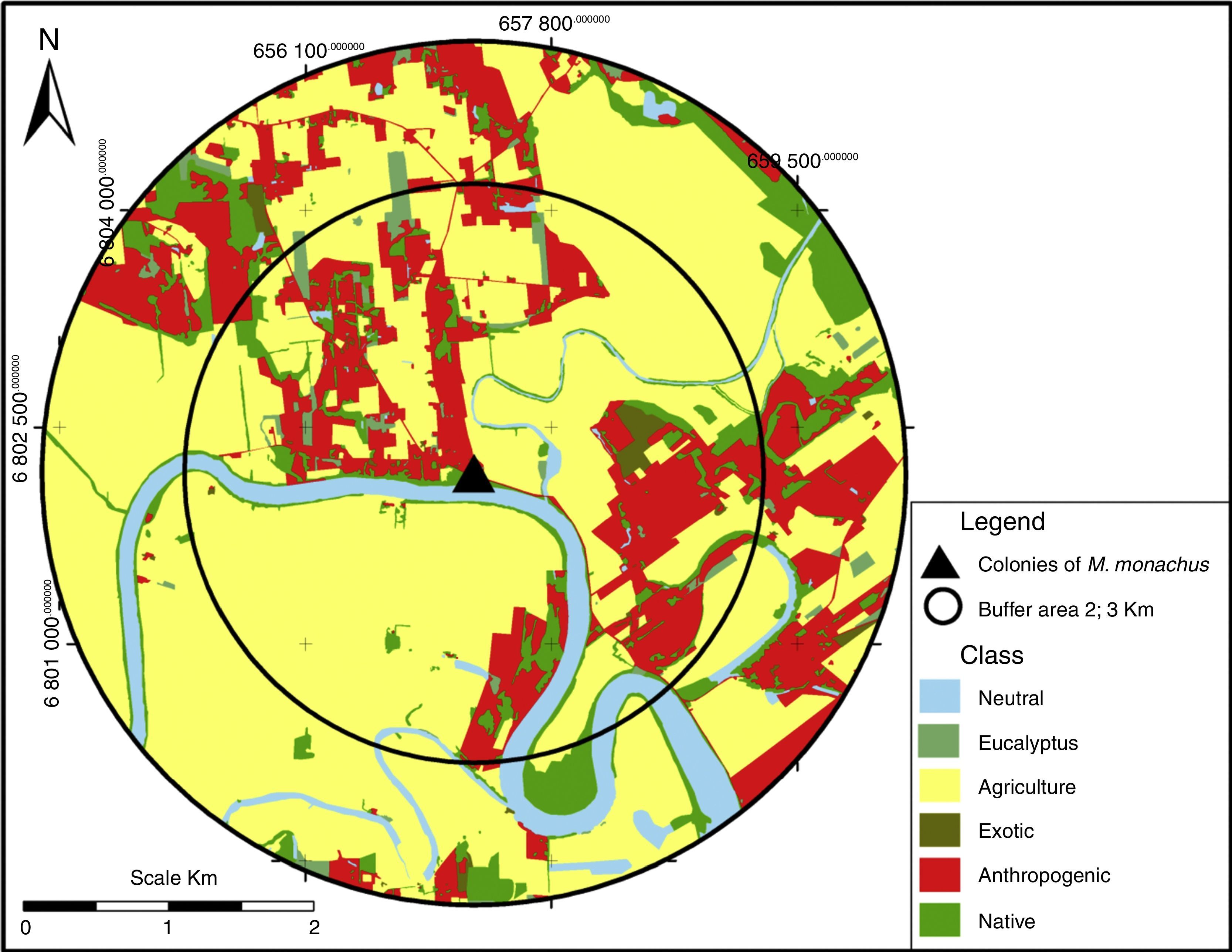
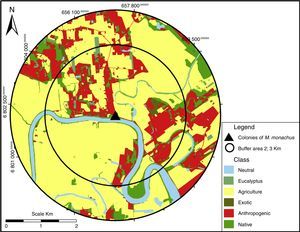
Landscape compositionThe composition of the landscape surrounding each of the 24 sites was obtained from land cover maps, generated by photo-interpretation of orthophotos with 0.37m resolution from 2010, projected in UTM SAD 1969 (Secretaria de Desenvolvimento Regional de Santa Catarina, SDS/SC – 2013). Here, we treat a “landscape” as a circular buffer area surrounding a focal site (Fig. 2). To characterize landscape composition, we calculated the area (in hectares) covered by different landcover types within two buffer areas of 2 and 3km radius centered at each site. All map processing procedures were performed in ArcGIS 9.3. Based on ground-truthing, all maps’ accuracies were >90%.
