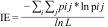- •
Pollination networks have long been studied by quantifying plant-flower visitor species interactions. Despite making considerable contributions, this ignores important steps of pollen movement from anthers to receptive stigmas and neglects the intraspecific variation of the interacting partners. Addressing specialization and niche partitioning regarding heterospecific pollen transport and transfer, is fundamental to untangle the mechanisms behind contrasts seen in the impact of alien species on native communities.
- •
We used two well-sampled datasets on pollen-transport and pollen-transfer networks to test how intraspecific variation in interaction specialisation affects invaded pollination networks. We considered different levels of biological organization: from species- to individual-based networks.
- •
We found significant intraspecific variation in the pollen loads and pollen deposition of the invasive plant Impatiens glandulifera; thus only a few individual pollinators and plant stigmas carried large amounts of alien pollen grains, potentially functioning as super-spreaders driving the invading process. Consequently, most individuals carried only a few, or no alien pollen at all, possibly buffering the negative effects of invasive plants at the population and community levels.
- •
Node and structural specialization were higher for individual-based and pollen-transfer networks, suggesting a lack of dominant, highly generalist links when downscaling from pollen-transport to pollen-transfer, and from species to individual-based networks.
- •
The high specialization, selectiveness and niche partitioning of plants, pollinators and their interaction revealed at the different stages of the pollination process and across distinct levels of biological organization, suggest important mechanisms associated with the (re) organization of population niches. Moreover, these mechanisms provide a promising approach towards a more comprehensive understanding of the dynamics of invasion biology from population to community and ecosystem functioning.
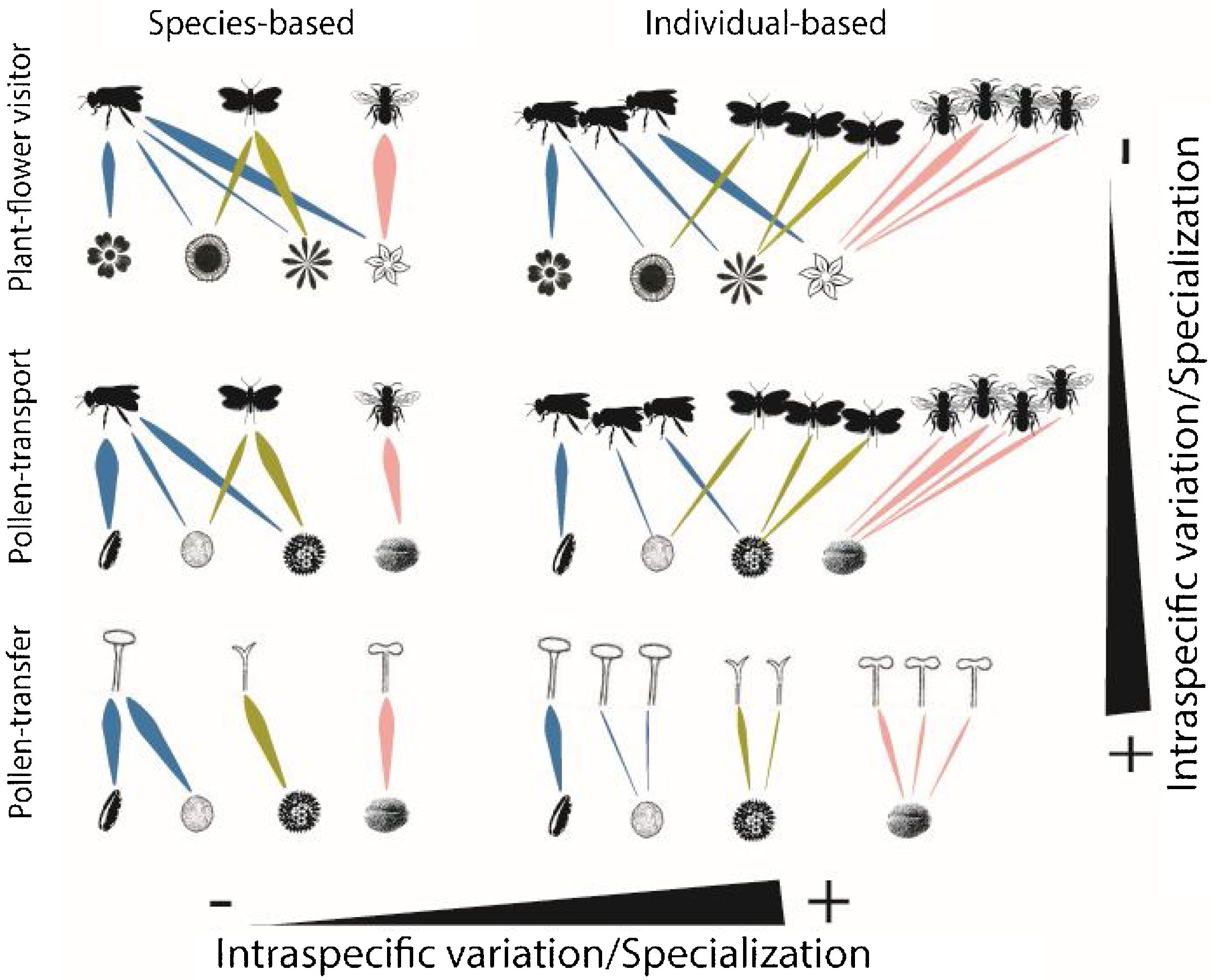
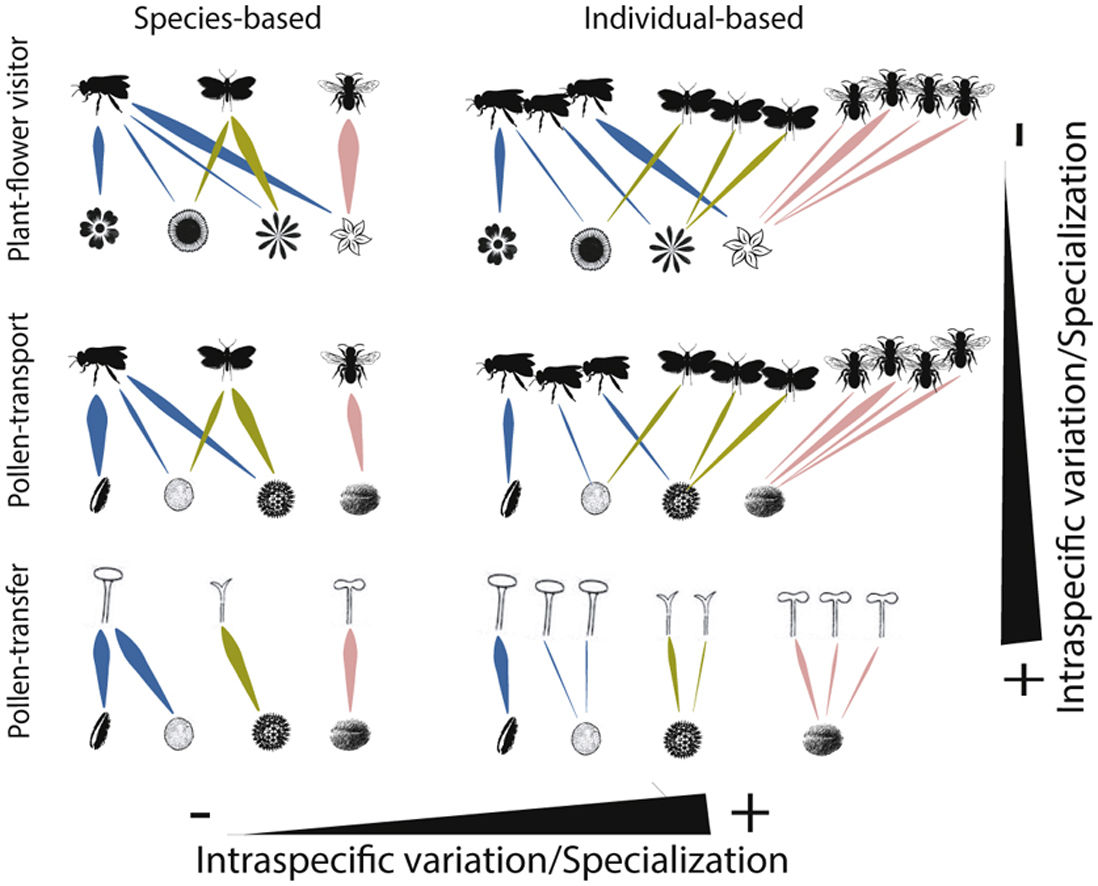
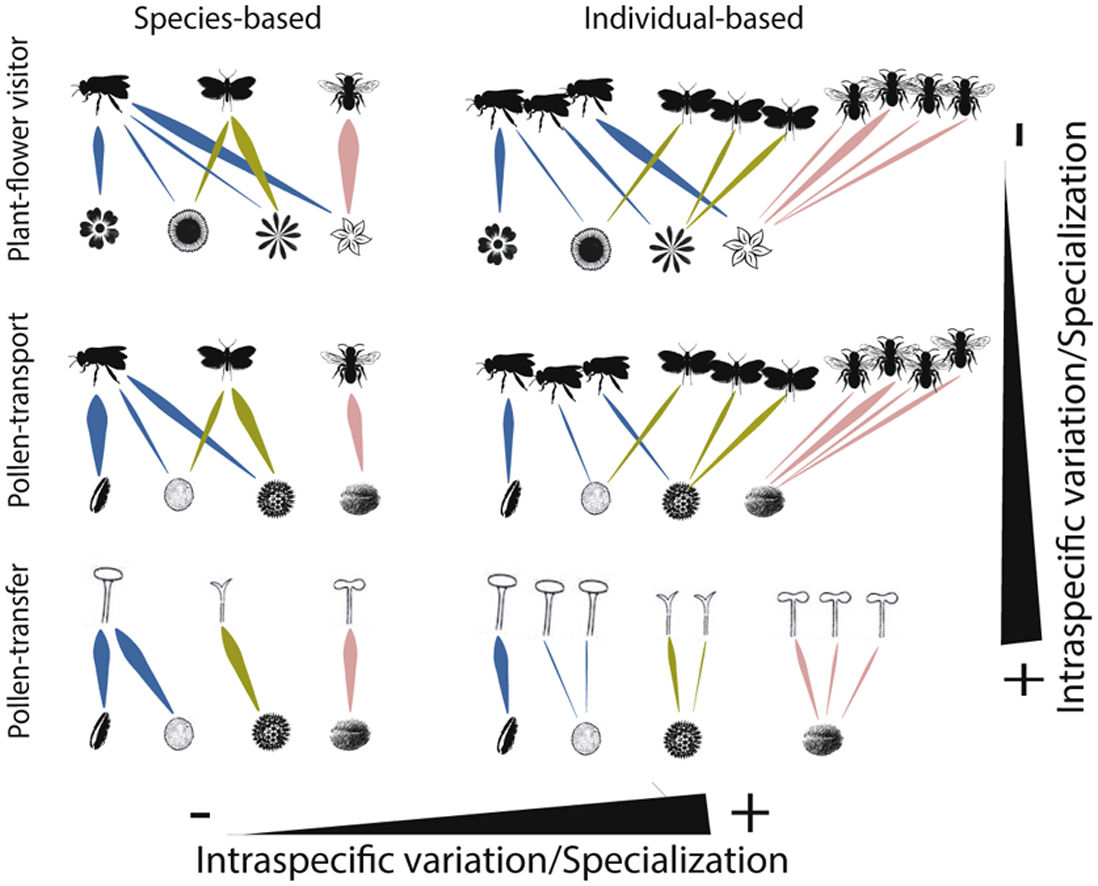
Schematic representation of the role of intraspecific variation and specialization in the distribution of links and nodes in pollination networks when distinct ecological processes (i.e., plant-flower visitor, pollen-transport and pollen-transfer) and levels of biological organization (i.e., species-based and individual-based networks) are taking into consideration.
