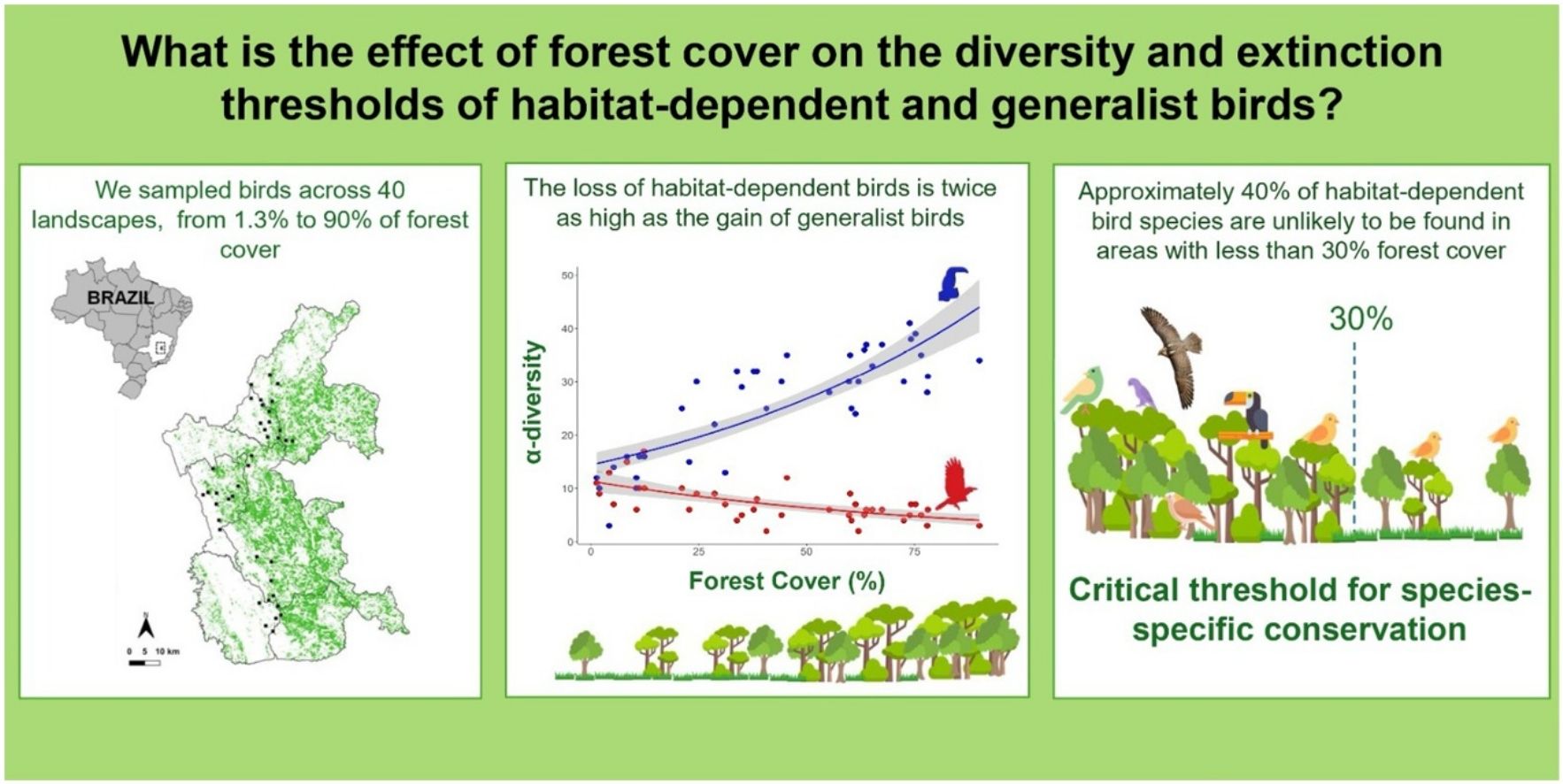
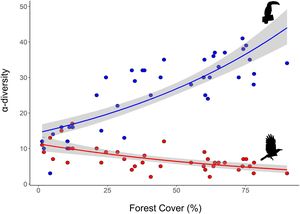
Species composition is influenced by the specific habitat requirements of each species. While habitat-dependent species are highly sensitive to deforestation, habitat-generalists are generally less affected. However, the effects of forest cover on determining species diversity and species-specific thresholds within remain poorly understood. In this study, we evaluate the impact of forest cover on the α- and β-diversity and species-specific threshold of habitat-dependent and habitat-generalist birds in the Brazilian Atlantic Forest. We sampled birds across 40 landscapes, ranging from 1.3% to 90% forest cover. Overall, our results demonstrate that a 10% reduction in forest cover is associated with the loss of two habitat-dependent species and the gain of one habitat-generalist species. We identified a critical species-specific threshold of 30% forest cover, where 18 out of 46 habitat-dependent bird species are lost. Our findings underscore the need to account for species requirements in response to habitat loss when planning conservation efforts. Thus, we suggest that a benchmark of 30% forest cover is more effective for conserving habitat-dependent birds than the 20% threshold currently proposed by Brazilian environmental law. We also suggest that incorporating species-specific extinction thresholds can serve as a powerful tool for shaping more targeted and effective environmental policies.