We classified the different landcover types into six classes: (1) Native – dense ombrophilous forest, young secondary vegetation, pioneer vegetation and sandbank; (2) Anthropogenic – rural areas with buildings, leisure areas, transport infrastructures and coal extraction areas; (3) Exotic – Pine plantations (Pinus spp.), banana plantations and orchards; (4) Eucalyptus – Eucalyptus plantations (Eucalyptus spp.) – considered a different category from “Exotic” because it has been suggested that Eucalyptus plantations offer suitable sites for nesting for M. monachus (Volpe and Aramburú, 2011); (5) Agriculture – agricultural fields (mostly corn, beans, cassava, sugarcane, and tobacco) and irrigated rice; and (6) Neutral – areas naturally devoid of vegetation (rivers, lakes, Atlantic Ocean, areas of dunes and pastures).
To quantify which components of landscape composition most strongly affects the occurrence of the bird species, we adopted a multi-scale approach, as different aspects of landscape composition may influence populations at different scales (Boscolo and Metzger, 2009). To do so, we quantified land cover composition at two spatial scales larger than the surveyed area, 2 and 3km, corresponding to the radius of the circular buffer centered at each site, resulting in landscapes of 1256 and 2825ha, respectively (Fig. 2; Table S1). These scales correspond, respectively, to the areas of more intensive use by colonies of M. monachus and the mean home range size of the species (Spreyer and Bucher, 1998). The sizes of the circular landscapes were assumed as sufficiently large to detect a possible influence of landscape composition on the occurrence of the species. Larger buffer sizes were not tested to avoid overlap among different landscapes.
Data analysisThe dependent variable in all analysis was the occurrence (i.e., presence or absence) of M. monachus at each of the 24 sites. Predictor variables were chosen based on our initial hypotheses and the existing knowledge on the biology of the species, as detailed below. These variables corresponded to four of the six landcover classes (Native, Anthropogenic, Agriculture and Eucalyptus), and their values corresponded to the area covered by the respective landcover class within the circular buffer areas. As the correlations among predictor variables were low for both scales analyzed (2 and 3km radius; Spearman correlation ranks<0.5), all of them were used as potential explanatory variables of the occurrence of the species across the 24 landscapes.
To determine how landscape composition affects the occurrence of the species, we built 13 candidate explanatory models, representing different hypotheses. Models were chosen a priori, based on the hypotheses being tested and the biology of the species. A null model, containing only the intercept and error as parameters, was also included to check if the most plausible models incorporating landscape composition fit the data better than purely random factors.
To determine which variables and spatial scales best explain species occurrence, we built binomial generalized linear models (GLM), assuming a binomial distribution of errors and using the logit as the link function. The most parsimonious models were selected based on the lowest Akaike's information criterion corrected for small sample sizes (AICc; Burnham and Anderson, 2002). For each model, we also calculated Akaike's weight of evidence (wi), which represent the likelihood that the model i is the best approximating model among the candidate models to explain our data. All analyses were performed in R 3.0.1 (R Core Team, 2014). Based on the single most plausible model, which had two explanatory variables (see the Results), we calculated the predicted probability of occurrence of M. monachus as a function of each explanatory variable, using the “conditional” option in the package visreg (Breheny and Burchett, 2016) in R. This analysis facilitates visualizing how each component of landscape composition affects species occurrence (as in Boscolo and Metzger, 2011).
ResultsThe model containing the Anthropogenic and Agriculture variables, measured on the largest spatial scale (3km), was clearly the best model to predict the occurrence of M. monachus in landscapes (Δi=0, wi=0.94; Table 1). The second model was much less plausible and also included the Anthropogenic and Agriculture variables, but measured on the scale of 2km. The null model was clearly implausible (Δi=11.49, wi<0.01).