Pollination ecology has advanced greatly over the last few decades by characterizing the network of interactions between plants and pollinators, an approach which has increased our understanding of the ecological and evolutionary patterns of mutualistic interactions (Memmott, 2002; Bascompte et al., 2003; Olesen et al., 2007; Arceo-Gómez et al., 2019). Pollination networks have also helped to untangle how animal-plant interactions respond to anthropogenic disturbance such as the invasion of alien species (Emer and Timóteo, 2020). However, most studies in pollination ecology are based on the interactions among species, ignoring the role of individual variation (but see, e.g., Dupont et al., 2011; Gomez et al., 2011; Gomez and Perfectti, 2012; Russel et al., 2021), which has greatly contributed to understand niche partitioning and foraging theory elsewhere (e.g., Araújo et al., 2011, Violle et al., 2012, Des Roches et al., 2018). Moreover, evidence is mounting against the use of plant-flower visitation as the only surrogate for pollination, which neglects important functional mechanisms that affect pollination effectiveness and plant fitness (Alarcón, 2010; Fang and Huang, 2013; King et al., 2013; De Santiago-Hernández et al., 2019; Lázaro et al., 2019). Among those mechanisms, the movement of conspecific and heterospecific pollen mediated by plant-animal interactions is a fundamental step in the pollination process (Wei et al., 2021). Understanding pollen transport and deposition at the community level can be done by quantifying pollen-transport and pollen-transfer networks, respectively.
