The objective of this study was to evaluate the combination of some sampling gears operated in different biotopes on fish species richness and species composition. Fish were collected from five different types of biotopes in the Upper Parana River floodplain, according to the most suitable sampling gear for the characteristics of each biotope. A total of 116 fish species were identified in the samples and the highest species richness (68 species) was recorded in streams sampled with boat electrofishing. Rivers and open lakes sampled with gillnets showed the greater similarity between the biotopes, while creeks sampled with electrofishing and open lakes sampled with gillnets showed the least. There were significant differences in species composition among the combinations of biotopes/fishing gears. The results of this study demonstrate the importance of using a variety of fishing methods to sample the different biotopes within a region. We emphasize the importance of well-conducted inventories that take into account the particularities of individual environments.
Initiatives for the conservation of fishery resources implemented by the electrical power companies in Brazil have been largely ineffective. This is mainly due to a lack of information about the species assemblages in the areas targeted by conservation initiatives, once the mitigation is used in areas already flooded. Moreover, the management measures are used opportunistically by having great popular acceptance, as the stocking and the building of fishways, both a posteriori of the reservoir (Agostinho et al., 2010).
In the 1990s, the Resolution 001/86 of the National Environmental Council (Conselho Nacional do Meio Ambiente (CONAMA)) established the need for an Environmental Impact Assessment (EIA) for projects involving the exploitation of or interference with water resources. However, in many cases, the assessment of impacts on aquatic communities and the decision making on measures to mitigate these impacts are still hampered by inadequate sampling procedures (Agostinho et al., 2007; Silveira et al., 2010).
With regard to methodological procedures, the selection of sampling techniques and equipments for inventories aiming to determine which and how many species are present at specific biotope should be based on a well-planned sampling design that takes into consideration the research questions, the habitats to be studied, the species, and the sampling period (Portt et al., 2006). In relation to fishing gears for sampling [e.g., gillnets (the most used, sometimes the only one), hooks, seines, fish-traps, cast nets, sieves, and electrofishing, among others], it is essential to consider the different biotopes to be sampled. Fishing devices select for different species, just as species select different habitats (Olin and Malinen, 2003). Fishing gear can be active or passive. Active gear is moved to capture fish. Passive gear is stationary and fish swim into it. Gears can vary in selectivity, according to the fish species and environment (Lapointe et al., 2006; Portt et al., 2006). Thus, the use of several fishing gears is fundamental to obtain high quality surveys and the use of a limited number of these gears is a key limitation in EIA sampling designs (Silveira et al., 2010).
The components of biodiversity (e.g., α, β and γ) are strongly underestimated when only a single fishing strategy or a limited number of strategies are used and when the sampled environments are all relatively similar to each other. Therefore, the objective of this study was to estimate α and γ diversity using combinations of fishing methods operated in different biotopes in the Upper Paraná River floodplain. It is expected that the methods are complementary to estimated γ diversity and the use of a single method or combinations of some methods underestimated it substantially.