Performance of models describing the influence of landscape composition on the occurrence of Myiopsitta monachus across 24 landscapes. Model variables correspond to the amount of different landcover classes (Native, Agriculture, Eucalyptus, and Anthropogenic), measured at two spatial scales (circular buffer areas with 2 or 3km of radius). Models are ranked according to the Akaike's information criterion corrected for small samples (AICc). LogLike=model log-likelihood, Δi=AICci−AICcminimum, wi=Akaike's weight of evidence.
| Model variables | LogLike | AICc | Δi | wi |
|---|---|---|---|---|
| Anthropogenic+Agriculture (3km) | −8.38 | 23.97 | 0 | 0.94 |
| Anthropogenic+Agriculture (2km) | −12.55 | 32.31 | 8.34 | 0.01 |
| Anthropogenic (3km) | −14.17 | 32.91 | 8.95 | 0.01 |
| Agriculture (3km) | −14.38 | 33.34 | 9.38 | 0.01 |
| Eucalyptus+Agriculture (3km) | −13.59 | 34.38 | 10.41 | 0.01 |
| Agriculture (2km) | −14.97 | 34.51 | 10.54 | <0.01 |
| Anthropogenic (2km) | −15.13 | 34.83 | 10.86 | <0.01 |
| Null model | −16.64 | 35.45 | 11.49 | <0.01 |
| Eucalyptus+Agriculture (2km) | −14.28 | 35.77 | 11.8 | <0.01 |
| Native (2km) | −16.39 | 37.35 | 13.38 | <0.01 |
| Eucalyptus (2km) | −16.47 | 37.51 | 13.54 | <0.01 |
| Eucalyptus (3km) | −16.55 | 37.66 | 13.7 | <0.01 |
| Native (3km) | −16.63 | 37.84 | 13.88 | <0.01 |
