Most conservation of biological diversity is achieved via networks of protected areas, including national parks, wilderness areas, and nature reserves. Protected areas are chosen predominantly for their vertebrates and vegetation, and typically it is assumed that other biota elements will be represented and managed appropriately. However, measures that are successful at conserving vertebrates or plants do not neccesary guarantee that highly-diverse taxa (e.g. insects) will be adequately maintained. Despite Mexico includes a large proportion of the biodiversity Mesoamerican hotspot, well-documented examples of insect diversity and distributions remain limited, and in many cases, sampling efforts have been biased towards ‘handy’ locations, thus limiting the role and importance of priority areas for conservation. We evaluated the coherence between conservation areas, the distribution, and diversity of a highly diverse Neotropical insect group: Erebidae moths. We considered two large spatiotemporal scales in Mexico (national and regional) to examine the consistency between the distribution of conservation polygons and moth diversity. We focused on patterns of moth diversity, endemism, spatiotemporal biases, and sampling effort. Moth species richness and sampling records are disproportionately distributed towards southern locations of Mexico (i.e., Neotropical region), but despite so, many species are only known from single records. 16% of Erebidae species are endemic to Mexico, and around 20% of these species occur in one single state (Chiapas). Intriguingly, 66% of endemic species have not been reported from protected areas in Mexico, and a similar proportion has been observed for the Chiapan region, where sampling was significantly biased towards a few Protected Natural Areas (PNAs) and vegetation types.
The assessment of insect diversity in Mexico is still far from being complete since managers continue to assume that conservation areas effectively protect a whole range of species. However, we detected that sampling biases follow a spatiotemporal trend, and it represents an important obstacle to offer a complete picture of the true richness of diverse moth faunas within conservation polygons. It is not enough to have many conservation areas or to protect a minimum proportion of land if we are not able to describe their diversity and representativeness. Conservation biologists should also consider taking actions involving policymakers and society to go beyond the global decline discourse and avoid turning it into a trite topic.
Increasing agriculture, livestock, urbanization, and mining have resulted in significant biodiversity loss in Mexico (Ceballos et al., 2015; CONABIO, 2020a). Over the last decade, almost 30% of the Mexican territory has turned into pastures and agricultural lands (SEMARNAT, 2020). Among the strategies to reduce anthropogenic effects on biodiversity, the Mexican government has implemented a Federal legislation framework, i.e., a Protected Natural Areas network (PNAs) (CONANP, 2021). PNAs in Mexico mean to preserve roughly 70% of representative natural habitats across the country. Mexican conservationists aimed to protect vulnerable habitats and preserve the integrity of biodiversity through increasing the number of natural reserves, namely terrestrial priority regions, terrestrial priority sites, and priority restoration sites (CONABIO, 2020b; CONANP, 2021; PRONATURA, 2020; TNC, 2020). However, many species inhabiting the 182 PNAs in Mexico remain poorly documented, particularly highly diverse insect groups. The link between protected areas and insect conservation research has become a major concern arising from their global decline (Cardoso and Leather, 2019; Hallmann et al., 2017; Thomas et al., 2019).
Insects represent the largest Mexican biodiversity component, with almost 48,000 species recorded (i.e., 66% of the documented fauna, SEMARNAT, 2020). Within this group, lepidopteran members include 30% of the insect richness in Mexico (Llorente-Bousquets and Ocegueda, 2008). Butterfly families such as Papilionidae, Pieridae, Nymphalidae, and Hesperiidae have received far more attention, and probably 90% of the species have already been listed (De la Maza et al., 1989, 1991; Llorente-Bousquets et al., 1990; 1997). Recent ecological assessments have suggested specific conservation recommendations for some key lepidopteran taxa (e.g., Almaraz-Almaraz et al., 2013; León-Cortés et al., 2004; Molina-Martínez et al., 2016; Ruiz-Utrilla et al., 2018). Moth groups instead have received little attention, and particularly highly diverse groups remain relatively understudied across important areas of Mexico (Richardson and Whittaker, 2010; Zahiri et al., 2012). Erebidae (Lepidoptera: Noctuoidea) moths comprise a highly diverse group in Mexico, including 1048 species, of which 42% have been recorded in southern Mexico, and of these, 34% of the species are restricted to this region. Indeed, previous research suggests that Arctiinae (Erebidae) moths are reliable surrogates for the total lepidopteran richness (see Summerville et al., 2004). However, several potential biases (e.g., a limited number of specialists involved, sampling locations, and poor taxonomy) prevent us from adequate interpretations of distributional patterns and diversity of this and other diverse moth groups (see Heppner, 2002; Lamas, 2021).
How effective are national/regional conservation areas at preserving biodiversity? Here we set out a benchmark assessment of large and regional-scale species richness patterns of a highly diverse moth group (Erebidae), aiming to evaluate whether conservation targets fit moth diversity hotspots. We built upon the historical and current state of the art for Erebidae moths in Mexico to address the following objectives and predictions: (1) to assess the diversity, completeness, and spatial distribution patterns for this group at the national and regional scales. We would expect important gaps for Erebidae diversity records, with the largest species richness occurring towards tropical areas (Conner, 2008). (2) To assess the potential spatiotemporal biases of Erebid diversity recorded in Mexico and Chiapas State. We would expect significant biases in sampling and collecting efforts, as observed in other lepidopteran groups across the country (Soberón et al., 2000).
We finally discuss the effectiveness of the current protected area network in a highly diverse region of Mexico -in Chiapas- regarding representativeness and conservation of Erebidae moth species. Taken together, we propose how to strengthen the current conservation framework to provide an integrated management perspective in future conservation programs for biodiversity as a whole (Myers et al., 2000).
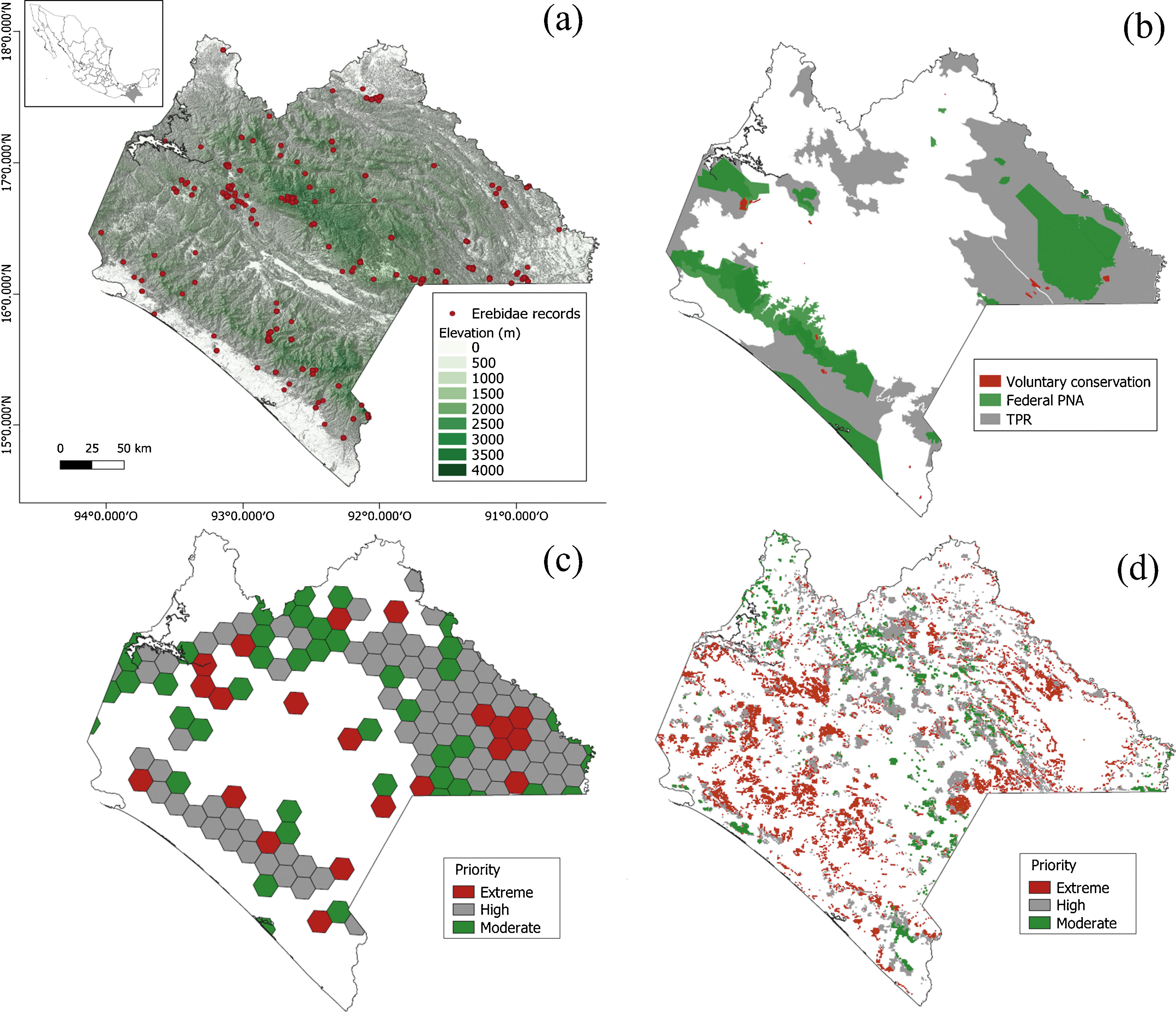
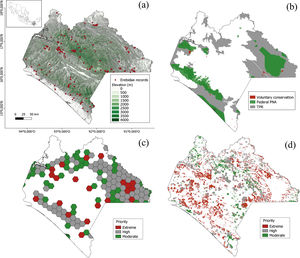
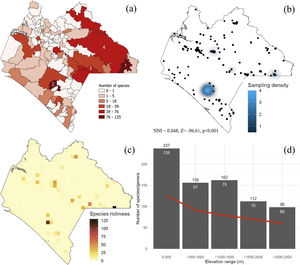
MethodsThe Chiapan regionThe Chiapas Mexican state is considered one of the highest species-diverse regions of all major biological and cultural groups in Mexico (CONABIO, 2013a; Fig. 1a). It includes 17 tropical and temperate vegetation associations, protects nine ethnic groups with different uses and customs (e.g., Tsotsiles, Tseltales, Choles, Lacandones, Zoques, and Mames), stands out upon important endemic flora and fauna elements, and concentrates 30% of Mexico’s freshwater (CONABIO, 2013a). In addition, it includes 19 federal protected areas (one of the highest number in Mexico) where angiosperm and vertebrate species have been the target of diversity and monitoring assessments (CONABIO, 2013b). Roughly 43% of the Erebidae species listed in the country occur in Chiapas, and 8% are endemic. Thus, the Chiapan region represents an informative example to examine the consistency between the presence and distribution of conservation polygons and Erebidae richness patterns; in doing so, we focused on species richness and endemism patterns, spatiotemporal biases, and sampling effort.