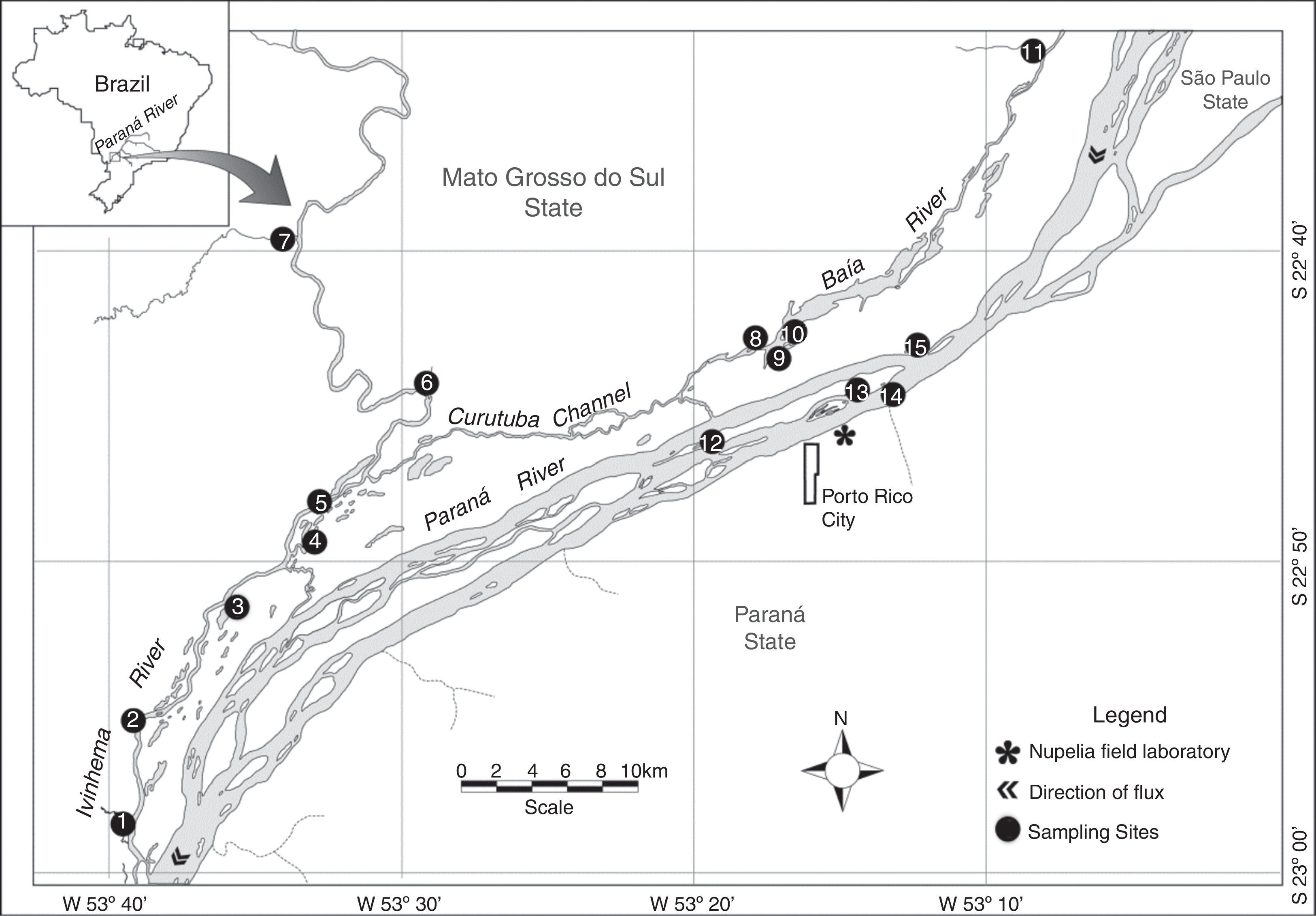
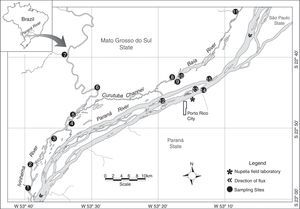
Materials and methodsStudy areaThis study was conducted in the floodplain of the Upper Paraná River, downstream Engenheiro Sérgio Motta Dam (locally known as Porto Primavera) and upstream Itaipu Reservoir. This 230-km reach represents the last significant dam-free stretch of the Paraná River within Brazil and plays a key role in maintaining aquatic biodiversity and fisheries of the region (Agostinho et al., 2000; Hoinghauss et al., 2009). The floodplain in the study reach is characterized by a high diversity of biotopes (see Supplementary material) and large variation in water levels, both in the Paraná River and two tributaries, the Baía and Ivinhema rivers (Fig. 1).
