One goal of ecological restoration is species conservation, so selecting tree species from local floras in restoration plantings is important to restore native species populations and avoid biotic homogenization. We evaluated if species planted to restore the Brazilian Atlantic Forest adequately represent the tree flora from local reference forests, comparing the tree seedlings selected for 1,073 restoration plantings with inventory data from 268 forest remnants, for three different Atlantic Forest types. We compared the floristic composition between plantings and remnants and calculated the Jaccard dissimilarity index to assess beta diversity among plantings, among remnants, and between plantings and remnants. Overall, plantings have lower beta diversity and higher nestedness than remnants. Furthermore, plantings form a single floristic group while remnants are split into three forest types. Plantings are more floristically similar to one another than to regional remnant forest types. Tree species selected for Atlantic Forest restoration poorly represent local floras, which could favor biotic homogenization. Incentivizing greater representation of local floras and threatened and endemic species is needed for forest restoration to facilitate biodiversity recovery at large spatial scales.
The reference ecosystem concept in ecological restoration is often defined based on one or a few reference sites (Gann et al., 2019), but ecosystems vary continuously over space, leading to natural differences in species composition across regions, i.e. beta diversity (Condit et al., 2002; Rosindell et al., 2012). Thus, a relevant question is how well restoration recreates beta diversity patterns at subcontinental scales. This question is timely as ecological restoration because ecological restoration is applied at increasingly large spatial scales (Aronson et al., 2020).
The representativeness of native species in ecological restoration initiatives is an issue that has been concerning the scientific community. Studies have identified that rare, endangered, and other groups of species are vulnerable in remaining forest fragments and not able to colonize areas undergoing restoration (Engert et al., 2020). In degraded and fragmented forest landscapes, planting native tree seedlings is a common and sometimes necessary restoration strategy (Rodrigues et al., 2009; Chazdon et al., 2021). These plantings introduce tree species with the objective of restoring basic ecological processes, such as the re-creation of a forest structure, elimination of invasive species, attraction of seed dispersers, and thus the promotion of natural regeneration processes (Holl, 1999). Studies have identified, however, that the arrival of new species in restoration plantings can take a long time or fail to occur (Reid et al., 2015; Shoo et al., 2015), so some species must be actively introduced. If they are not, their populations will be confined to dwindling and often degraded forest remnants (Suganuma and Durigan, 2021). However, current restoration practices are characterized by poor representation of the full set of regional species in the pool of species planted (Jalonen et al., 2018; Ladouceur et al., 2018; Almeida et al., 2024). Most planted species are abundant in forest remnants and are dispersed by wind rather than by animals (Brancalion et al., 2018; Engert et al., 2020). In addition, most planted species are pioneers with small seeds and rapid growth (Holl et al., 2017; Brancalion et al., 2018).
Biotic homogenization is the process of gradual replacement of endemic species by widespread ones over time (Olden and Rooney, 2006), and it can occur either through the increase in the abundance of invasive or generalist species (Beauvais et al., 2016). Thus, if restoration initiatives promote planting a small set of species throughout a region, and colonization by species from the surrounding native sites is limited, the landscape can become dominated by a restricted set of species, leading to biotic homogenization (McKinney and Lockwood, 1999; Olden and Rooney, 2006). Although the process of biotic homogenization is known for some regions, such as the Atlantic Forest (Zwiener et al., 2018; de Lima et al., 2020), we lack a comprehensive understanding on how restoration efforts may affect this process. This evaluation is important to define strategies and public policies to make restoration initiatives more effective in promoting regional biodiversity conservation.
The Atlantic Forest is a highly diverse but highly fragmented biome, with less than 16% of the original forest cover remaining, which is concentrated in small and isolated remnants (Ribeiro et al., 2009). To reverse this scenario, the Atlantic Forest has been undergoing large-scale restoration in recent decades (Rodrigues et al., 2009; Brancalion et al., 2013). We evaluated whether lists of species planted for restoration in the Brazilian Atlantic Forest represent the natural variation in tree species composition found in nearby forest remnants. We hypothesized that plantings are more floristically similar to each other than to forest remnants due to limitations imposed by the species pool currently available for planting (i.e., trees in nurseries; Ladouceur et al., 2018) and because some species are widely used in restoration plantings across the Atlantic Forest. Thus, we expected a lower beta diversity among and within restoration plantings compared to native forest remnants.