Over the past two decades, several studies have shown the ongoing effects of habitat loss on biodiversity (e.g., Banks-Leite et al., 2014; Boesing et al., 2018; Fahrig, 2003). Habitat loss affects several biological processes, such as dispersal, intra/interspecific interactions, extinction and colonization rates (Püttker et al., 2020; Thompson et al., 2017). Consequently, habitat loss is reported as the main cause of changes in community composition between different landscapes (Roque et al., 2018; Si et al., 2016).
Empirical studies have shown that a minimum amount of native habitat cover is critical to ensure the maintenance of species richness and ecosystem functions (i.e., extinction thresholds: Pardini et al., 2010; Banks-Leite et al., 2014; Brejão et al., 2018). Thus, ensuring a suitable habitat amount is critical to avoid compositional shifts in species assemblages in working landscapes (Baguette et al., 2013; Cardinale et al., 2011). In this context, most studies in tropical regions have identified an abrupt shift in communities with thresholds ranging from 50% to 20% of habitat cover (Pardini et al., 2010; Banks-Leite et al., 2014; Boesing et al., 2018). Although thresholds stand out as a valuable tool to determine conservation targets, few studies have assessed them at the species level (but see Brejão et al., 2018), considering the species-specific traits and species-specific responses to habitat change.
Besides knowing about the differences in community composition between landscapes, i.e., β-diversity, it is essential to assess the drivers of this diversity component. Different mechanisms can be accessed to infer the processes shaping changes on β-diversity, including species gain/loss (βrich) and replacement (βrepl) (Montaño-Centellas et al., 2021). In βrich, β-diversity is explained by processes related to environmental filtering, in which a decrease or increase in species number follows changes in environmental conditions across landscapes (Soininen et al., 2018). Distinctly, in βrepl, β-diversity is explained by processes related to species interactions or random events, in which different landscapes can sustain a similar number of species, but with different traits (Cardoso et al., 2014; Si et al., 2016; Montaño-Centellas et al., 2021). Thus, accessing the species loss/gain and species replacement may unravel the dynamic of communities along with directional gradients such as habitat cover loss (Si et al., 2016; Montaño-Centellas et al., 2021).
In general, habitat-dependent bird species are more sensitive to habitat loss due to their higher dependency on specific resources and/or environmental conditions and lower dispersal capacity (Si et al., 2016; Neate-Clegg, 2024). Therefore, their distribution is mainly restricted to areas with higher habitat cover (Martensen et al., 2012). On the other hand, habitat-generalist species are generally less affected by changes in habitat suitability due to their higher resilience to anthropogenic changes and dispersal capacity (Carrara et al., 2015; Liao et al., 2017). As a result, habitat loss may impose varying limitations on the occurrence of different species (Liao et al., 2017; Boesing et al., 2018).
Here, we investigated how habitat loss impacts both α-diversity (local species richness) and β-diversity (compositional differences between sites) of habitat-dependent and habitat-generalist bird species. We hypothesize that (I) habitat cover affects positively α-diversity of habitat-dependent species and has no or negative effects on habitat-generalists. (II) Differences in habitat cover have a higher effect on β-diversity of habitat-dependent than habitat-generalist species because habitat-dependent species are more sensitive to habitat changes than habitat-generalists. (III) Species gain/loss (βrich) will succeed over the replacement (βrepl) component of β-diversity along the habitat cover gradient. Finally, (IV) we hypothesize that extinction thresholds will be species-specific. Evaluating such thresholds at the species level can contribute to developing more effective management strategies.