Study area with the 15 sampling sites. (1) Curupaí Stream; (2) Peroba Creek; (3) Ventura Lake; (4) Patos Lake; (5) Ivinhema River; (6) Lambaci Creek; (7) Guiraí Stream; (8) Guaraná Lake; (9) Baía River; (10) Fechada Lake; (11) Perdiz Stream; (12) Osmar Lake; (13) Pau Veio Lake; (14) Paraná River; (15) Garças Lake.
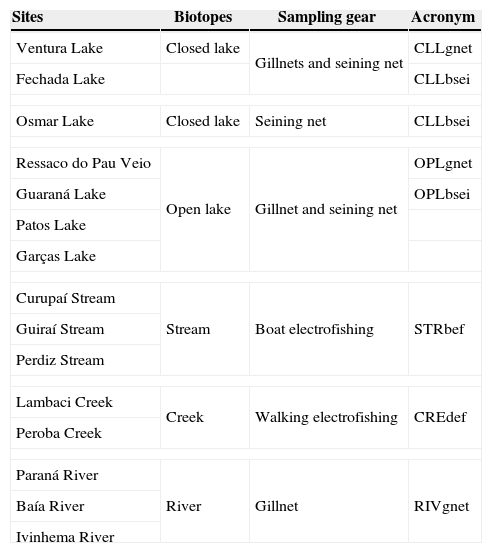
Fish samples were collected at 15 sites in May–June (dry season) and November–December (wet season) 2012, totaling 42 samples (Table 1). The areas explored (Biotopes) included floodplain lakes that remain isolated from the main channel for most of the year (closed lakes; Ventura, Osmar, and Fechada), lakes that are connected to the main channel throughout the entire hydrological cycle (open lakes; Ressaco do Pau Veio, Guaraná, Patos, and Garças), three streams (Curupaí, Guiraí, and Perdiz), and two creeks (Lambaci and Peroba), in addition to the main channels of the Paraná, Baía, and Ivinhema rivers (Fig. 1). The sampling gears used included gillnets (Fig. S1), seining nets (Fig. S2), walking and boat electrofishing (Figs. S3, S4a, and S4b), and longline. Data obtained with the latter were not used due to low catch rates (see Supplementary material for details).
List of sites, biotopes, and sampling technique employed in every one of them. It is also supplied with the acronyms used along the text.
| Sites | Biotopes | Sampling gear | Acronym |
|---|---|---|---|
| Ventura Lake | Closed lake | Gillnets and seining net | CLLgnet |
| Fechada Lake | CLLbsei | ||
| Osmar Lake | Closed lake | Seining net | CLLbsei |
| Ressaco do Pau Veio | Open lake | Gillnet and seining net | OPLgnet |
| Guaraná Lake | OPLbsei | ||
| Patos Lake | |||
| Garças Lake | |||
| Curupaí Stream | Stream | Boat electrofishing | STRbef |
| Guiraí Stream | |||
| Perdiz Stream | |||
| Lambaci Creek | Creek | Walking electrofishing | CREdef |
| Peroba Creek | |||
| Paraná River | River | Gillnet | RIVgnet |
| Baía River | |||
| Ivinhema River | |||
Species richness (α diversity) was calculated for each sample. The number of species registered only using gillnets (the most used sampling gear in most surveys) was compared to against the number of species captured with seining nets and electrofishing. The number of species sampled and the number of unique species were also counted for each biotope/sampling gear combination. The number of species identified by each combination of biotopes and sampling gears was also counted (using the Complementarity Index) as the shared species by each pair that can be expressed as similarity (Jaccard similarity index; details in Colwell and Coddington, 1994). In addition, the percentage that each combination sampled in relation to the total richness (γ diversity) was also computed.
As the Complementary Index deals with combinations (Pairs) of biotope/fishing technique, we applied non-metric multidimensional scaling (NMDS) (Legendre and Legendre, 1998) using a Jaccard similarity (Resemblance) matrix (presence and absence data to minimize the effect of gear selectivity), to ordinate the captured fish assemblages in all combinations of biotopes and fishing gears simultaneously. A PERMANOVA main-test was applied to the resemblance (Jaccard similarities) matrix to test for differences in the assemblages captured for the combination of biotopes and fishing gears, and a posteriori tests were subsequently used to quantify the degree by which each factor differed (Anderson et al., 2008), totaling 21 pairwise comparisons.