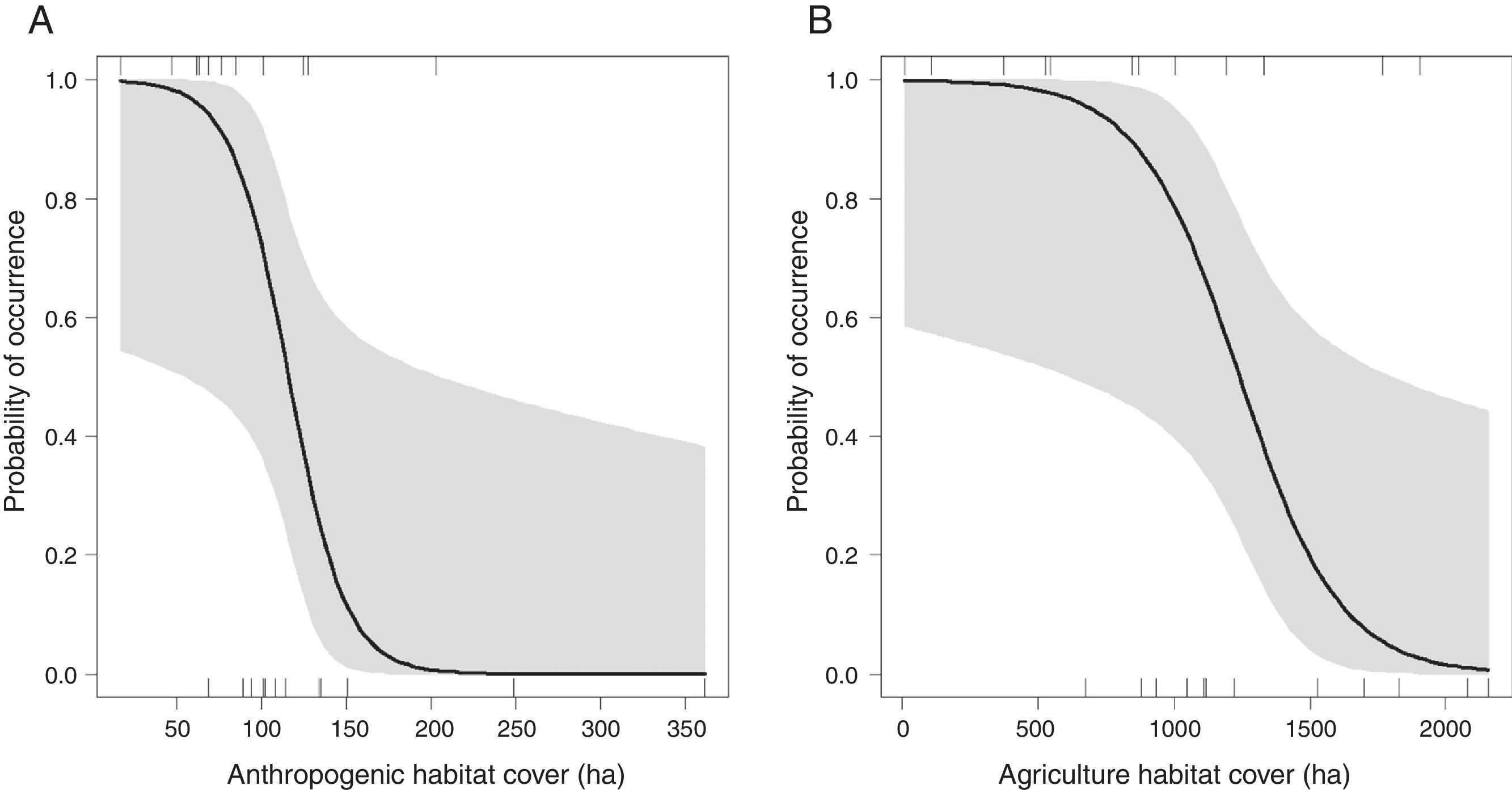
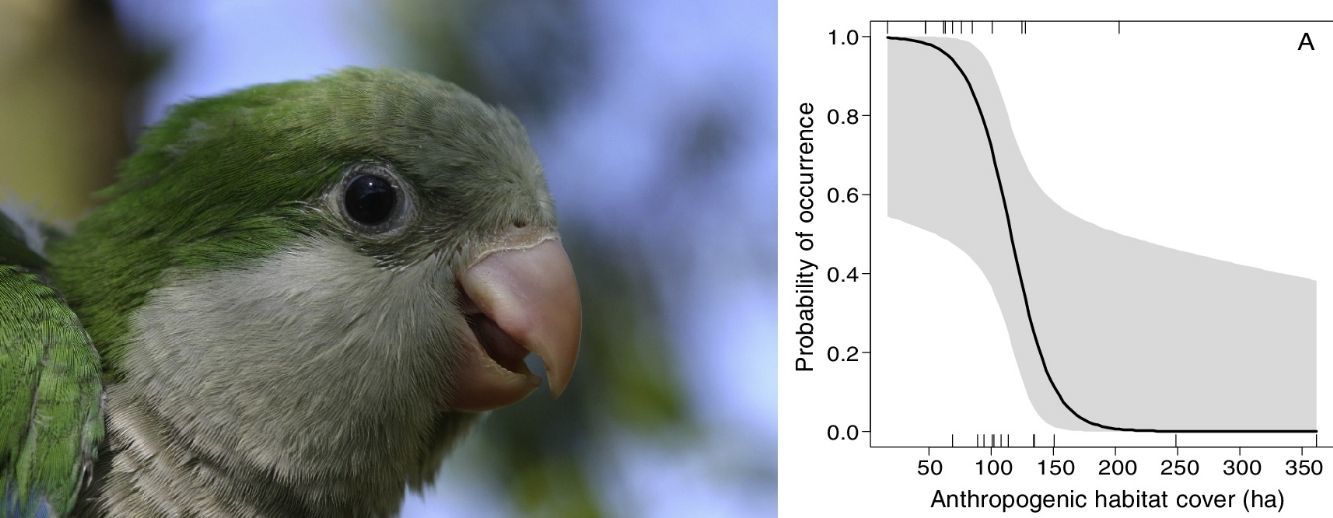
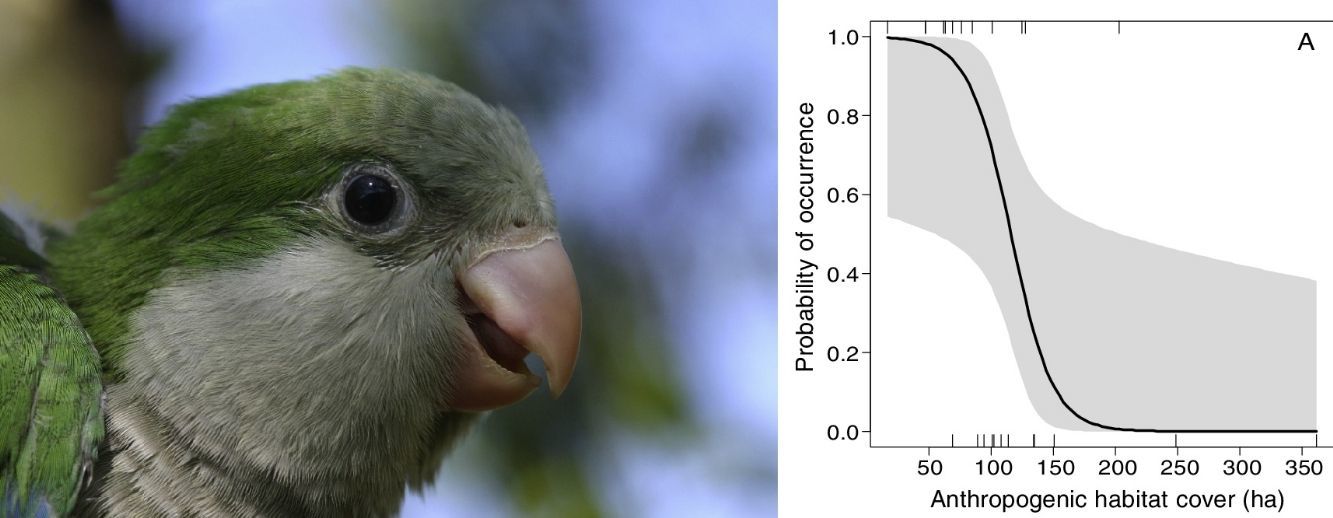
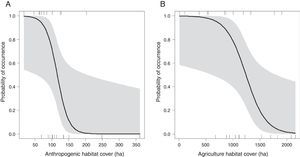
The occurrence of M. monachus was negatively affected by the increase in the area covered by the Anthropogenic class, with the species occurring only in landscapes with less than 200 hectares of anthropogenic area (Fig. 3A). Accordingly, the selected model (Anthropogenic+Agriculture (3km)) indicated that the increase in anthropogenic areas in the landscape reduces the probability of occurrence of the species (Fig. 3A).
Effects of the amount of anthropogenic (A) and agricultural areas (B) in the landscape on the occurrence of Myiopsitta monachus. The black line indicates the mean predicted probability of occurrence as a function of the amount of each habitat in the landscape, according to the most plausible model explaining species occurrence. This model included as explanatory factors two variables, the amount of anthropogenic habitat and the amount of agriculture habitat, measured at a circular landscape with 3km of radius. The gray area indicates the 95% confidence interval. The vertical lines in the rugged plot indicate the observed amounts of habitat in the 24 studied landscapes (12 with and 12 without M. monachus nests).
Similarly, the area covered by the Agriculture class had a negative effect on the occurrence of M. monachus. The species was always present when the area covered by Agriculture in the landscape was relatively small (<500ha; Fig. 3B), but was absent in the landscape when Agriculture area exceeded 2000ha (Fig. 3B). Accordingly, the selected model (Anthropogenic+Agriculture (3km)) indicated that the increase in agricultural areas in the landscape reduces the probability of occurrence of the species (Fig. 3B).
DiscussionOur study provides evidence that landscape composition may affect the occurrence of a widespread invasive species across landscapes of the Atlantic Forest hotspot. The occurrence of the Monk Parakeet, M. monachus, in landscapes located outside the native distribution range of the species, is negatively related to the amount of disturbed environments, more specifically the amount of anthropogenic and agricultural areas in the surrounding landscape. The role of landscape composition as a regulator of the occurrence of native species in landscapes has been demonstrated in many studies (Cushman and Mcgarigal, 2004; Pardini et al., 2010), but our study is one of the first to show that landscape composition may also affect patterns of presence or absence of invasive bird species in landscapes.