Both pollen transport and pollen transfer can be modified by the presence of invasive plant species (Jhonson and Ashman, 2019; Parra-Tabla et al., 2020) that might competitively exclude native species depending, in part, on how they share pollinators’ niche (Pauw, 2013; Arceo-Gómez et al., 2019; Ashman et al., 2020). Theory predicts that pollinators’ niche partitioning is an important driver of plant species coexistence and diversity (Pauw, 2013; Bergamo et al., 2020, Wei et al., 2021). Pollinators’ niche partitioning is associated with the plant specialization continuum in terms of interacting partners that ultimately influences heterospecific and conspecific pollen movement and therefore, plant fitness (Pauw, 2013, Armbruster, 2017, Bergamo et al., 2020; Wei et al., 2021). Thus, high pollinators’ niche overlap in invaded communities can be hypothesized to increase heterospecific pollen movement that would affect the structure of pollen-transport and pollen-transfer networks. Usually, the impact of alien plants is considered as the average or summed impact on native plant species, i.e., the impact of alien plant species x on native species a, b, c and d. By summarizing information at the species level, intraspecific variation is lost, and it is assumed that all individuals contribute equally to niche partitioning and foraging strategies thereby neglecting the potential impacts of the pervasive individual variation in nature (Van Valen, 1965; Bolnick et al., 2011; Violle et al., 2012; Des Roches et al., 2018). For example, ignoring the individual variation in pollinator behavior (Bosch et al., 2009; Harder and Aizen, 2010; Song and Feldman, 2014; Russel et al., 2021) or floral constancy (Raine and Chittka, 2007; Huang et al., 2015) by summing or averaging these in species-based networks can distort our understanding of pollination outcomes. Similarly, by assuming that flower-visitation is a surrogate for pollination, we neglect other factors that influence efficient pollen transfer (King et al., 2013; Ballantyne et al., 2015), such as heterospecific pollen movement mediated by shared pollinators that visit distinct plant species simultaneously (Morales and Traveset, 2008; Bergamo et al., 2020), and the molecular and physiological interactions that take place at the pollen-stigma interface (Hiscock et al., 2002; Ashman and Arceo-Gómez, 2013).
Pollination scientists are gradually overcoming these challenges by analyzing different levels of biological organization (Guimarães, 2020), moving from traditional flower-visitor networks to pollen-transport networks that consider the transport of pollen on animal’ bodies (e.g., Lopezaraiza-Mikel et al., 2007; Devoto et al., 2011; Travesest et al., 2014; Zaho et al., 2018) and pollen-transfer networks that look at the animal-mediated movement of pollen from anthers to stigmas of distinct plant species (e.g., Fang and Huang, 2013; Arceo-Gómez et al., 2018, Parra-Tabla et al., 2020). However, collecting and processing data for pollen-transport and pollen-transfer networks is already very challenging and time-consuming, and it complicates matters further if intraspecific variation and the dynamics of invasive species also need to be considered. Consequently, most studies to date investigate either one type of network (pollen-transport, pollen-transfer) or level (species-based, individual-based) and the effects of invasive species, when present, are analyzed within and not among those levels. Here though, we test how an alien plant species shapes invaded network structure at different levels of biological organization, considering variation in specialization at the node-level (individual-based and species-based networks) and in two types of networks (pollen-transport and pollen-transfer networks).