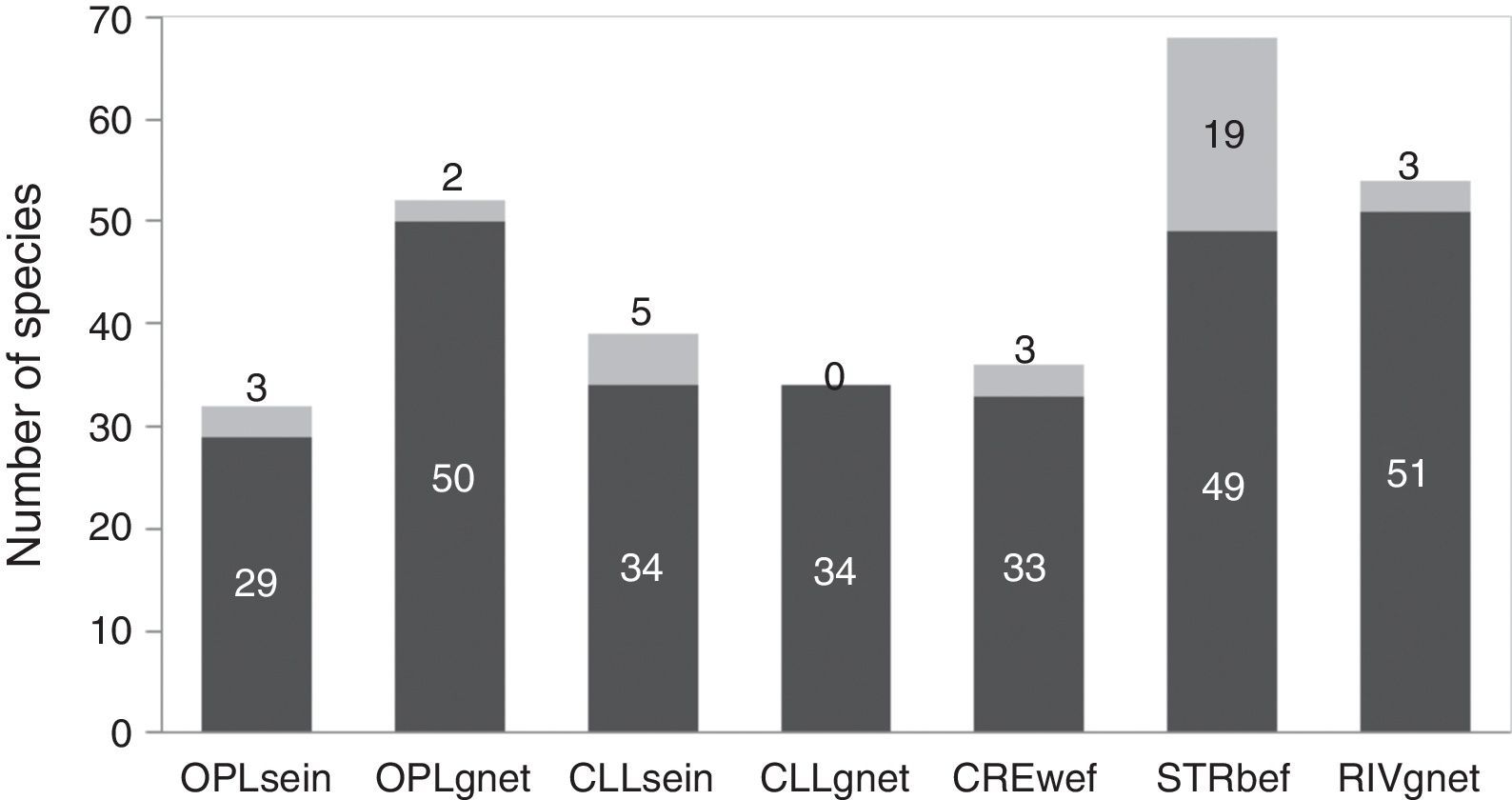
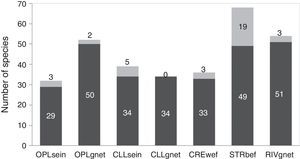
ResultsWe captured 116 fish species distributed in eight orders and 30 families. If the samplings were conducted only with gillnets (operated in open and closed lakes and river channels), the number of species would be 65 (only 56.0% of the total richness – γ diversity). Using only seining nets, this value was 49, whereas only with electrofishing the number was 73 (see Supplementary material for details). The last fishing gear was employed only in streams and creeks, usually not considered in surveys conducted to produce EIA. Out of the 116 species caught, OPLsein sampled 27.58%, OPLgnet 44.82%, CLLsein 33.62%, CLLgnet 29.31%, RIVgnet 46.55%, CREwef 31.03% and STRbef 58.62%.