Material and methodsStudy region and databaseWe used a database containing lists of seedling species from 29 nurseries selected for restoration plantings in 1,073 Atlantic Forest sites planted in 2002–2018 (Fig. 1, Table S1). Plantings were done through the “Click Árvore” and “Florestas do Futuro” restoration programs, coordinated by the Brazilian NGO SOS Mata Atlântica (https://www.sosma.org.br). Each list in the database contained the number of seedlings per species destined for planting in each site. As such, the database did not describe species performance in the field or species that successfully established or colonized sites naturally. Although we investigated planting lists and not the complete tree composition (including natural regeneration) some years after planting, our approach is relevant because planted trees may impact future composition in fragmented and long-disturbed regions, such as the Atlantic Forest, where seed dispersal, and consequently natural regeneration, may be limited in space and time (Holl, 1999).
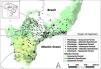
Location of Atlantic Forest plantings and remnant forests included in this study. Plantings and remnants lying outside of the Atlantic Forest official limits in the map are small enclaves of Atlantic Forest in other floristic regions. Adapted from Almeida et al. (2024).
We used data from remnant Atlantic Forest fragments in the Neotropical Tree Communities database (TreeCo) as reference ecosystems for species composition. This database compiles observations of individual tree species’ abundances in inventories of eastern South American vegetation (de Lima et al., 2015, 2020). From TreeCo, we selected 268 forest surveys (total of 286,660 trees measured), which (1) included trees with diameter at breast height ≥5 cm, (2) were conducted in fragments without strong disturbances, and (3) had clear information on the forest type surveyed. Remnants had from 0.1 to 5.04 ha of sampling area (Table S2). We included surveys in three Atlantic Forest types: rainforest, seasonal forest, and Araucaria forest. These three forest types, which are the major ones in the Atlantic Forest biome, differ in temperature, rainfall regime, latitude, altitude and, therefore, also in their tree species composition (Oliveira‐Filho and Fontes, 2000). Based on the TreeCo vegetation type classification, our dataset contained 85 rainforest, 27 Araucaria forest, and 156 seasonal forest fragments. We classified restoration plantings as one of the three forest types by placing their geographical location into the official delimitation map of the Atlantic Forest regions in Brazil, produced in accordance with the Brazilian Atlantic Forest Law (11428/2006). This resulted in 81 restoration plantings in rainforest, 958 in seasonal forest, and 34 in Araucaria forest.
Data analysisTo assess if forest restoration plantings are floristically more similar to themselves than to remnant forest, we analyzed their beta diversity, i.e., the dissimilarities among species composition in restoration plantings and forest remnants across space. Beta-diversity analyses were performed by comparing species composition between all pairs of sites, totaling 898,470 pairwise values. In this analysis, individual sites represented replicates of alpha diversity. Additionally, for an overview of beta diversity at a larger scale, we also calculated beta diversity at the level of forest type, using the compiled list of species in each of the six combinations of forest type (rainforest, seasonal forest, or Araucaria Forest) and land use (planting or remnant) as a replicate of alpha diversity.
To estimate beta diversity, we used the Jaccard dissimilarity index, which is based on the presence or absence of a given species in a site. The Jaccard dissimilarity index ranges from 0 when two samples have identical species lists to 1 when no species are shared. We also tested the abundance-based Bray Curtis index; however, we decided to use the Jaccard dissimilarly index because (1) both returned similar overall results, (2) partitioning of beta diversity into species replacement or subsets of species from richer sites is more common and easier to understand in presence-absence indexes; and (3) the use of a presence-absence index may better represent the species composition of tree plantings where survival was <100%. To further understand how species composition varied among groups, we split the values of beta diversity obtained from the Jaccard dissimilarity index into turnover, which represents the replacement of some species by others, and nestedness, that is, difference due to subsets of species composition from sites with greater richness (Baselga, 2010). We used the function “beta.pair” in the betapart R package to run the beta diversity analysis (Baselga and Orme, 2012).