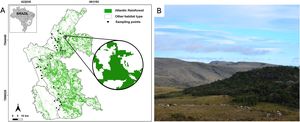
MethodsStudy areaWe conducted this study at the Espinhaço Mountain Range in the state of Minas Gerais, southeastern Brazil (19°07’01” – 18°48’24” S and 43°43’49” – 43°74’22” W; Fig. 1) within the Atlantic Forest range – a highly threatened and fragmented biome that had already lost ∼ 72% of its original distribution (Rezende et al., 2018). The studied Atlantic Forest remnants are embedded in a mix of natural grassland (campo rupestre; rocky outcrops grasslands) and managed pastures. The forest remnants are distributed in continuous and naturally fragmented forest patches forming forest islands (Fig. 1a). In these cases, the forest islands are typically found in erosion valleys devoid of boulders and are generally associated with headwaters and small streams along the edges of campo rupestre formations. (Coelho et al., 2018a). Although the region exhibits some level of natural fragmentation, the existing fragments have experienced significant anthropogenic degradation over recent decades, particularly through size reduction due to cattle ranching (Coelho et al., 2018b; Kuchenbecker et al., 2023). The surveyed forest fragments are located between 900 and 1400 m a.s.l. The climate is type Cwb (subtropical highland climate with dry winters) according to Köppen’s classification. Average daily temperature varies seasonally, ranging from 14.7 to 30.3 °C in the summer (wet season) and from 9.2 to 29.5 °C in the winter (dry season; da Silva et al., 2019).
Study area in the Brazilian Atlantic Forest within the Espinhaço Mountain Range in Minas Gerais state. (A) Map of the area showing the forest remnants (green) and the 40 sampling points (black squares). The zoom-in circle represents a 1000 m-radius buffer around a sampling point (forest patch). B) Illustration of the landscape with one of the surveyed forest patches. Photo credits: Paulo Siqueira.
We selected 40 forest fragments at least 1000 m from each other (Supplementary Table S1). For each fragment, we assessed the forest cover in a buffer of 1000 m radius (hereafter referred to as landscapes; Fig. 1a). We used a 1000-m radius since this value corresponds to the range of movement of most bird individuals within the same forest fragment (Marini, 2010). Given the strong correlation between forest cover and connectivity (Villard and Metzger, 2014), we adopted forest cover as a parsimonious yet ecologically meaningful metric. Forest cover was calculated using land-cover and land-use maps from the MapBiomas project, analysed with the plugin LecoS (Jung, 2016) in the QGIS software (QGIS Development Team, 2021). The landscapes used ranged from 1.3% to 90% of forest cover.
Bird species sampling and classificationWe surveyed bird communities at each forest fragment with point counts (Bibby et al., 2000). At each one of the 40 forest fragments, we established one point count in the interior of the fragment at least 50 m from any forest edges. The point count method consists of observing and hearing individuals of bird species at each sampling point for 10 min over a 50-m radius. Only individuals perching and/or singing within the detection radius were considered. Birds observed above the canopy were not considered. To enhance cryptic and rare species detection, we resampled each point count four times from July 2020 to May 2021 (two times in the dry season and two times in the wet season). Surveys were conducted during the first 5 h after sunrise (between 5:00 and 10:00 AM), since this is the period of highest bird activity (de Araújo et al., 2021). Samplings were not performed under rainy conditions.
We consulted specialized literature to classify species into habitat-dependent or habitat-generalists (Silva, 1995; Alexandrino et al., 2016). Habitat-dependent species (forest-dependent) occur preferably in the forest interior and rarely occur in other environments (Morante-Filho et al., 2016). Habitat-generalist species occur in both primary and secondary forests (especially at edges) and open areas (Morante-Filho et al., 2016).
Data analysisWe estimated sampling sufficiency as the sum of data on habitat-dependent and habitat-generalist bird species recorded across the four sampling campaigns by constructing rarefaction curves and extrapolating (double) the total abundance data (Chao et al., 2014). We calculated sampling coverage by using Hill numbers of q = 0 (species richness) to estimate sampling sufficiency (Chao et al., 2014). We performed this analysis using the “iNEXT” R package (Hsieh et al., 2016).
To address how habitat-dependent and habitat-generalist α-diversity (species richness) respond to variations in forest cover, we used two generalized linear models (GLMs). We modelled habitat-dependent α-diversity using a negative binomial error distribution, and habitat-generalist α-diversity using a Poisson distribution to control for overdispersion (quasi-Poisson). We selected the best distribution errors for each model based on data assumptions (Olsson 2002) and verified model residuals (Crawley, 2012) using the “RT4Bio” R package (Reis Jr et al., 2015).