The analysis of the different combinations of biotopes and fishing gear employed showed that streams sampled with boat electrofishing (STRbef) yielded the greatest number of species (S=68), while sampling with gillnets in closed lakes presented the lowest number (S=34) and the only combination without unique species (Fig. 2).
Number of species sampled in each biotope with each fishing gear, with unique species shown in gray. OPLsein=open lakes using seining nets; OPLgnet=open lakes using gillnets; CLLsein=closed lakes using seining nets; CLLgnet=closed lakes using gillnets; CREwef=creeks using walking electrofishing; STRbef=streams using boat electrofishing; RIVgnet=rivers using gillnets; numbers at the top of each bar indicate unique species.
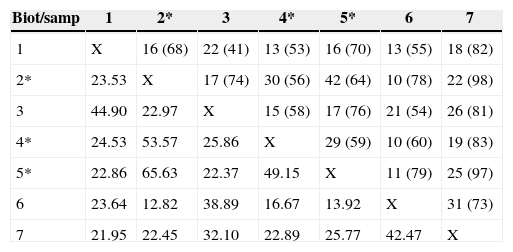
The pair of combinations that resulted in greater species richness was OPLgnet and STRbef (S=98, 22 shared; similarity=22.45), while the pair with lower richness was OPLbsei and CLLbsei (S=49, 22 shared; similarity=44.90) (Table 2). Overall, lower values of similarity (Complementary Index) were observed with the combinations of gillnets and other biotope/sampling gear, especially with walking electrofishing in creeks (Table 2). Only 2 species were shared by all combinations (biotope plus gear). The number of shared species was higher between OPLgnet and RIVgnet (42 of 64 species, 65.6%) and lower between OPLgnet and CREdef (10 of 78 species, 12.8%).
Number of species shared and the total number of identified species (in brackets) with the pairs of combination of biotope with more fishing gear (Biot/Samp) (upper half to the matrix) and percentage of similarity between pairs (Jaccard Similarity Index; lower half of the matrix). Open lake/seining net=1; open lake/gillnet=2; closed lake/Seining net=3; closed lake/gillnet=4; river/gillnet=5; creek/walking electrofishing=6; stream/boat electrofishing=7 (*sampling with gillnets).