Erebidae data source and curationTo elaborate our species checklist, we followed the last phylogenetic classification of Erebidae proposed by Zahiri et al. (2012). The current classification of Erebidae recognizes 18 subfamilies: Scoliopteryginae, Rivulinae, Anobinae, Hypeninae, Lymantriinae, Pangraptinae, Herminiinae, Aganainae, Arctiinae, Calpinae, Hypocalinae, Eulepidotinae, Toxocampinae, Tinoliinae, Scolecocampinae, Hypenodinae, Boletobiinae, and Erebinae.
We gathered occurrence, diversity, and endemic data for adult Erebidae moths from public web repositories, i.e., the Global Biodiversity Information Facility (GBIF, 2020) and the National Information System on Biodiversity (SNIB, 2021). Such online resources contain data of Erebidae from different entomological collections: Natural History Museum of Mexico City (MHNCM), Institute of Biology at Universidad Nacional Autónoma de México (IBUNAM), Entomological Collection at El Colegio de la Frontera Sur (ECO-SC-E), Arthropod Collection-Colorado State University (CSU), Entomology Collection-University of Minnesota, St. Paul (UMSP), Collection of Entomology-Yale Peabody Museum of Natural History, Yale University (YPM). Published checklists and related documents were consulted (Balcázar and Beutelspacher, 2000; Brown and Faulkner, 1997; Hernández-Baz, 2012; Hernández-Baz and Granados, 2004; Salas-Araiza et al., 2015; (Turrent-Díaz and Pescador, 2013) Vincent and Laguerre, 2014; Zaspel and Branham, 2008). Records from our field surveys in Chiapas were also included. Since moth records could derive from single collections or studies with several transects surveyed at different times, we collated the total number of records into one single historical sampling period (i.e. 1908–2020).
To provide high resolution and reliable faunistic data, we restricted our search to taxonomically valid scientific names. We carefully inspected the taxonomic status of each species and discarded possible misidentifications and synonymies. The taxonomy of Erebidae has suffered recent modifications (Zahiri et al., 2012), so we revised the status of other groups now included in Erebidae to remove or add species to the database accordingly. We aligned our dataset according to best standard practices to avoid duplication, special characters, misspellings, and doubtful georeferences (de Jonge and van der Loo, 2013; see Tellez et al., 2020 for detailed techniques of spatial data preprocessing). Data cleaning was performed using QGIS v. 3.10.6 and R v. 4.0.2 (QGIS Development Team, 2020; R Core Team, 2020). In total, we recorded 19,590 records of Erebidae moths across Mexico and from which 2691 corresponded to Chiapas’ State.
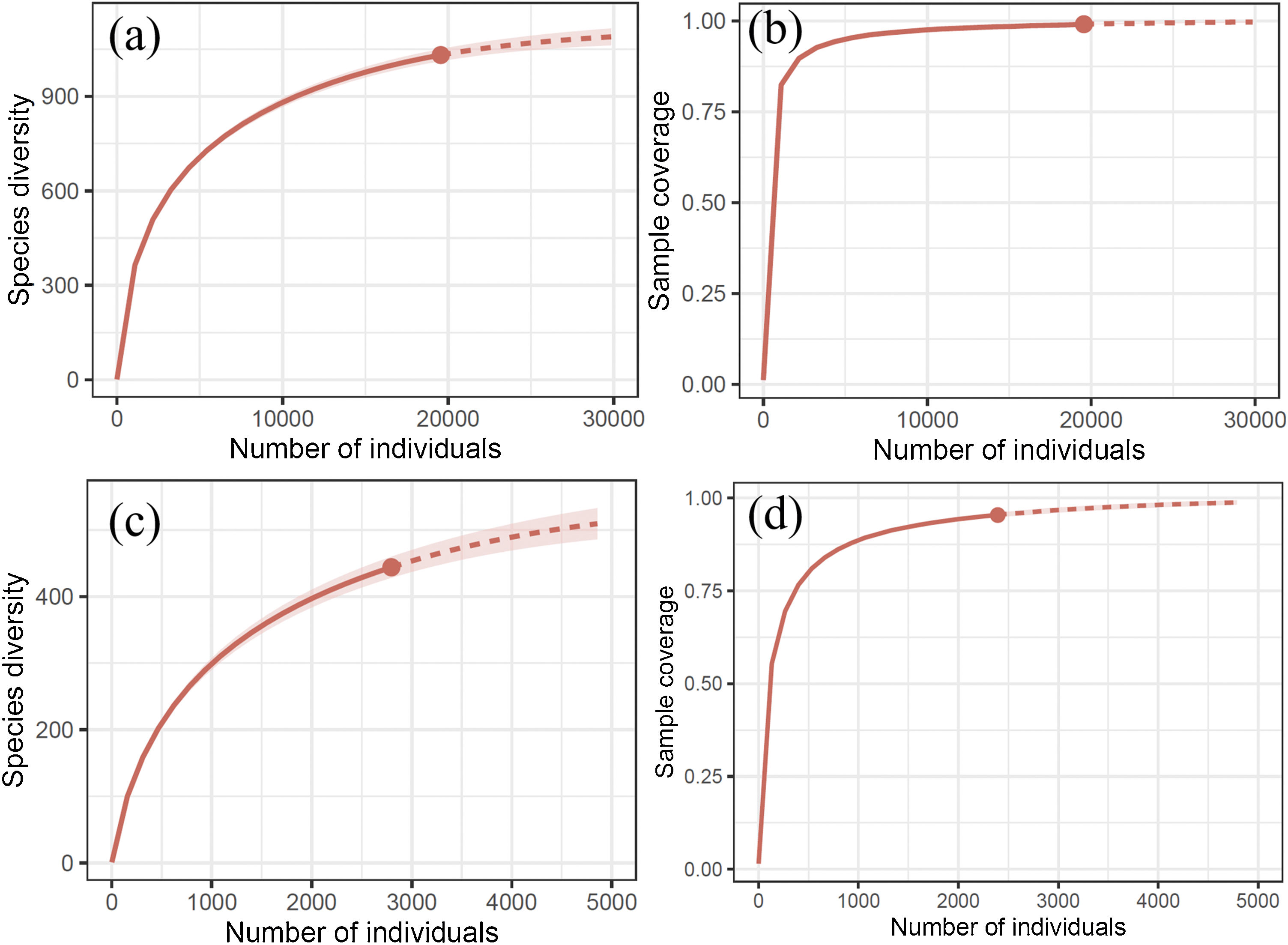
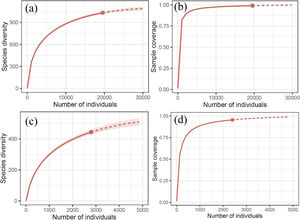
Diversity, completeness, and spatial distribution patternsDiversity information, including the number of subfamilies, genera, and species, was obtained by filtering our database. The asymptotic estimators of species diversity (i.e., Chao1, Chao2, ACE, ICE, and Jacknife2) were calculated using the package fossil (Vavrek, 2011). To evaluate the temporal changes of the Erebidae diversity, we plotted the historical collecting efforts and fitted a linear regression to evaluate changes of species diversity values (H') over time. Diversity completeness was evaluated using species rarefaction curves with extrapolated individual-based (abundance) data. Fitted curves were computed in R with the package iNext (Hsieh et al., 2020).