We estimated differences in habitat-dependent and habitat-generalist bird species composition by calculating the β-diversity and its components (Podani and Schmera, 2011; Legendre, 2014), and we tested its response to habitat coverFor this approach, we first calculated the Sorensen’s dissimilarity index using the “BAT” package (Cardoso et al., 2015) since it gives higher weight to species shared among sampling units. We then decomposed the total β-diversity (Sorensen’s dissimilarity) of habitat-dependent and habitat-generalist assemblages into richness difference (βrich) and species replacement (βrepl) (Legendre, 2014) to assess which of the two components is responsible for differences in bird composition (β-diversity) associated with forest cover. Finally, we used partial Mantel tests to analyze whether differences in β-diversity and the components (species replacement and richness difference) were either positively or negatively correlated with the matrix of forest cover variation (calculated using Euclidean distance). Partial Mantel tests were also used to verify spatial autocorrelation between sampling locations using “vegan” R package (Oksanen, 2019).
To assess species-specific thresholds across the forest cover gradient, we performed the Threshold Indicator Taxa Analysis (TITAN; Baker and King, 2010) using the “TITAN2” R package (Baker et al., 2015). TITAN detects changes in species distribution across environmental gradients, in our case the forest cover gradient. Such changes are assessed by indicator-species scores (IndVal), which compare the frequency of species records among sampling points. We then used bootstraps to calculate the confidence interval of the location of change points for each taxon along the forest cover gradient (Baker and King, 2010). We performed all analyses in R software version 4.21 (R Development Core Team, 2021).
ResultsWe obtained 3662 records from 132 bird species. Of these, 86 species (65.2%) were classified as habitat-dependent and 46 (34.8%) as habitat-generalist (Supplementary: Table S2). The Golden-crowned Warbler (Basileuterus culicivorus) was the most frequently recorded bird species (n = 403).
As expected, we found opposite effects of forest cover on α-diversity of habitat-dependent and habitat-generalists. The decrease of forest cover across sampling points negatively influenced α-diversity of habitat-dependent bird species (R² = 0.58, p < 0.001) and positively influenced α-diversity of habitat-generalist species (R2 = 0.42, p < 0.001) (Fig. 2).
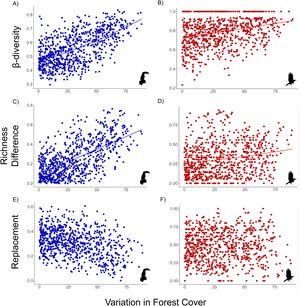
The β-diversity of habitat-dependent (R2 = 0.6, p = 0.001) and habitat-generalist (R2 = 0.28, p = 0.001) assemblages was positively correlated with variation in forest cover (Fig. 3a, d). Within the β-diversity partitioning, the βrich component was positively associated with forest cover for both habitat-dependent (R2 = 0.62, p = 0.001) and habitat-generalist (βrich: R2 = 0.19, p = 0.003) (Fig. 3b,e). The βrepl component did not exhibit significant relationships with the variation in forest cover for both habitat-dependent (R2 = −0.35, p > 0.05) and habitat-generalist species (βrepl: R2 = 0.02, p > 0.05) (Fig. 3c,f).
Relationship between the variation of forest cover and the β-diversity (A and B) and its components – richness difference (βrich: C and D) and species replacement (βrepl: E and F) – for habitat-dependent (blue dots) and habitat-generalist (red dots) assemblages. Solid line: p < 0.05; no line: p > 0.05.
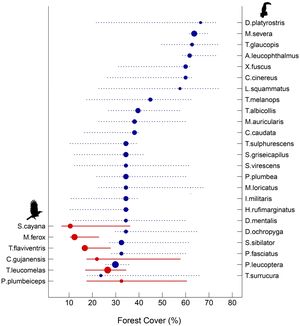
For the species-specific threshold across the forest cover gradient, 30 bird species (23% of the total) showed significant changes, of which 24 presented a negative response to decreased forest cover and six presented positive responses (Fig. 4). Overall, habitat-dependent species showed negative responses, with the greatest changes in distribution values found for the Planalto Woodcreeper (Dendrocolaptes platyrostris) and the Tufted Antshrike (Mackenziaena severa), both showing distribution species-specific thresholds higher than 60% of forest cover (Fig. 4, Supplementary: Table S3). Conversely, all species that had a positive response to forest cover loss were habitat-generalists. The lowest values were found for the Burnished-buff Tanager (Stilpnia cayana) and the Short-crested Flycatcher (Myiarchus ferox), which showed distribution species-specific thresholds at 12% forest cover (Fig. 4, Supplementary: Table S3).