A common view in the literature on biological invasions is that human-disturbed areas are more susceptible to invasion (Song et al., 2005; Lockwood et al., 2007). However, in apparent contradiction to this hypothesis, the occurrence of M. monachus was negatively (rather than positively) correlated with the amount of agricultural and anthropogenic areas (Figs. 2 and 3). These areas are represented mostly by cultivated land, rural areas with buildings, roads and coal extraction sites, all of which probably do not provide suitable sites for foraging or nesting for the Monk Parakeet. Thus, an increase in these areas in landscapes is likely to reduce the amount of suitable habitat, which in the case of M. monachus is represented mostly by open areas with sparse trees (Bucher and Aramburú, 2014; Viana et al., 2016). Such habitats are present, for example, in urban parks and backyards, allowing the occurrence of M. monachus even in large urban centers (South and Pruett-Jones, 2000; Chace and Walsh, 2006). In the studied landscapes, the species builds nests only in sparse Eucalyptus trees within grassland areas (Viana et al., 2016). The amount of grassland areas in these landscapes is negatively correlated with the amount of agricultural plus anthropogenic areas (r=−0.70; p<0.001; N=24). This negative correlation potentially contributes to explaining the negative effects of agricultural and anthropogenic areas on M. monachus occurrence. Grassland areas, per se, are not a plausible explanatory variable in our dataset (I. R. Viana, unpublished data), probably because many of the grassland areas are devoid of sparse Eucalyptus trees. Unfortunately, scattered Eucalyptus trees are difficult to identify with the imagery available, precluding quantification of the coverage of grassland areas with Eucalyptus trees in the studied landscapes. Thus, we suggest, as a hypothesis for future studies, that the probability of occurrence of M. monachus in rural landscapes is positively related to the amount of grassland areas with sparse Eucalyptus trees.
Although a potential preference of M. monachus for building nests in Eucalyptus plantations has been suggested by previous studies (Volpe and Aramburú, 2011; Bucher and Aramburú, 2014), we did not find evident effects of the area covered by Eucalyptus plantations in the landscape on the occurrence of the species. This probably occurred because the species does not build its nest in continuous Eucalyptus plantations; in the 12 landscapes where M. monachus occurred, it built nests only in sparse Eucalyptus trees located in open areas (IR. Viana, unpublished data). Thus, the species is unlikely to be affected by the total area occupied by Eucalyptus plantations at the landscape scale, although individual Eucalyptus trees may be used and thus important at a local scale.
The amount of native habitat in the landscape also had no evident effect on the occurrence of M. monachus. As the species does not occur in the studied native habitats (IR. Viana, unpublished data), and it is generally expected that invasive species benefit from disturbed habitats (see Discussion above), we expected a negative effect of the amount of native habitat on its occurrence. The lack of important effects of the amount of native habitats may reflect the relatively heterogeneous nature of the studied landscapes, since the matrix is composed by a mosaic of different types of non-native areas (e.g., rural areas with buildings, agriculture, exotic plantation, rivers). The amount of native habitat may be more important in landscapes with a more homogeneous matrix (e.g., dominated only by grasslands), where it may have stronger, negative effects on the occurrence of this and other invasive species.
In our study, the model selection indicated that the occurrence of the species was more strongly related to landscape composition measured at the largest spatial scale analyzed (3km). This scale nearly corresponds to the home range of the species (3–5km radius; Martín and Bucher, 1993; Spreyer and Bucher, 1998; Silva et al., 2010). Thus, the 3-km scale is probably more likely to capture the influence of landscape composition on the establishment of individuals in particular sites. We recommend using this scale in attempts to quantify landscape composition and predict the occurrence of M. monachus across different landscapes.
In this study, we showed that landscape composition may be a useful predictor of the occurrence of invasive species across landscapes. We suggest that future studies adopt a landscape-ecology approach for the study of biological invasions, complementarily to more local and biogeographical approaches that are frequently used. The application of a landscape perspective to more species, at different stages of the invasion process and in different types of landscape, may enhance our ability to predict and mitigate the impacts of biological invasions on native habitats and species.
Conflicts of interestThe authors declare no conflicts of interest.
We thank two anonymous reviewers and Alexandre Uezu for valuables comments on a previous version of this paper and DS for English and style revision of this manuscript. IRV was supported with scholarships from Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina and Coordenação de Aperfeiçoamento Pessoal de Nível Superior. JAP was supported with a scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (process n. 2013/03457-1).