To examine diversity patterns for Erebidae in Mexico, we stored georeferenced records using 1° × 1° grid cells (~100 km × 100 km), which is an adequate resolution scale to provide a complete picture of country-scale richness patterns (Cruz-Cárdenas et al., 2013). For the case of Chiapas, our records were stored in 10 km × 10 km grid cells to plot species richness and spatial patterns of sampling points. We used bdvis package (Barve and Otegui, 2018) to display the temporal sampling patterns. Plotted maps were obtained using R v.4.0.2 with the libraries sf, maps, maptools and grid (Becker and Wilks, 2018; Bivand and Lewin-Koh, 2020; Pebesma, 2018; R Core Team, 2020).
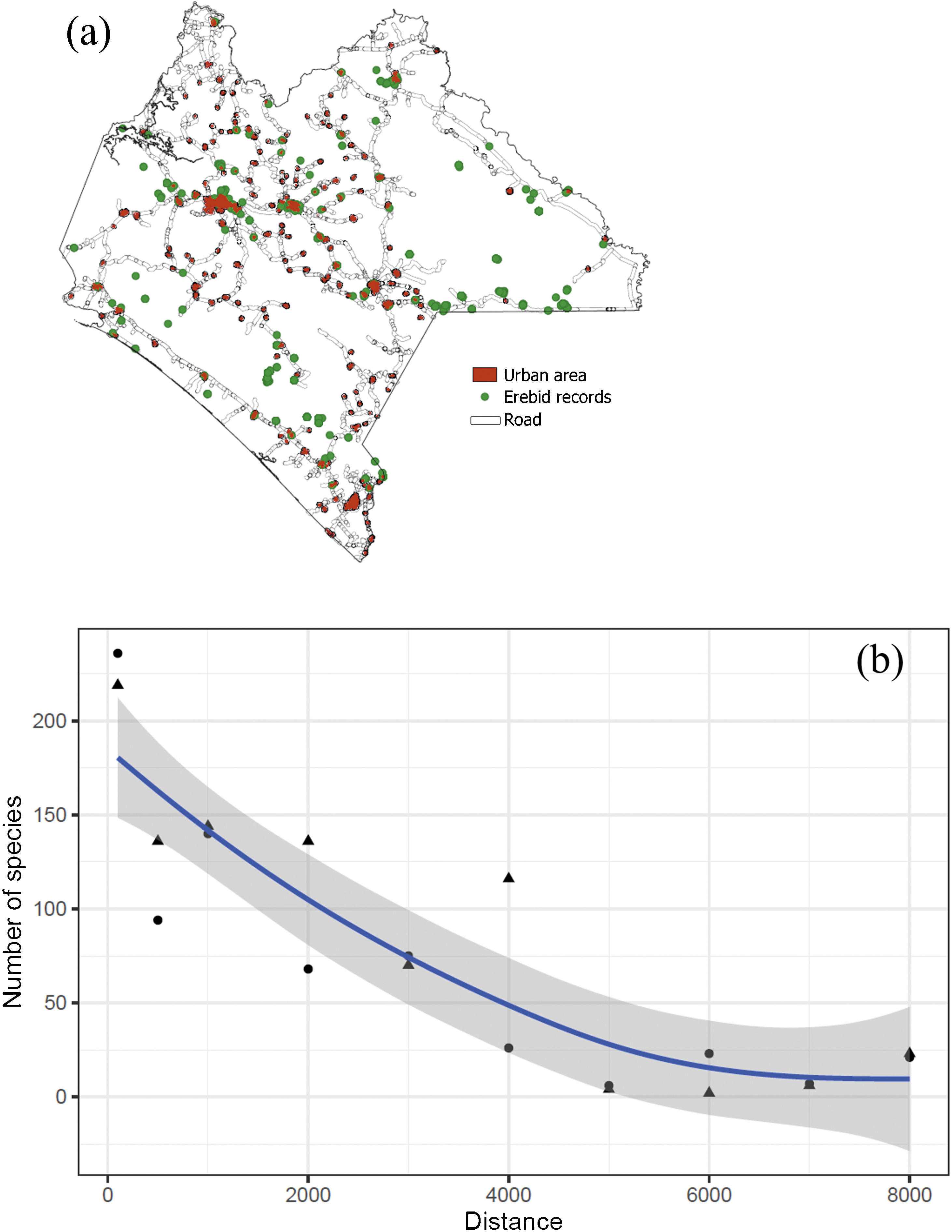
Moth spatial sampling biasesTo detect spatial biases of Erebidae records in Chiapas, we created a Kernel map for the point pattern analyses and estimated the Nearest Neighbor Index (NNI). The NNI measures the distance between each feature centroid and its nearest neighbor. Values near zero indicate clustering, whereas values >1 mean dispersion. This procedure allowed us to provide a p-value assuming that spatial points are randomly distributed (Acevedo, 2013). Maps were produced using QGIS, and R. Point pattern analyses were computed in R with the packages sp, tmap, and spatstat (Baddeley et al., 2015; Pebesma and Bivand, 2005; QGIS Development Team, 2020; R Core Team, 2020; Tennekes, 2018). We tested whether richness and species distribution in Chiapas were spatially biased towards road infrastructure (including Federal, state-owned, and municipal paved roads) and human settlements (urban and rural locations). Species richness values of Erebidae were calculated and compared based on four arbitrary distance values from both roads and settlements: 100 m, 500 m, 1 km, and 5 km, and the estimated proportion richness from a given distance range. We also evaluated the relationship between Erebid richness and the distances to roads and human settlements using a robust regression (see below). Map and distance measurements were computed in QGIS (QGIS Development Team, 2020). Both road and human settlement shapefiles were retrieved from the INEGI’s repository (2020).
In addition, we considered elevational values and categorical features for each Erebidae record by extracting the spatial information from a Digital Elevation Model (DEM), including land cover type (INEGI, 2020), climatic classification, and conservation polygons (CONANP, 2021). This approach allowed us to determine whether faunistic records of Erebidae in Chiapas were biased towards specific elevational ranges, land use types, biomes, or conservation polygons (see Supplementary data). We projected the records on the DEM and extracted the elevation values from each location using the Point Sampling Tool plugin from QGIS (Fig. 1a). We collated data into five elevational ranges: 0−500 m, >500−1000 m, >1000–1500, >1500–2000 and >2000–2500, which we correlated against species richness values. Statistical tests (chi-square, Spearman's rank correlation, and robust regression) were computed in R and spatial analyses were performed in QGIS (QGIS Development Team, 2020; R Core Team, 2020). All linear regressions were fitted with Huber weighting, a procedure to avoid the influence of outliers or high leverage data points (Rawlings et al., 1998). Robust regressions were carried out using MASS (Venables and Ripley, 2002). All shapefiles were obtained from map repositories of INEGI (2020) and CONANP (2021).