Threshold Indicator Taxa Analysis (TITAN) of 30 bird species that showed significant changes in frequency and abundance over the forest cover gradient. Blue circles represent negative (z−) indicator taxa associated with habitat-dependent species affected by forest cover (right axis), while red circles represent positive (z+) indicator taxa linked to habitat-generalist species in response to forest cover (left axis). Circles are sized in proportion to z scores. Lines overlapping each circle represent 5 and 95% percentiles among 500 bootstrap replicates.
We found that the loss of habitat-dependent species and gain of habitat-generalist species following forest loss was the primary cause of the increase in β-diversity, being higher for habitat-dependent species. Therefore, there is no sufficient compensatory gain or replacement of habitat-dependent bird species by habitat-generalist species in highly deforested landscapes. We also found that 30 bird species (23% of all species) showed species-specific thresholds, from which 18 exhibited a significant reduction in their occurrence in landscapes with less than 30% of forest cover.
The absence of sufficient compensatory gain or replacement of habitat-dependent by habitat-generalist birds represents a novel finding that has not been previously reported, particularly in highly deforested landscapes (e.g., Morante-Filho et al., 2015, 2016, 2018). We demonstrate that habitat-dependent bird species decline twice as rapidly as habitat-generalist species increase for every 10% reduction in forest cover. Many studies have linked changes on β-diversity following habitat loss to species replacement only (Morante-Filho et al., 2015, 2016). However, without analysing partitioned β-diversity, such inferences may be problematic, as in addition to replacement, species loss or gain may also be associated with community changes.
As expected, differences in forest cover had a higher effect on β-diversity of habitat-dependent than habitat-generalist species. Given that species loss/gain is the main cause of differences in β-diversity along the forest cover gradient, environmental filtering seems to be a deterministic factor playing a great role in driving the dynamics of the studied assemblages. Habitat loss has a large effect on the movement and establishment of habitat-dependent birds, i.e. dispersal events, making them more susceptible to local extinction events by either deterministic or stochastic processes (Uezu and Metzger, 2011; Saura, 2021). Therefore, such populations tend to be smaller or absent in highly deforested landscapes (Si et al., 2016; Liao et al., 2017). On the other hand, habitat-generalist species show higher dispersal capacity in open areas (Morante-Filho et al., 2016), and also tend to be superior competitors when searching for resources in areas with decreased native habitat cover (Wiens et al., 2010). Thus, landscapes subjected to structural changes due to reduced forest cover tend to be hostile for many habitat-dependent species, but suitable to most habitat-generalist species. The degree of change in forest cover may determine species-specific thresholds and might thus lead to abrupt shifts in species composition (Püttker et al., 2020). Hence, we corroborated our hypothesis of the existence of individual species extinction thresholds across the forest cover gradient. The distribution of species-specific thresholds ranged from ∼73% to ∼23% among habitat-dependent species and from ∼32% to ∼10% among habitat-generalist species. We found 22 species with threshold values higher than 30% of forest cover; eight of them showed values higher than 50%. A study using other taxa (fishes) showed variation in species-specific thresholds, in which more sensitive species showed thresholds less than 20% forest cover loss (Brejão et al., 2018). These results demonstrated that community-level thresholds might mask the response of more sensitive species for which the thresholds are always high, such as the endemic Tufted Antshrike (Mackenziaena severa).
Implications for conservationWe emphasize that conservation measures considering habitat-dependent species instead of community-wide approaches can better identify the needs of more sensitive species. In addition, our findings unravel that habitat-dependent and habitat-generalist assemblages yield contrasting species composition patterns in response to habitat loss. Thus, considering community-wide approaches might lead to biased conservation strategies, as convergent species responses may obscure or moderate compositional shifts in biological communities (Brant et al., 2021; da Silva et al., 2019). Furthermore, partitioned β-diversity effectively captures changes in bird assemblages along forest cover gradients. We highlight the importance of species-specific thresholds over community-wide approaches to accurately link forest cover loss with species declines. This method enables targeted conservation strategies, which are crucial for protecting biodiversity in fragmented ecosystems, especially for the most threatened species.