To assess the ordination of plantings and remnants according to their floristic composition, we used non-metric multidimensional scaling (NMDS). We used the Jaccard dissimilarity matrixes as inputs for plotting an NMDS using the three forest types (rainforest, seasonal forest, and Araucaria forest) for plantings and remnants. NMDS is an ordination method that plots dissimilar objects far apart in the ordination space and similar objects close to one another, thus it preserves the ordering relationships among objects (Legendre and Legendre, 2012). Ordinations were plotted using the vegan package’s “metaMDS’’ procedure with the maximum number of runs set to 100 (Oksanen et al., 2022).
We compared between-group dissimilarities using the non-parametric permutational test PERMANOVA, implemented using the “adonis’’ procedure in the vegan package in R (Anderson, 2001). We set maximum number of runs to 999 and used Bonferroni corrections to adjust p-values in multiple comparisons. All analyses were performed in R version 4.3.0. (R Development Core Team, 2023) and interpreted following language of evidence (Muff et al., 2021), where p values < 0.001 represent very strong evidence, 0.001–0.01 strong evidence, 0.01–0.05 moderate evidence, 0.05–0.1 weak evidence, and 0.1–1 no evidence.
ResultsWe found 423 species in the plantings and 1,891 in the remnants, totaling 1,954 species, with 81% of the species found in remnants absent in plantings (Table S3). In the site-to-site comparison for the whole Atlantic Forest (without splitting into forest types), the mean Jaccard dissimilarity index for remnants is 0.91 while for plantings it is 0.70 (Fig. 2A). In addition, beta-diversity is higher for planting-remnants and remnants-remnants comparisons than it is for planting-planting comparisons (Table S4). These results indicate that overall remnants are more floristically diverse than plantings.
Boxplot of beta diversity expressed by the Jaccard dissimilarity index and its turnover and nestedness values for plantings (orange) and remnants (green) for the whole Atlantic Forest (A–C) and for its three different forest types: Araucaria forest (AR, D–F), rainforest (RF, G–I), and seasonal forest (SF, J–L). In the analysis, each site (planting or remnant) was compared for beta diversity with each other site.
In site-to-site comparison within forest types, the mean Jaccard dissimilarity index is higher for remnants than for plantings in the three forest types (Fig. 2, Table S5), indicating that remnants are more floristically diverse than plantings across the Atlantic Forest biome. Turnover values are higher than nestedness (Fig. 2, Table S5), which indicates that at the scale of individual sites, dissimilarities in tree species composition are more related to species replacement from one site to another than to representation of species subsets.
At the higher level comparing the entire flora of different forest types, again remnants have higher beta diversity and turnover than plantings (Figure S1). However, at this scale, turnover is the main component of beta diversity only for remnants while for plantings it is nestedness (Figure S1). Turnover is also higher than nestedness when plantings are compared to remnants of the same forest type, except for the seasonal forest (Table S6). In addition, when we compare seasonal forest remnants to Araucaria forest and rainforest plantings, we unexpectedly also have a higher nestedness than turnover (Table S6), suggesting that a subset of the seasonal forest flora is predominating in the restoration of all forest types.
Our NMDS analysis split remnants into the three forest types: rainforest, seasonal forest, and Araucaria forest, but grouped all plantings together (Stress = 0.165, Fig. 3). In addition, we found strong evidence that forest type, land use (planting or remnant), and their interaction influence the floristic dissimilarities between groups (Table 1). Thus, plantings form a single group that was not differentiated by the regional forest type associated with their geographic location. In other words, plantings in one forest type are more floristically similar to plantings in other forest types than they are to forest remnants within their own forest type.