Conservation areasWithin the national conservation framework and global agreement to preserve biological biodiversity, the Mexican federal legislation has established 182 conservation polygons, including terrestrial and marine ecosystems, as Protected Natural Areas (see SEMARNAT, 2020). Additionally, several scientists supported by federal commissions and NGOs representatives have proposed complementary areas to strengthen conservation programs and preserve ecological integrity (Arriaga et al., 2000; CONANP, 2021). Thus, we focused on four major categories of conservation areas: Protected Natural Areas, Terrestrial Priority Regions, Terrestrial Priority Sites, and Priority Restauration Sites (Fig. 1b–d). Protected Natural Areas aim to protect the natural resources and wildlife in pristine or threatened areas; terrestrial priority regions prioritize the recognition of regions holding outstanding ecological and biological richness; terrestrial priority sites identify sites facing high deforestation and environmental degradation, and priority restauration sites detect sites deserving attention to revert the environmental impacts based on fine-scale ecosystem analyses (Arriaga et al., 2000). We used QGIS to extract all conservation polygons using Chiapas State's mask (QGIS Development Team, 2020).
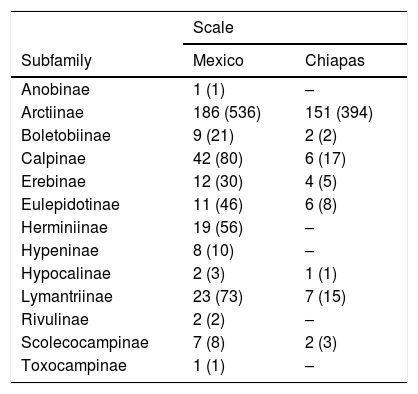
ResultsRichness and completenessOur overall estimators of species richness in Mexico and Chiapas showed different values for either incidence or abundance-based data (Table 1). We recorded 323 genera within 13 subfamilies of Erebidae in Mexico and 179 genera within eight subfamilies in Chiapas. Members of Arctiinae are the best represented at generic and specific levels at both scales (Table 2). We estimated that the total observed species richness of Erebidae in Mexico ranged between 64%–94% and ~58%–80% in Chiapas (Fig. 4a–d).
Diversity estimator across Mexico and Chiapas for Erebidae moths. Values in parentheses indicate standard error. Abundance (abun) and incidence (inc) estimators.
| Scale | Species | Chao1 | ACE | Chao2 | ICE | Jack2(abun) | Jack2(inc) |
|---|---|---|---|---|---|---|---|
| Mexico | 1048 | 1116(16.8) | 1090 | 1478(65.2) | 1182 | 1202 | 1611 |
| Chiapas | 445 | 552(24.4) | 498 | 694(53.6) | 576 | 631 | 772 |
Representation of lower taxonomic levels of Erebidae in Mexico and Chiapas. Number of genera and species are indicated outside and inside the parentheses respectively.
| Scale | ||
|---|---|---|
| Subfamily | Mexico | Chiapas |
| Anobinae | 1 (1) | – |
| Arctiinae | 186 (536) | 151 (394) |
| Boletobiinae | 9 (21) | 2 (2) |
| Calpinae | 42 (80) | 6 (17) |
| Erebinae | 12 (30) | 4 (5) |
| Eulepidotinae | 11 (46) | 6 (8) |
| Herminiinae | 19 (56) | – |
| Hypeninae | 8 (10) | – |
| Hypocalinae | 2 (3) | 1 (1) |
| Lymantriinae | 23 (73) | 7 (15) |
| Rivulinae | 2 (2) | – |
| Scolecocampinae | 7 (8) | 2 (3) |
| Toxocampinae | 1 (1) | – |
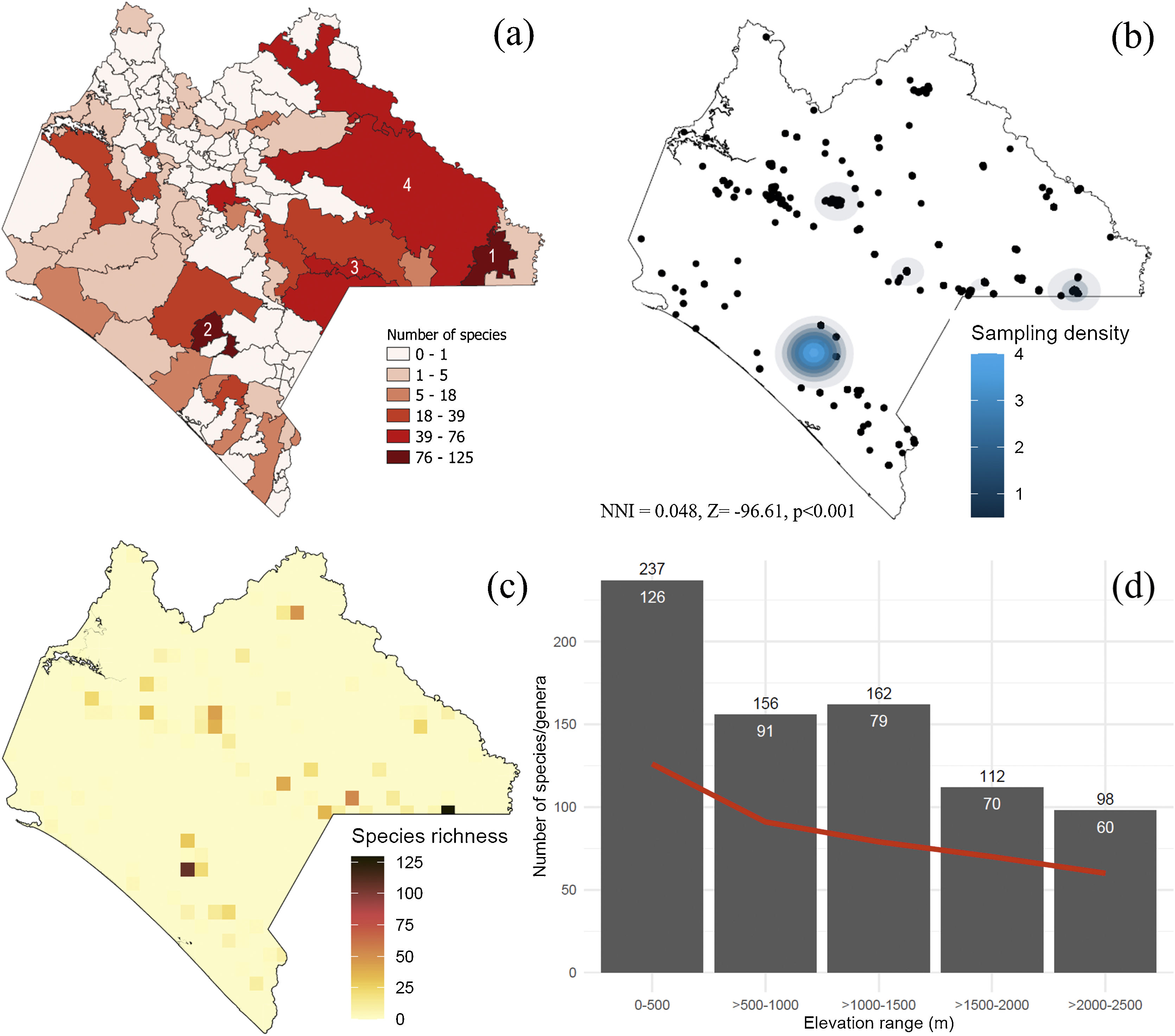
Species richness of Erebidae in Chiapas. (a) Municipalities. 1 = Marqués de Comillas, 2 = Ángel Albino Corzo, 3 = La Independencia, 4 = Ocosingo. (b) density of sampling records, (c) species richness distribution. Each grid cell is 10 km2. Grids were only generated upon sampling locations. (d) Species richness along the elevational range. The red line represents the generic richness trend. Number of genera and species are indicated in white and black, respectively. NNI = Nearest Neighbor Index showing that sampling records are far to be random.