The first individual-based pollen-transport networks hosting multiple plant species was constructed by Tur et al. (2014). The results from their two field sites revealed higher node specialization in the pollinators' use of pollen (i.e., pollinators’ niche partitioning) than that seen in species-based networks. Such intraspecific variation in specialization may be more common and more important than previously thought in structuring higher species-species level interactions, determining niche partitioning and optimal foraging strategies (Araújo et al., 2011; Violle et al., 2012; Des Roches et al., 2018) in invaded communities. In turn, Lopezaraiza-Mikel et al. (2007) showed that in the presence of the highly invasive plant species, Himalayan balsam, Impatiens glandulifera (hereafter balsam), pollen-transport networks are dominated by alien pollen grains which are transported around on pollinators` bodies. Thus 96% of pollen grains on the insect’s body were balsam at the invaded sites. Given how generalized pollination systems are assumed to be (Waser et al., 1996; Waser and Ollerton, 2006) and that balsam tends to be highly abundant in invaded sites, functioning as a ‘magnet’ for pollinators (Chittka and Schurkens, 2001), it seems likely that these pollen grains will be transferred to the stigmas of the native plants, potentially negatively affecting native plant reproduction by stigma clogging (Morales and Traveset, 2008). However, research by Emer et al. (2015) found that, contrary to expectation, there were very low rates of alien balsam deposition on stigmas, and pollen deposition was restricted to a small subset of species. Both Lopezaraiza-Mikel et al. (2007) and Emer et al. (2015) studied pollinators at the species level, but their field recordings were made at the individual level. Moreover, both collected data in the same general area, thus providing extensive overlap in plant and pollinator species. These records provide an excellent opportunity to investigate the differences in intraspecific variation and pollinators’ niche partitioning between species-based and individual-based networks in two distinct but complementary levels of the pollination process: pollen-transport (using Lopezaraiza-Mikel et al., 2007) and pollen-transfer to stigmas (using Emer et al., 2015).
Here our aim is to determine the magnitude of intraspecific variation in determining specialization in multiple aspects of invaded pollination systems, from pollen-transport to pollen-transfer networks, scaling-down from species to individual levels. Specifically, we have four objectives: 1) quantify intraspecific variation in the balsam pollen load of the two commonest insect pollinators at the eight field sites used by Lopezaraiza-Mikel et al. (2007); 2) quantify the intraspecific variation in balsam pollen deposition on stigmas of the three commonest plant species studied at the 20 sites investigated by Emer et al. (2015); and to test for differences in 3) species and individuals specialization in terms of interacting partners, considering the commonest pollinator and plant species in both network types, and 4) network specialization in terms of selectiveness, evenness and niche partitioning of the overall plant-pollinator interactions, considering the individual- and the species-based networks for both pollen-transport and pollen-transfer networks. Because pollinator behavior varies according to their nutritional needs (Gegear and Thomson, 2004; Russel et al., 2021), levels of fidelity and grooming (Brosi and Briggs, 2013; Willmer and Finlayson, 2014), we expect to find (1) high variation among individuals in the amount of balsam pollen found on their bodies. Associated to that, negative density-dependent effects (Bergamo et al., 2020) and trait variation in floral morphology can affect pollinator effectiveness (Lázaro et al., 2019), ultimately affecting the amount of pollen grains arriving at the stigmas, therefore we expect (2) that a few individual plants would have significantly higher amounts of balsam pollen on their stigmas, deviating from a normal distribution and with values higher than expected by chance. Consequently, (3) node specialization, considering its total number of distinct pairwise interactions and interaction selectiveness, will be higher at the individual-based networks because of the higher niche partitioning expected at this level, for both pollen-transport and pollen-transfer networks. Finally, (4) network specialization will be higher for individual-based, pollen-transfer networks as we expect that the niche of individual plant stigmas regarding pollen deposition would overlap less than species-based, pollen-transport networks, and the links between plant stigmas and pollen types are less diverse and more evenly distributed at this level of biological organization due to the physiological and chemical mechanisms in place to avoid heterospecific pollen deposition (Allen et al., 2011; Ashman et al., 2020).
Materials and methodsWe used two independent datasets both of which studied plant-pollinator networks invaded by Impatiens glandulifera, namely the eight pollen-transport networks published by Lopezaraiza-Mikel et al. (2007), and the 20 pollen-transfer networks published by Emer et al. (2015). Lopezaraiza-Mikel et al. (2007) collected data from an experiment composed of four experimental plots, in which flowers of I. glandulifera were manually removed; these were paired with four adjacent control plots. Insects visiting flowers in the study sites were collected and swabbed with fuchsin jelly to identify and quantify the pollen types transported on the insect`s bodies, thereby obtaining pollen-transport networks. Emer et al. (2015) collected data from 20 sites – 10 invaded and 10 non-invaded by I. glandulifera, with fieldwork conducted in the same city (Bristol, England) as Lopezaraiza-Mikel et al. (2007). Emer et al. (2015) built pollen-transfer networks by sampling stigmas of the flowering plants at each site to identify and quantify the pollen types deposited in each stigma, thus forming plant-plant interactions represented by the deposition of shared pollen types on plant stigmas. Pollen types were identified at the species and genera level, morphotypes and non-identified pollen grains. Balsam pollen is quite distinct from the native species, which facilitates the distinction between invasive and native pollen grains.