While the loss of habitat-dependent species cannot be compensated for habitat-generalists, the latter may thrive in more fragmented or deforested landscapes. This shift carries implications for ecosystem functioning and the delivery of ecosystem services (Gardner et al., 2019). For instance, habitat-generalist bird species have a key role in restoring degraded areas since they are great seed dispersers – moving seeds from more source patches to more deforested areas (Carlo and Morales, 2016). In this study, we observed several habitat-generalist species that are seed dispersal agents and can help in the environmental restoration process, including the Purple-throated Euphonia (Euphonia chlorotica), the Pale-breasted Thrush (Turdus leucomelas), and the Creamy-bellied Thrush (Turdus amaurochalinus). Additionally, all recorded habitat-generalist species showed no exclusive association with grasslands or rocky outcrops, with the sole exception of the Tropical Kingbird (Tyrannus melancholicus) – a generalist species observed twice in forest-fragment clearings. Therefore, these species may also be especially relevant in keeping the functional connectivity between forest patches (Baguette et al., 2013).
Environmental changes due to forest loss, which may also impact forest microclimate, is associated not only with the exclusion of species – mainly habitat-dependent – but also, to changes in ecosystem services. For instance reduction of frugivorous species may change the forest dynamics, including reduced carbon sequestration and decreased food availability for more specialized species (Gardner et al., 2019). We found that major recognized seed dispersers from the Atlantic Forest, such as the Red-ruffed Fruitcrow (Pyroderus scutatus) and Dusky-legged Guan (Penelope obscura), were absent in landscapes with less than 30% of forest cover. Therefore, these more sensitive species should be of special concern for conservation measures in the region.
This study examined both landscapes with fragments formed by human activities and those naturally fragmented, but which have been transformed by anthropogenic pressures over the past century (Coelho et al., 2018b; Kuchenbecker et al., 2023). In fact, approximately half of the studied landscapes exhibited more than 40% of human-modified land uses (Supplementary Table S1), which have also affected forest corridors and gallery forests by decreasing connectivity between remaining forest fragments (Coelho et al., 2018b). While forest cover served as our primary landscape metric and correlates strongly with other landscape characteristics (Villard and Metzger, 2014), we recognize that connectivity and other spatial factors may also influence the distributions of some species. For example, the occasional presence of the Cerrado-associated Helmeted Manakin (Antilophia galeata) in three study landscapes suggests potential connectivity between the Cerrado and the Atlantic Forest biomes. Our results indicate that while forest cover effectively predicts species presence, other fragment characteristics (e.g., connectivity) may also be ecologically significant for certain species and should be considered in future research.
The Brazilian Forest Code (Law 12.651/2012) determines that all rural properties within the Atlantic Forest domain must maintain a legal reserve with native vegetation, accounting for 20% of the total property size. We found that 18 (13.6%) of habitat-dependent species show distribution thresholds above 30% of forest cover. Regarding community thresholds, several other studies have also reported such tipping points above the 30% benchmark (Martensen et al., 2012; Boesing et al., 2018). Thereby, the benchmark of 20% of forest cover established by Brazilian law may not provide sufficient protection for a large number of habitat-dependent bird species. Therefore, we highlight the importance of evidence-based laws and suggest the development of other studies evaluating the extinction threshold to support decision-making.
Declaration of Generative AI and AI-assisted technologies in the writing processAs non-native English speakers, during the preparation of this work the authors used ChatGPT and Grammarly to further correct grammar and enhance the readability of specific sections of the manuscript. The authors take full responsibility for the content of the publication.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the financial support by Funbio (01/2019) and Rufford Foudation (31129-1). Additionally, we extend our thanks to Lorena Bueno, Matheus Belchior, Vanessa Monteiro, Marina Lodi, Karen Caldeira and Pedro Anselmo for their fieldwork support. We are also grateful for the insightful comments on the earlier versions of the manuscript provided by Dr. Marco Aurélio Pizo, Dr. Marcelo Vasconcelos, Dr. Milton Barbosa Junior, Wallace Beiroz Imbrosio da Silva, Dr. Eliana Cazetta and Dr. Paloma Marques Santos. PRS would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship. FSN acknowledges the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 306218/2023-2) for the fellowship grants. TVF acknowledges the financial support from the - CNPq (428298/2018-4) andCAPES (PDPG-13179).