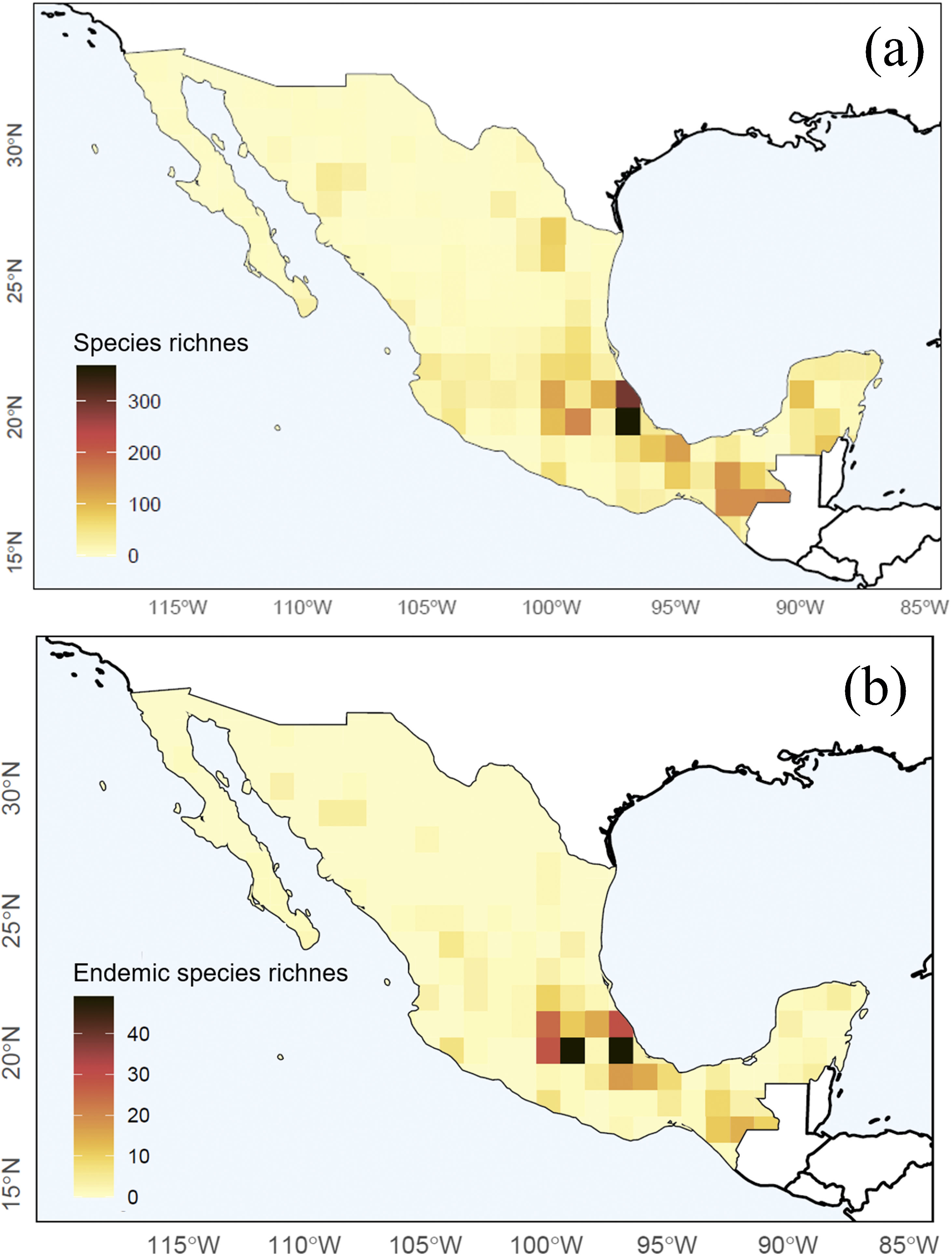
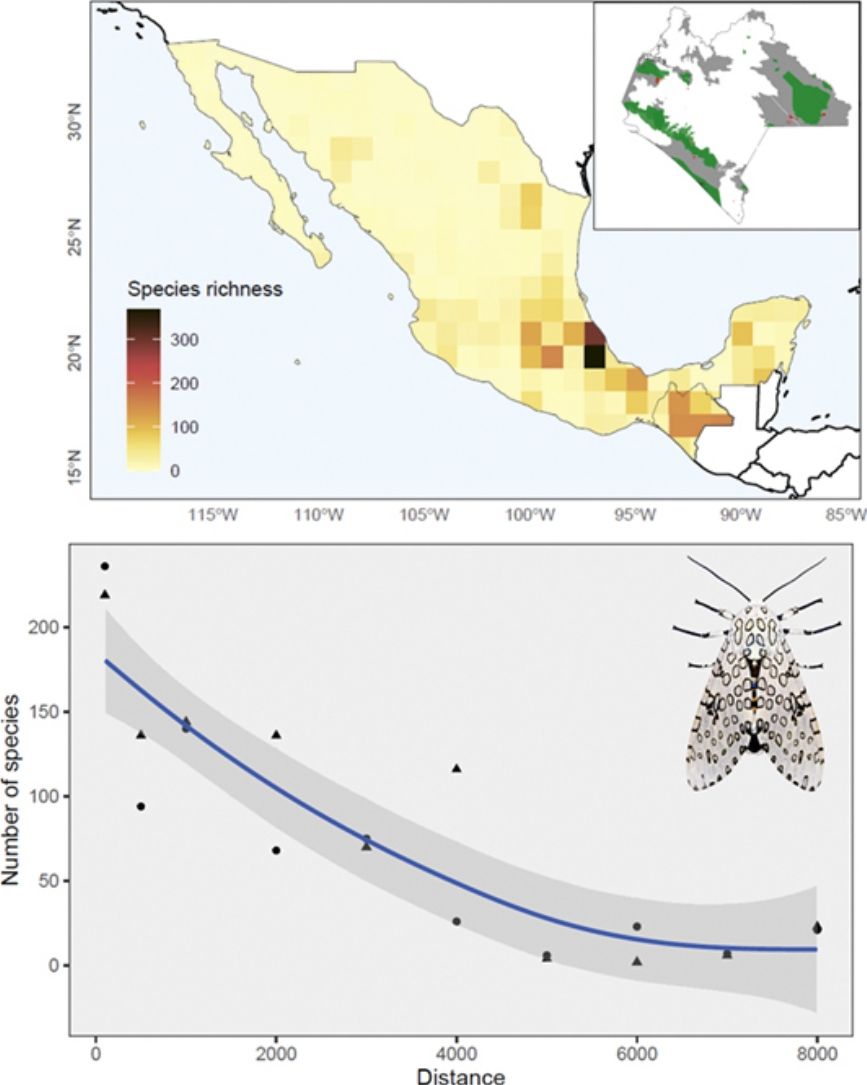
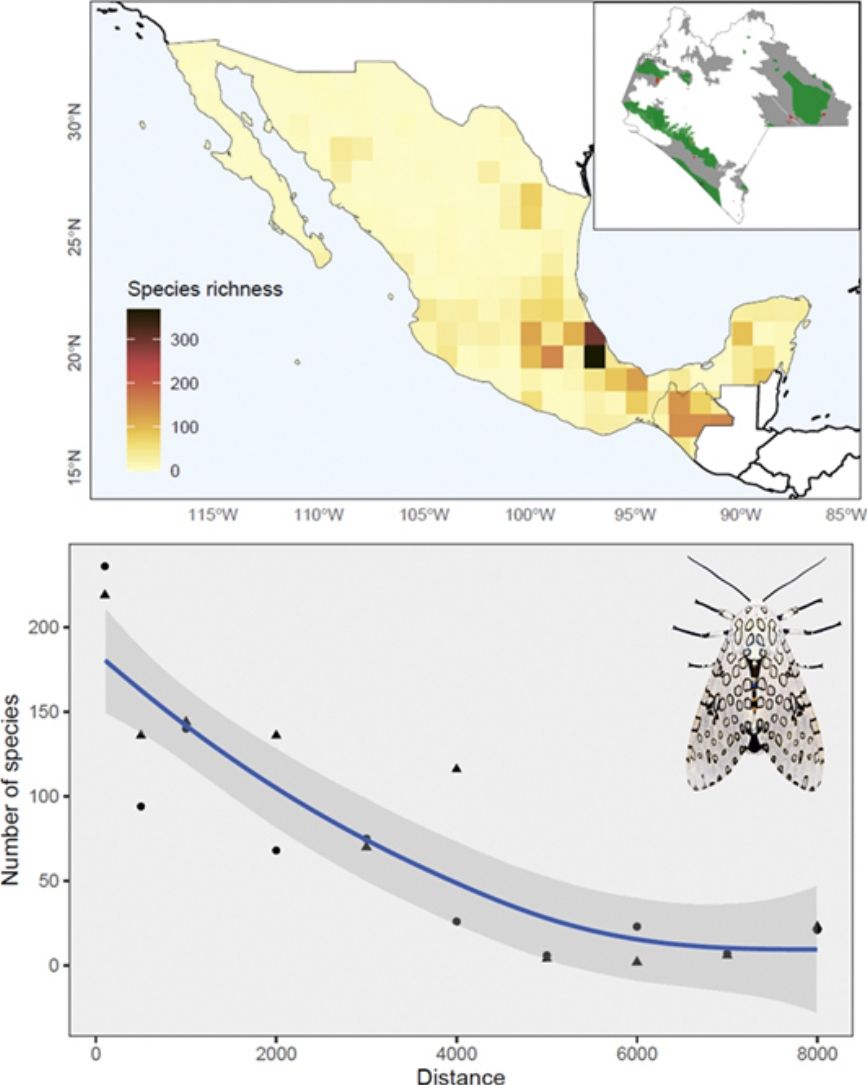
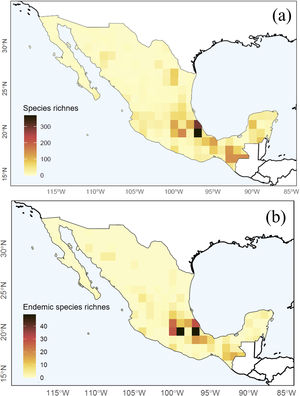
The Nearctic region of Mexico (the northernmost area) recorded a limited number of species (27%) and sampling records in general (13%). At the national scale, a couple of species-rich areas corresponded to Sonora and Nuevo León states. Although most species and records occurred in the Neotropical region (95% and 87%, respectively), species richness in this area (i.e. southern Mexico) is relatively patchy, with areas having few or no species records. The states of Yucatán (19%), Veracruz (16%), and Chiapas (14%) included the largest number of Erebidae records; Veracruz (51%), Chiapas (43%), and Oaxaca (18%) concentrated the highest diversity of species. Central Veracruz included the highest density of species (368 species/100 km2) (Fig. 2a). Ascalapha odorata L. 1758, Estigmene acrea Drury (1773), Utetheisa ornatrix L. (1758) (84%), and Hypercompe scribonia (Stoll) (78%) corresponded to widespread species across the country. In contrast, only 1.5% of the species occurred in >50% of the states, and 49% are known from a single federal state (singletons). In total, we recorded 172 endemic Erebidae species to Mexico, and the highest endemism level was found in Central Mexico, in the states of Veracruz (71 species), Estado de México (37), Chiapas (34), and Puebla (31) (Fig. 2b, see Supplementary data for an expanded list of regional endemism).