| Biot/samp | 1 | 2* | 3 | 4* | 5* | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 | X | 16 (68) | 22 (41) | 13 (53) | 16 (70) | 13 (55) | 18 (82) |
| 2* | 23.53 | X | 17 (74) | 30 (56) | 42 (64) | 10 (78) | 22 (98) |
| 3 | 44.90 | 22.97 | X | 15 (58) | 17 (76) | 21 (54) | 26 (81) |
| 4* | 24.53 | 53.57 | 25.86 | X | 29 (59) | 10 (60) | 19 (83) |
| 5* | 22.86 | 65.63 | 22.37 | 49.15 | X | 11 (79) | 25 (97) |
| 6 | 23.64 | 12.82 | 38.89 | 16.67 | 13.92 | X | 31 (73) |
| 7 | 21.95 | 22.45 | 32.10 | 22.89 | 25.77 | 42.47 | X |
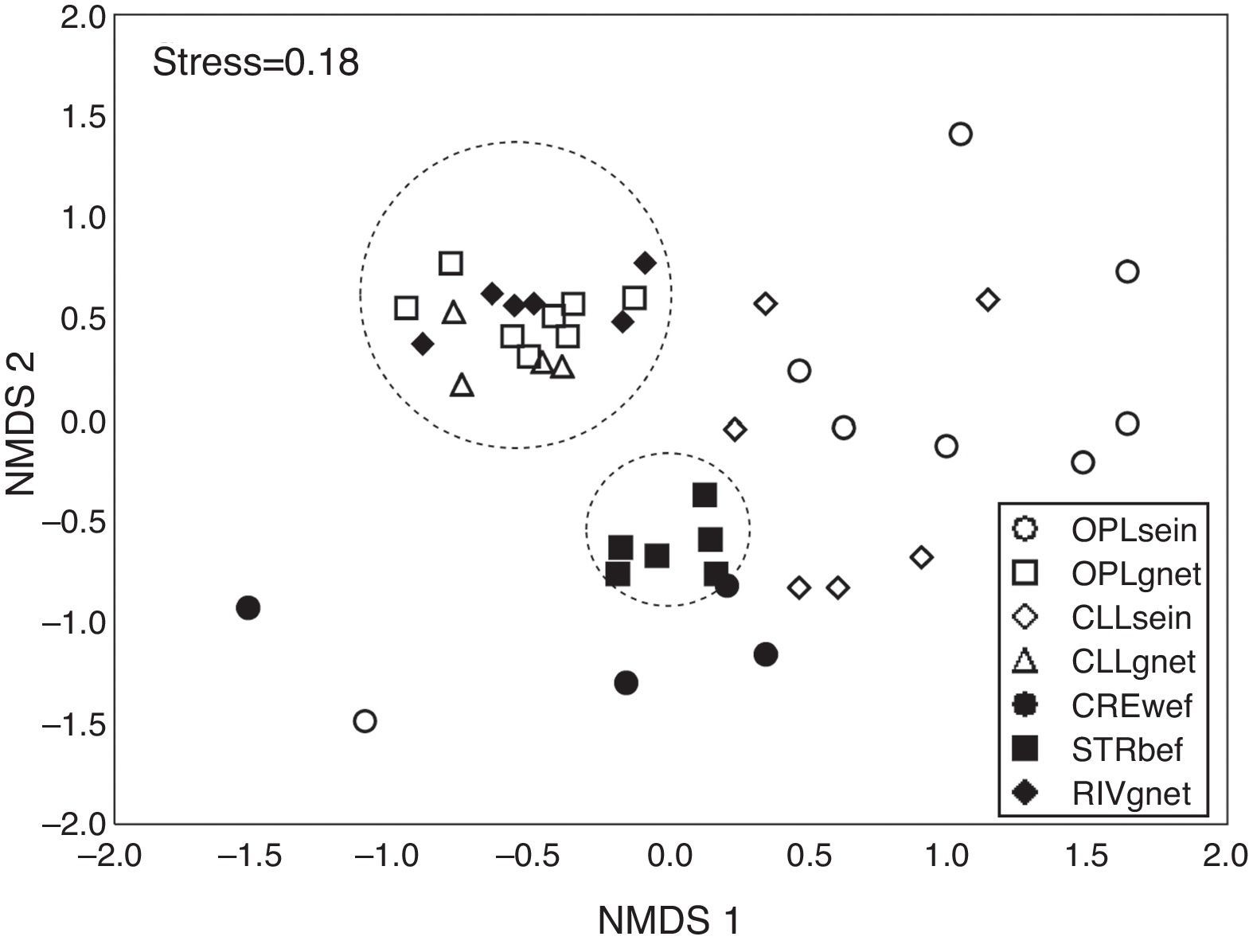
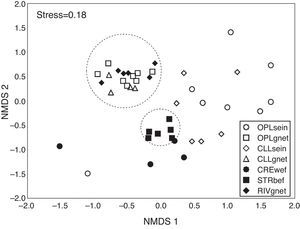
The ordination resulted from the NMDS (Stress=0.18) clearly showed a clustering of samples from biotopes sampled using gillnets and samples obtained by boat electrofishing in streams. In contrast, samples from open and closed lakes and creeks sampled with walking electrofishing were scattered across the plot, indicating high heterogeneity within each sample and among the different sampling methods (Fig. 3).