Results of the permutational multivariate analysis of variance (PERMANOVA) using Jaccard dissimilarity index. The PERMANOVA test used 1,000 simulations based on species data sets to detect differences in groups of rainforest, seasonal forest and Araucaria forest restoration plantings and remnants in the Atlantic Forest in Brazil. The p-value corresponds to the PERMANOVA testing for significant difference between groups: ***very strong evidence (p < 0.001); * moderate evidence (0.01 > p < 0.05). Bonferroni correction was used for adjusting p-values in multiple comparisons.
| Comparison | d.f | F | R2 | p-value |
|---|---|---|---|---|
| Type of forest (Araucaria forest, rainforest, or seasonal) | 2 | 18.11 | 0.02 | 0.001*** |
| Land use (planting, remnant) | 1 | 119.94 | 0.09 | 0.001*** |
| Type of forest vs. land use | 2 | 9.91 | 0.01 | 0.001*** |
| Araucaria forest planting vs. Araucaria forest remnant | 20.24 | 0.26 | 0.015* | |
| Araucaria forest planting vs. rainforest planting | 1.78 | 0.02 | 0.465 | |
| Araucaria forest planting vs. rainforest remnant | 22.04 | 0.16 | 0.015* | |
| Araucaria forest planting vs. seasonal forest remnant | 16.30 | 0.08 | 0.015* | |
| Araucaria forest planting vs. seasonal forest planting | 1.48 | 0.001 | 1.000 | |
| Araucaria forest remnant vs. rainforest planting | 22.14 | 0.17 | 0.015* | |
| Araucaria forest remnant vs. rainforest remnant | 9.64 | 0.08 | 0.015* | |
| Araucaria forest remnant vs. seasonal forest planting | 32.36 | 0.03 | 0.015* | |
| Araucaria forest remnant vs. seasonal forest remnant | 10.14 | 0.05 | 0.015* | |
| Rainforest planting vs. rainforest remnant | 32.14 | 0.16 | 0.015* | |
| Rainforest planting vs. seasonal forest planting | 1.50 | 0.001 | 0.660 | |
| Rainforest planting vs. seasonal forest remnant | 25.15 | 0.10 | 0.015* | |
| Rainforest remnant vs. seasonal forest planting | 79.78 | 0.07 | 0.015* | |
| Rainforest remnant vs. seasonal forest remnant | 14.12 | 0.06 | 0.015* | |
| Seasonal forest planting vs. seasonal forest remnant | 84.35 | 0.07 | 0.015* |
Our results support the hypothesis that, in the Brazilian Atlantic Forest, plantings for forest restoration are floristically more similar to one another than to forest remnants of the same forest types. This trend could be even more pronounced if we had the complete list of species from remnants. Remnant species composition data come from samples and not from complete censuses, thus we likely underestimate remnant species richness and rare species presence; and rare species are frequently absent in restoration plantings (Almeida et al., 2024). Plantings are more frequently dominated by the same species than forest remannts, so there is a lower beta diversity among plantings, and they form a single floristic group across the Atlantic Forest. In other words, remnant forest communities shift strongly across geographic and environmental gradients, but the same tree species are often planted in restoration sites regardless of their geographic location. As such, tree planting-based restoration is failing to reproduce regional beta diversity patterns that characterize the Atlantic Forest biome.
Most of the difference in beta-diversity between restoration plantings and remnants was represented by turnover, indicating that the replacement of species plays a greater role than nestedness in their dissimilarity. However, when we consider only plantings, nestedness plays a greater role in their difference, which means that the less diverse groups are impoverished subgroups of the larger groups of species. Nestedness indicates that restoration plantings may be contributing to biotic homogenization, although this pattern is likely counterbalanced at some sites by natural regeneration inside the plantings, which was not accounted for in our analysis of tree planting lists.
Planting the same set of species across diverse regions could be an artifact of a framework species restoration strategy, in which a relatively small set of species with selected traits (e.g., rapid growth, fruit production) are planted to quickly recreate forest structure and the basic conditions to facilitate recolonization of restoration sites by plants and animals (Viani et al., 2015a; Elliott et al., 2022). If this recolonization process occurs, with natural regeneration of the more distinctive regional species from different forest types, planting the same set of species across diverse landscapes might not lead to biotic homogenization. However, restoration plantings are usually done in fragmented landscapes with low natural regeneration capacity, where the time needed for plantings to reach species compositions similar to reference forests is long and uncertain (Suganuma and Durigan, 2015; Beltrán et al., 2022). In such landscapes, the recolonization of restoration plantings by species from some functional profiles, such as slow-growing species and species dispersed by gravity or large mammals, will rarely fully happen (Pohlman et al., 2021; Suganuma and Durigan, 2021). In other words, some species will only reach restoration sites if they are introduced by plantings, which our analysis finds, is not occurring.