Diversity of Erebidae in ChiapasOut of the 1048 Erebid species listed in Mexico, 42% have been recorded in Chiapas, and 34% of the species only occur in this region. Most endemics recorded from Chiapas were listed in three local municipalities: La Independencia (n = 11 species), Ángel Albino Corzo (8) and Ocosingo (8). Chiapas holds the highest number of species known from a single location (El Triunfo). Cosmosoma teuthras cingulatum Butler and Dysschema leucophaea Walker are widely distributed species across the Chiapan region, whereas 39% of them are known from a single location. We found records of Erebidae in 42% of the municipalities, of which 72% contained less than 10 erebid species. The municipalities of Marqués de Comillas (125 species) and Ángel Albino Corzo (122) recorded the largest number of species (Fig. 3a). The latter contains the highest number of records (39%). Accordingly, sampling records are significantly clustered across the state (χ2 = 7560.1, df = 19, p < 0.001) (Fig. 3b), showing areas of high species diversity (Fig. 3c). We failed to detect an area effect on species richness values (t = 9.34, s.e. = 7.89, d.f. = 44, p = 0.769) but the number of records was highly correlated with Erebid species (S = 351.1, rho = 0.97, p < 0.001). Moreover, species richness and genera decreased with increasing elevation (S = 40, rho = −0.96, p = 0.01), with the highest diversity being recorded between sea level and 500 masl (Fig. 3d).
Sampling effort and biasesThe historical Erebidae moth sampling encompassed an approximate 112-year time span of observations (1908–2020), with only 102 years containing georeferenced sampling records. Historically, most of these surveys were carried out during late springs and summers, especially in May and July (Supplementary data). In Chiapas, we identified a 94-year period of historical records (1926–2020), of which only 71 years contained local occurrence records. Moreover, both sampling records and species diversity of Chiapas have positively increased during the last century (t = 3.42, d.f. = 69, p = 0.001), with the highest frequency of surveys from the mid-'50s to '80s (Supplementary data).
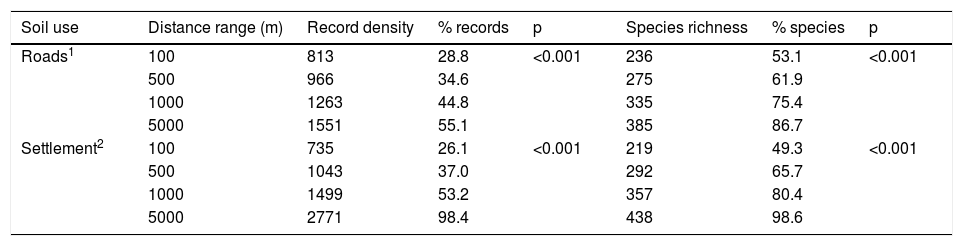
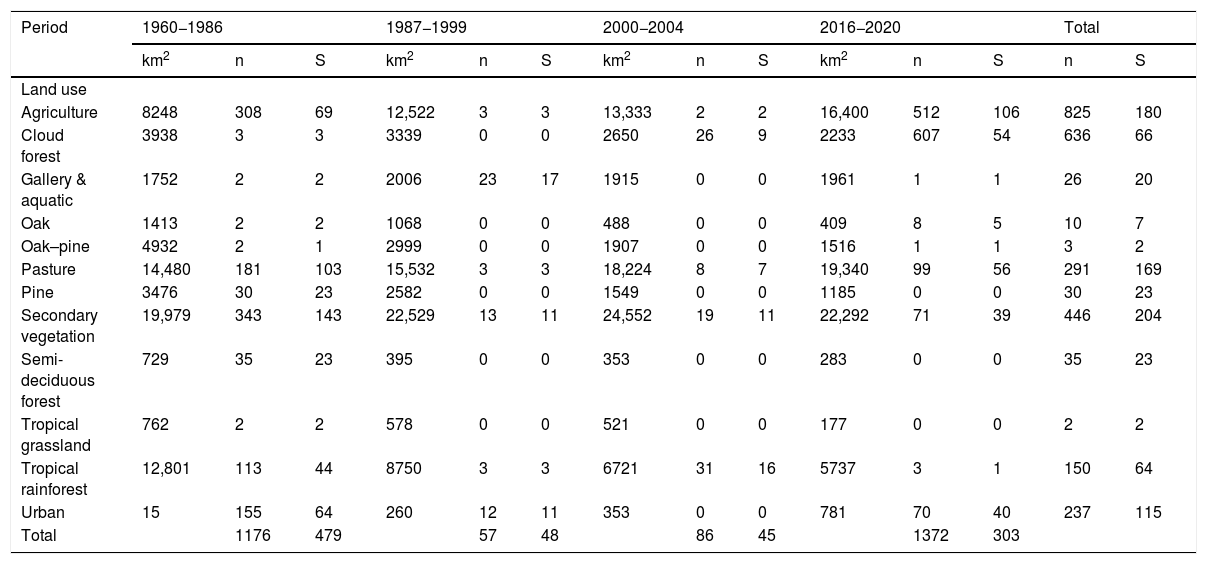
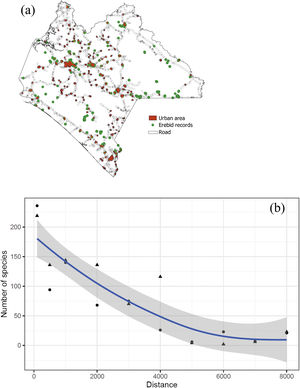
In Chiapas, we found a disproportionate number of records and species richness towards human settlements (χ2 = 25.2, df = 2, p < 0.001) and roads (χ2 = 59.9, df = 2, p < 0.001) (Table 3; Fig. 5a). In both cases resulting in a strong inverse relationship (S = 306.4, rho = −0.85, p = 0.001 and S = 512, rho = −0.79, p = 0.003, respectively). Roughly half of these species have been collected within the shortest distance range (100 m) from either human establishments or roads and more than three-quarters of species were found within a 1000 m distance range (Fig. 5b). Moreover, the proportion of records was unevenly distributed among land-use types (χ2 = 3776.3, df = 11, p < 0.001). Most species occurrences were recorded in agricultural fields, and most were associated with secondary vegetation habitats. In addition, a disproportionate number of occurrences have been recorded during 1960−1986 and from the 2016 to 2020 periods (Table 4). Similarly, neither the number of records nor the species were evenly distributed among climatic conditions, being most species generally recorded from tropical climates (see Supplementary data).