Ordination resulted from the application of the non-metric multidimensional scaling (NMDS) to the presence×absence data for the combination of biotope/fishing gear. OPLsein=open lakes using seining nets; OPLgnet=open lakes using gillnets; CLLsein=closed lakes using seining nets; CLLgnet=closed lakes using gillnets; CREwef=creeks using walking electrofishing; STRbef=streams using boat electrofishing; RIVgnet=rivers using gillnets.
The PERMANOVA showed significant differences among the combinations of biotopes/fishing gears (pseudo-F=3.46; p (perm)<0.01). The pairwise post hoc tests indicated statistically significant differences between most of the biotope/fish gear combinations. However, there was no statistically significant difference between open lakes sampled using gillnets and closed lakes sampled using gillnets and between open lakes sampled with gillnets and rivers sampled with gillnets (Fig. 3). Species composition differed between open and closed lakes samples, and within a given biotope (open or closed lake), the samples differed by type of fishing gear used, as shown in the post hoc PERMANOVA test (Table S1, Supplementary material).
Despite low catch rates (18 individuals), six species were caught with longlines, including four migratory species of high commercial interest (Pseudoplatystoma corruscans (Spix & Agassiz 1829), Pterodoras granulosus (Valenciennes 1821), Pinirampus pirinampus (Spix & Agassiz 1829) and Pseudopimelodus mangurus (Valenciennes 1835) (exclusive for this fishing method).
DiscussionFish fauna inventories, especially those performed for impact assessments (EIA) for large engineering projects have been criticized for using an insufficient number of samples and a short study period (Agostinho et al., 2007). Indeed, the results of this study show that imposing limits on the types of biotopes sampled or the fishing gears used may lead to underestimation of the diversity of fish in aquatic inventories.
All fishing gears have restrictions and limitations. Active gears will collect more species than passive gears, highlighting the need to use them as complementary. This can be exemplified with the samplings carried out in open lakes with gillnets and in the streams sampled with boat electrofishing, which together captured 98, but only 22 shared. Moreover, only two species were present in the catches of all combinations. Therefore, fishing strategies should be selected according to the sampling goals and the characteristics of the biotope and biota. There is no single, universal method of investigating the ichthyofauna (Penczak et al., 1998).
The combinations with higher similarity in species composition (65.6%) were open lakes/gillnets and rivers/gillnets. This result shows that regardless of the biotope, gillnets sampled similar portions of the assembly. Gillnets are highly selective and a passive method that generally is not effective for sampling fish with low mobility or slender shapes (Growns et al., 1996; Lapointe et al., 2006; Portt et al., 2006). The choice of a wide range of mesh sizes for gillnets minimizes selectivity by ensuring the capture of fish of varying sizes and life stages. Furthermore, their use is important in inventories because the catches contain species not caught by other methods (Penczak et al., 1998). Conversely, the lowest similarity was between streams/boat electrofishing and open lakes/gillnet. This reinforces the importance of sampling different habitats, adapting methods to their characteristics.
Only two pairs of combinations of biotopes/fishing gears did not show statistically significant differences in assemblage composition: open lakes/gillnets and closed lakes/gillnets; open lakes/gillnets and Rivers/gillnets. This means that the composition of the fish assemblage of open lakes is similar to rivers and closed lakes, at least with respect to the portion likely to be caught with gillnets. Despite this, sampling in these lakes cannot be ignored because five species were unique to gillnetting samples and three species were unique to seining nets’ samples in this biotope. This result should be considered carefully for future studies, especially when the purpose of sampling is to complete a fish fauna inventory.