Biotic homogenization is not limited to tree plantings; it is also predicted to occur in native forest remnants due to climate change and human actions, including seed disperser decline (Nielsen et al., 2019; Fricke and Svenning, 2020). In the Atlantic Forest, biotic homogenization is driven by the expansion of small-seeded pioneer species that proliferate in forest edges and small fragments (de Lima et al., 2020), a process that can lead many species to become locally extinct (Zwiener et al., 2018). Small-seeded pioneers are also predominant in forest restoration plantings, a result of natural regeneration and the ease of collecting large quantities of small seeds for nursery propagation (Holl et al., 2017; Brancalion et al., 2018; Engert et al., 2020, Almeida et al., 2024). These observations from disturbed, regenerating, and restored forests further aggravate our concern that planted forests will remain floristically distinct from remnants.
Many tropical fragmented landscapes, including the Atlantic Forest, have experienced an erosion of biodiversity, particularly for late-successional, large-seeded, and endemic tree species in forest remnants. This pattern is exacerbated in the more fragmented regions where active restoration efforts are concentrated (de Lima et al., 2020). Regional declines of select tree types suggest that even if tree plantings and natural regeneration adequately represented the flora of local remnants, there would still be low representation in the long-term of some groups that are already missing or poorly represented in degraded landscapes. There is no easy solution to countering biotic homogenization in restoration, but improving the representation of local floras in plantings would help, as would promote landscape restoration approaches that aid the recovery of degraded fragments (Viani et al., 2015b) and the increase of forest cover to levels that can increase the probability that rare and endemic species arrive and survive in remnant and restored forests (de Lima et al., 2020; Hansen et al., 2020).
Our results indicate that forest restoration plantings require improvement to effectively contribute to biodiversity conservation in the United Nations Decade on Ecosystem Restoration and the 30 × 30 Biodiversity Target. In the world of large-scale restoration initiatives, the reference ecosystem concept needs to consider macroecological patterns, like species turnover through space (Condit et al., 2002). Failing to address this concern means that restoration is at risk of exacerbating biotic homogenization (Holl et al., 2022), and failing to contribute to the conservation of endemic, threatened and specialist species. Information on species with limited capacity to colonize plantings (Suganuma and Durigan, 2021) and lists of species with high conservation values for restoration (de Lima et al., 2020) are important, but they are only the first part of the plan to solve this issue. Additionally, it is important to increase the regional, endemic and/or threatened species in the pool of species available in nurseries and used for active restoration practices. This inevitably involves raising awareness, but also public policies and economic incentives that can help nurseries and practitioners to diversify their production and uptake in large-scale restoration initiatives.
CRediT authorship contribution statementCrislaine de Almeida: Conceptualization, Methodology, Formal analysis, Data Curation, Writing - Original Draft. J. Leighton Reid: Methodology, Writing – Reviewing and Editing. Renato A. Ferreira de Lima: Data Curation, Writing - Review & Editing. Luis Fernando Guedes Pinto: Resources, Writing- Reviewing and Editing. Ricardo Augusto Gorne Viani: Conceptualization, Methodology, Formal analysis, Writing- Reviewing and Editing, Supervision.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study was partly financed the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (Capes) - Finance Code 001. We are thankful to the SOS Mata Atlântica nongovernmental organization for aiding this research and to Virginia Tech for hosting Crislaine de Almeida. Ricardo A.G. Viani is thankful to the grant #2022/13398-1 and grant #2018/18416-2, São Paulo Research Foundation (FAPESP), and to the National Council for Scientific and Technological Development (CNPq; grant #308777/2023-9). The compilation of the species abundances and traits stored in TreeCo database was funded by the grant #2013/08722-5, São Paulo Research Foundation (FAPESP), but many other agencies (e.g., Capes, CNPq, FAPEMIG, FAPERJ, FAPESC, etc.) funded the hundreds of researchers that produced the forest remnants data used in this study. We thank Jordan Coscia for the help in data analysis.