Correspondence between the soil use and the sampling efforts for Erebidae moths. Both the density of records and the number of species are accumulated values as the distance range increases. The p values represent the chi-square test (d.f. = 3) evaluating the proportion of records and species richness found at each distance category (i.e., not accumulated values).
| Soil use | Distance range (m) | Record density | % records | p | Species richness | % species | p |
|---|---|---|---|---|---|---|---|
| Roads1 | 100 | 813 | 28.8 | <0.001 | 236 | 53.1 | <0.001 |
| 500 | 966 | 34.6 | 275 | 61.9 | |||
| 1000 | 1263 | 44.8 | 335 | 75.4 | |||
| 5000 | 1551 | 55.1 | 385 | 86.7 | |||
| Settlement2 | 100 | 735 | 26.1 | <0.001 | 219 | 49.3 | <0.001 |
| 500 | 1043 | 37.0 | 292 | 65.7 | |||
| 1000 | 1499 | 53.2 | 357 | 80.4 | |||
| 5000 | 2771 | 98.4 | 438 | 98.6 |
Summary of sampling records (n) and species (S) of Erebidae moths collected at different vegetation types in Chiapas over the past 60 years. The period corresponds to the period INEGI surveyed each vegetation (see text). During 2009-2011, there is only one record (1 species) in the tropical rainforest. The area (km2) is indicated to each vegetation class (numbers have been rounded). The agricultural land use was categorized by merging both intensive and extensive management.
| Period | 1960−1986 | 1987−1999 | 2000−2004 | 2016−2020 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| km2 | n | S | km2 | n | S | km2 | n | S | km2 | n | S | n | S | |
| Land use | ||||||||||||||
| Agriculture | 8248 | 308 | 69 | 12,522 | 3 | 3 | 13,333 | 2 | 2 | 16,400 | 512 | 106 | 825 | 180 |
| Cloud forest | 3938 | 3 | 3 | 3339 | 0 | 0 | 2650 | 26 | 9 | 2233 | 607 | 54 | 636 | 66 |
| Gallery & aquatic | 1752 | 2 | 2 | 2006 | 23 | 17 | 1915 | 0 | 0 | 1961 | 1 | 1 | 26 | 20 |
| Oak | 1413 | 2 | 2 | 1068 | 0 | 0 | 488 | 0 | 0 | 409 | 8 | 5 | 10 | 7 |
| Oak–pine | 4932 | 2 | 1 | 2999 | 0 | 0 | 1907 | 0 | 0 | 1516 | 1 | 1 | 3 | 2 |
| Pasture | 14,480 | 181 | 103 | 15,532 | 3 | 3 | 18,224 | 8 | 7 | 19,340 | 99 | 56 | 291 | 169 |
| Pine | 3476 | 30 | 23 | 2582 | 0 | 0 | 1549 | 0 | 0 | 1185 | 0 | 0 | 30 | 23 |
| Secondary vegetation | 19,979 | 343 | 143 | 22,529 | 13 | 11 | 24,552 | 19 | 11 | 22,292 | 71 | 39 | 446 | 204 |
| Semi-deciduous forest | 729 | 35 | 23 | 395 | 0 | 0 | 353 | 0 | 0 | 283 | 0 | 0 | 35 | 23 |
| Tropical grassland | 762 | 2 | 2 | 578 | 0 | 0 | 521 | 0 | 0 | 177 | 0 | 0 | 2 | 2 |
| Tropical rainforest | 12,801 | 113 | 44 | 8750 | 3 | 3 | 6721 | 31 | 16 | 5737 | 3 | 1 | 150 | 64 |
| Urban | 15 | 155 | 64 | 260 | 12 | 11 | 353 | 0 | 0 | 781 | 70 | 40 | 237 | 115 |
| Total | 1176 | 479 | 57 | 48 | 86 | 45 | 1372 | 303 | ||||||
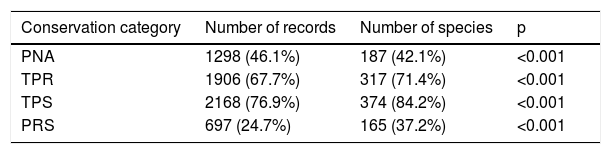
More than half of the records and species listed in Chiapas corresponded to non-protected areas. Native Erebidae species have been recorded in three PNA’s: El Triunfo (8 species), Lagunas de Montebello (2) and Bonampak (3). We failed to gather Erebidae records in private protected areas (Table 5). Among the PNA’s, most records (39%) and species (24%) have been collected at El Triunfo biosphere reserve, whereas 37% of PNA’s remained without information, and PNA size was not associated with the number of records (S = 944.9, rho = 0.17, p = 0.48). As for the terrestrial priority sites, the Lacandon tropical rainforest region recorded the highest number of species (38%), but the El Triunfo-La Encrucijada-Palo Blanco region contained 40% of species records. 25% of the Chiapan territory had no records of Erebidae. According to the terrestrial priority sites and priority restoration sites, most records were found in those sites (polygon classes) under the category of extreme priority (see Supplementary data).
Erebidae records and species across conservation polygons in Chiapas. The numbers in parentheses indicate the percentage of records and species found in each conservation category. The p values represent the chi-square test evaluating the proportion of records and species richness between conservation polygons. Protected Natural Areas (PNAs), Terrestrial Priority Regions (TPR), Terrestrial Priority Sites (TPS), and Priority Restauration Sites (PRS).
| Conservation category | Number of records | Number of species | p |
|---|---|---|---|
| PNA | 1298 (46.1%) | 187 (42.1%) | <0.001 |
| TPR | 1906 (67.7%) | 317 (71.4%) | <0.001 |
| TPS | 2168 (76.9%) | 374 (84.2%) | <0.001 |
| PRS | 697 (24.7%) | 165 (37.2%) | <0.001 |
Our results showed that moth recording in Mexico has been spatial and temporally inconsistent and typically closely related to distributional patterns of plant richness inventories (Cruz-Cárdenas et al., 2013). In particular, the pattern of endemic Erebidae resembles the distribution of other plant taxa (Sosa et al., 2018), and the low richness of these moths in northern Mexico could be attributed to their Neotropical affinity (Conner, 2008). Despite this, we identified two Erebid-rich spots in Sonora and Nuevo León States located in the Nearctic region. The distribution of these moth populations corresponds to floristic species-rich areas (Cruz-Cárdenas et al., 2013), reinforcing the idea of a relationship between Erebidae and plant diversity. Considering the floristic richness in other states within the Neotropical region, i.e., Oaxaca (Rzedowski, 2006), a greater number of moth species would be expected, but the lack of complete inventories prevents us from estimating accurate levels of diversity for these moths in Neotropical Mexico.
Since the diversity and abundance of insects might be shaped by vegetation richness patterns (Vogel, 2017), it is likely that changes in vegetation cover and microclimate have negatively affected populations of Erebidae, especially for those species with restricted distributions and specific host plant associations (Green et al., 2011; Hernández-Baz et al., 2014). Even though the unpalatability of larvae (i.e., unpleasant taste) and the generalist feeding habits of many species (Powell and Opler, 2009), members within the Tribe Lithosiini exhibit an important degree of habitat specialization, i.e., most of these species feeding on lichens, liverworts, algae, mosses (Conner, 2008), and their ecological specialization pose them as threatened species in fragmented Mexico. Although plant-moth associations are critical to understand their ecology and prevent their decline, the dietary source is missing for several species since studies usually do not involve rearing methods. For instance, caterpillar monitoring has allowed identifying a severe decline of dietary and ecological specialist moths in Costa Rica caused by an increase of human population density and intensive agriculture (Wagner et al., 2021). We thus believe that rearing studies or in the best case, barcoding techniques, would be an important step towards tailoring conservation recommendations for specific moth guilds in Mexico (including our group).
Chiapan diversityRoughly 43% of the Erebidae species listed in the country occur in Chiapas, and 8% are endemic. Veracruz and Chiapas hold the highest biodiversity levels (including other arthropod members) across the country (Balcázar and Beutelspacher, 2000; SEMARNAT, 2020). In addition, the diversity of other invertebrate i.e., spiders, harvestmen, scarab beetles, and lepidopteran species (CONABIO, 2013b; León-Cortés, 2013), and vertebrate groups i.e., mammals (e.g. Jenkins and Giri, 2008) is exceptionally high in Chiapas, Although Chiapas stands out for the most remarkable Lepidoptera diversity in the country (≈1733 species), Lepidoptera records mostly represent those of diurnal families Hesperiidae (462), Nymphalidae (379), Lycaenidae (333) (León-Cortés et al., 2005). In contrast, many moth families from Chiapas remain only known from a few species records (León-Cortés, 2013).