The use of electrofishing was restricted to creeks and streams, where it is generally considered the most appropriate method (Growns et al., 1996; Knight and Bain, 1996; Penczak et al., 1998). Despite using the same method, the similarity between creeks and streams was only 42.2%, indicating heterogeneity in species composition between the biotopes (high β diversity), confirmed by post hoc analysis of PERMANOVA. This method is not selective in more structured environments because the shock induces fish to follow the electric current. It also has the advantage to sample both sedentary and active fish, unlike gillnetting, in which capture depends on the movement of the fish (Growns et al., 1996).
In this study, streams sampled by boat electrofishing accounted for 58.6% of the total number of species collected and yielded the largest number of unique species, showing statistically significant differences in α diversity and composition from the other biotope/fishing gear combinations. Creeks and streams are rarely included in environmental studies for the purposes of licensing and project permitting even though creeks and streams, which have high ecological and taxonomic diversity, may disappear completely following the formation of reservoirs (Winemiller et al., 2008).
Although the fish captured using longlines were not included in the analyses, this gear should be used in a complementary manner with other techniques in sampling procedures because it often captures species that are occasionally not captured using other methods, as in the case of a long-distance migratory specie (Pseudopimelodus mangurus) in this study. Thus, longlines should be used, especially in rivers where migratory species may be vulnerable to the impact of large engineering projects.
The sampling of different biotopes is essential for conducting reliable fish fauna inventories and realistic impact assessments and requires the use of several gears that are appropriate for the physical characteristics of a given environment. Each gear captures a different portion of the fish assemblage, resulting in varying assessments of habitat (Achleitner et al., 2012). Therefore, regional richness (γ diversity) is dramatically underestimated when a limited number of sites and types of fishing gears are used for sampling fish. The highest number of combined methods possible should be used to obtain a γ diversity estimate that is as close as possible to the true value. The use of combined methods to obtain a more representative sample also enables a better understanding of the local fish assemblage, fish dynamics, and habitat use, with direct implications for developing successful management measures for conservation (Lapointe et al., 2006).
The use of only a few sampling methods in fish fauna inventories is usually the result of the short timelines required by the licensing process of engineering projects and the desire of the companies completing the projects to maintain low costs. As shown in a technical report for engineering projects, sampling methodologies rarely include the use of more than two fishing gears, and samples are typically taken from various sites within the same biotope, with emphasis on the main channel of a river and gillnet sampling (IAP, 2013). In many cases, these studies do not even include essential areas for the life cycles of large-sized migratory fish, including spawning and nursery areas, in their sampling design. Failing to sample the habitats of large migratory fish goes against the recommendations of the Coordinating Committee of Environmental Activities in the Electric Sector (Comitê Coordenador das Atividades de Meio Ambiente do Setor Elétrico [COMASE]) for studies conducted in preparation for the implementation of engineering projects (Agostinho et al., 2007).
The results of this study, which was conducted in an environment that exhibits large environmental heterogeneity and retains many natural features, may serve as a reference for other surveys by showing the contribution of diverse habitat types to the regional pool of species and emphasizing the importance of including all biotopes in a species inventory. Moreover, the results of this study demonstrate that fishing gears for sampling should be appropriate to the unique characteristics of each environment as well as to the objectives of the study.
Conducting high-quality inventories with well-planned sampling designs are fundamental given the accelerated pace at which projects that affect water bodies and their fish assemblages are licensed and implemented. The recommendations presented in this study will enable a better assessment of project impacts, greater transparency in the decision-making process, and better efficiency in the development of mitigation measures.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thanks CAPES-PROEX for financial supporting, CNPq for scholarship to A. G. O, PELD-site 6, NUPÉLIA and anonymous referees.