On the other hand, there are key ecological regions within Chiapas, presumably harboring a great richness of moths. Previous surveys from the Lacandon forest suggest that nearly half of the sphinx moth richness from the country has been documented in this area (León-Cortés et al., 1998; León-Cortés and Pescador, 1998). Besides, this large rainforest region concentrates the highest butterfly diversity of Mexico (Arriaga et al., 2000), and we found the highest diversity of Erebidae (38.5%) occurring in this region too (see Supplementary data). Our observations are in line with previous studies suggesting that, for a broad range of plant and vertebrate communities (e.g., Jenkins and Giri, 2008), the Chiapan region can be considered as a biodiversity hotspot, reflecting the influence of the remaining largest portion of tropical rainforest in northern Mesoamerica (Dirzo and Domínguez, 1995). The highest Erebid species records from Chiapas were concentrated between sea level and 500 m height and then decreased with increasing elevation. In this regard, it is hard to disclose whether physiological limitations, ecological specialization, or host-larva associations are shaping the elevational pattern of Erebidae (Green et al., 2011). The elevational gradient can be one of the major predictors of moth diversity in tropical ecosystems (Maicher et al., 2019), as it is for other lepidopterans (Beck et al., 2016; Molina-Martínez et al., 2016). However, there is no general principle ruling this correlation. For instance, in the Ecuadorian Andes, Geometridae moths did not correlate with an elevational gradient, whereas the Pyralidae richness peaked at 1000 m and then decreased with increasing elevation; similarly, Arctiinae diversity showed a marginally decreasing pattern (Fiedler et al., 2008). In a general perspective, Beck et al. (2016) found a mid-domain global pattern in geometrid moths regardless of the geographical location. However, species diversity responses may be due to the taxonomic resolution and moth life history and sampling design effects (McCain and Grytnes, 2010).
Sampling gaps and completenessWe found evidence of strong sampling biases that could be limiting our interpretation of recognizing either large or small-scale spatiotemporal patterns for Erebidae in Mexico. Nonetheless, the estimated diversity of Erebidae is still higher than the observed so far; roughly 200–300 species remained unsampled from other locations or habitat types, especially in northern Mexico, where sampling effort has been insufficient. These results, however, could reflect sampling artifacts. For instance, many Lepidopteran species records from Central Mexico have often been attributed to recurrent expeditions since the 18th century (Luis-Martínez and Llorente-Bousquets, 1990). Thus, as perhaps seen in other faunistic cases, the current pattern of Erebidae has been historically influenced by rather biased sampling efforts across the country. Other contemporary trends have been observed in the Mexican butterfly richness, i.e., the strong sampling biases towards roads (Soberón et al., 2000), and confirmed for Chiapas’ butterfly inventories (León-Cortés et al., 2005). Likewise, we observed a marked influence of human infrastructure, particularly a significant decrease in species richness as the distance from roads and settlements increases.
In sum, our results proved that sampling records for our study group are significantly patchy and influenced by seasonal collects, revealing many gaps across vegetation types, climate, and conservation polygons. Furthermore, knowledge of global biodiversity has been typically biased toward three major taxa: Aves, Liliopsida, and Mammalia (Troudet et al., 2017), and perhaps such over-representation reflects the interest of society and not the result of scientific interest (Wilson et al., 2007). Spatial biases are also present at regional scales, highlighting, for example, the strong influence of roads and airports in the sampling of terrestrial mammals (Pereira et al., 2021), turtles (Steen and Smith, 2006) and amphibians (Petrovan et al., 2020).
The coherence between protected areas and moth diversityIn Chiapas, efforts to protect vulnerable ecosystems have led to establishing many federal PNA’s, designating 15% of the Chiapan territory as ecological reserves along with different voluntary conservation polygons (CONANP, 2021). Despite these and other initiatives aiming at preserving the biodiversity of Chiapas, landscape fragmentation has exponentially increased during the last decades, with prominent effects on insect diversity (CONABIO, 2013b). For example, 50% of the papilionid species from the Chiapan central depression might have significantly reduced their populations because of habitat fragmentation and habitat loss (Molina-Martínez and León-Cortés, 2006).
A disproportionate sampling effort for these moths towards a limited number of protected areas has been observed in other taxa, including Arctiinae moths (Hernández-Baz, 2012). Occurrence biases towards the El Triunfo biosphere reserve could be explained by the large number of Arctiini moth records recently included by us as part of a large moth inventory, but 37% of PNA’s network in Chiapas remain with virtually no faunistic records of Erebidae. Similarly, an unequal number of records have been observed across the Terrestrial Priority Regions, and 25% of these polygons remained with no Erebidae records. As terrestrial priority sites and priority restoration sites preserve type areas, most species (58% and 30% respectively) were found within the extreme priority polygons (Supplementary data). Therefore, Erebidae moths have been recorded from areas facing high environmental degradation in Chiapas State (Arriaga et al., 2000), and terrestrial priority sites could represent a practical standpoint for their regional conservation. Despite so we are unable to identify a decline of Erebidae moths throughout the country, and there is a matter of some urgency to reinforcing a monitoring scheme at various spatial scales in places with different degrees of fragmentation and habitat change, so population changes can be detected through time (see León-Cortés et al., 1999). Finally, we would expect many species occurring in other Mexican states with several terrestrial priority sites and priority restoration sites polygons under the extreme priority class (i.e., Oaxaca, Veracruz) (CONABIO, 2020b).
Conclusions and recommendationsBiodiversity conservation in Mexico has focused on protecting both locations and species, through the Protected Natural Areas network (PNAs) (CONANP, 2021) and the National Endangered Species Act (SEMARNAT, 2002), respectively. However, our analysis indicated that southern Mexico reserves are unrepresentative of key diverse areas for our study moths and that a large proportion of endemic species (66%) are outside protected areas. Most of the 172 Erebidae endemics occur in the Central Mexican plateau, but only 12% of the PNA’s included records of these restricted species. In Chiapas, among the critical regions to preserve biodiversity, cloud forests have been recognized as biologically relevant (González-Espinosa et al., 2012) because of their disproportionate levels of diversity and because of the ancestral populations that occur there (Arriaga et al., 2000; Conner, 2008). Our observations suggest that substantial tracts of forest in the Chiapan region have been severely transformed, which raises conservation concerns for regional biodiversity as a whole: thirteen percentage of the genera of the endemic flora of Mexico and roughly 20% of its fauna are considered vulnerable and restricted to these ecosystems (Sánchez-Ramos and Dirzo, 2014).
Given the intense sampling biases on Erebidae moths, we recommend the following aspects for their conservation: (1) Sampling design and monitoring. Researchers and moth enthusiasts should consider registering and monitoring Erebids at various spatial scales and representative vegetation types (e.g., montane and aquatic vegetation) and realm (studies in the Mexican Nearctic region are much needed). (2) Ecological specialization. Generating host plant use information and habitat suitability surveys would assess the ecological specialization of rare and or spatially restricted moths. Rearing programs supported with DNA barcoding could be helpful tools to retrieve such data. (3) Strengthening socio-political instruments. Although financial support remains a major limitation to the Mexican PNA’s, federal and local government organisms (including reserve managers) should build a trusted alliance with academia to prioritize an integrative assessment of biodiversity without overlooking insect faunas.
Overall, diverse insect groups have largely been ignored in the Mexican conservation agenda (SEMARNAT, 2002); instead, most programs involve preventing whole-scale ecosystem destruction, combating pollution, or taking deliberate measures to conserve 'representative' biodiversity elements (e.g., vertebrates or plants). Nature reserves are chosen and valued predominantly for their vertebrates and vegetation (see Ceballos, 2007), and typically it is assumed that other biota elements will be well represented and managed appropriately. However, measures that are successfully conserving vertebrates or plants do not always guarantee that insect populations will be adequately maintained (León-Cortés et al., 2004; Axmacher et al., 2011). Global insect decline has been increasingly recognized, pointing out the lack of reliable population estimates and the incomplete values of species richness (Didham et al., 2020a, 2020b). Understanding species diversity patterns and their change remain as top challenges for conservation planning (Richardson and Whittaker, 2010). In Mexico, one of the shortcomings of tailoring integral conservation strategies is the limited evidence to protect insect populations, essentially because insects have been poorly understood and valued, and population monitoring is urgently needed to assess species spatiotemporal variations (Ferrier and Drielsma, 2010).
Declaration of competing interestThe authors declare no conflict of interest.
AF-B received a post-doctoral scholarship (grant number 30223), through a grant from the National Council for Science and Technology in Mexico (CONACYT) to JLL-C (258792: CB-2015-01). MM-R received a doctorate scholarship (grant number 670510) from the National Council for Science and Technology in Mexico (CONACYT).