For the objectives 1, 2, and 3 described in detailed below, we only used sites with at least five individuals of the most common species – these being the pollinators Apis mellifera and Bombus pascuorum from Lopezaraiza et al. (2007) and the plants Calystegia sepium (Convolvulaceae), Epilobium hirsutum (Onagraceae), and Circaea lutetiana (Onagraceae) from Emer et al. (2015). These were chosen as target species based on their high abundance and widespread nature, thus providing sufficient replicates for statistical purposes.
Building the individual-based and the species-based networksWe studied individuals of pollinator and plant species in both datasets to evaluate intraspecific variation and specialization in pollen-transport and pollen-transfer comparing these to the species-based networks in the original studies. All individuals within a given species were considered as independent nodes in these networks. Networks were defined as weighted Aij adjacency matrices, thus considering the frequency of interactions among nodes. For pollen-transport networks, i represents a pollinator species in the species-based networks and an individual pollinator in the individual-based networks. Then, the aij element corresponds to the amount of pollen loads on pollinators’ body at the species and individual levels, respectively. For pollen-transfer networks, i is a plant species in the species-based networks, and an individual plant at the individual-based networks. The aij element is the amount of pollen grains deposited on plants’ stigmas at the species and individual levels, respectively. The amount of pollen grains on stigmas is estimated by the average pollen load of three open flowers per individual plant (if that was not possible, we considered the number of pollen grains present in one or two flowers, as available in the sampling area). Thus, the aij is the sum of the average pollen load of all individuals of a given plant species in the species-based, pollen-transfer networks and the average pollen load per individual plant at the corresponding individual-based networks. In all cases, j corresponds to a pollen type, keeping the number of columns in the adjacency matrix constant in both individual- and species-based networks (Fig. 1, Table S1).
A hypothetical diagram showing how the different types and levels of animal-mediated pollination are assembled in ecological networks and the role of intraspecific variation on the arrangement of network links. Species-based networks aggregate all the individual information of each plant and pollinator species of a given community into a single unit – the species. Individual-based networks consider each individual of a given species as a single unit in the network, therefore taking into account the intraspecific variation that may affect structural patterns. Plant-flower visitor networks use flower visitation as a proxy for pollination; pollen-transport networks consider the pollen load on pollinator’s body after a flower-visitation event while pollen-transfer networks consider the pollen deposition on stigmas as a baseline in which pollination takes place.
Balsam pollen load on pollinators’ bodies was measured as the number of balsam pollen grains counted on the body of each individual of A. mellifera and B. pascuorum recorded in the pollen-transport networks. Balsam pollen deposition on plant stigmas was measured as the number of balsam pollen grains deposited on individual stigmas of C. sepium, E. hirsutum and C. lutetiana likewise recorded in the pollen-transfer networks. Both individual pollen load and pollen deposition were compared to the quantities recorded for the corresponding species level data (see section on the statistical analyses).
Objective 3. Testing for differences in species and individuals’ specializationWe estimated node specialization considering the target pollinator (A. mellifera and B. pascuorum) and plant species (C. sepium, E. hirsutum, C. lutetiana) by calculating their k degree and d’ specialization index (Blüthgen et al., 2006). Here, degree is the number of distinct pollen types found on pollinator’s bodies and/or deposited on plant stigmas for pollen-transport and transfer networks respectively, at the individual- and species-based levels. For all cases, d’ specialization estimates how selective a given plant or pollinator individual/species is regarding the potential partners available and is based on the full communities in which they were sampled.
Objective 4. Testing for differences in network specializationConsidering the entire dataset of both target studies, i.e., the networks containing all the plant and pollinator species sampled, we aim to understand whether changing from species- to individual-based networks, and from pollen-transport to pollen-transfer networks increases overall network structural specialization and pollinators’ niche partitioning. The results are expected to help to understand the generalism/specialism conundrum in pollination systems (Waser et al., 1996; Armbruster, 2017; Ashman et al., 2020). To do this, we used three complementary, non-correlated metrics, that give us different perspectives about the overall structural specialization, as follows:
i) H’2 specialization: this metric describes how the observed distribution of interactions deviates from an expected distribution based on the number of interactions per species in the network (Blüthgen et al., 2006). The higher the pairwise species selectivity, the higher its contribution to the overall network specialization. This index is based on the Shannon entropy, constraining the total number of interactions for each species; its final value is scaled between H’2min and H’2max such that the higher the value, the higher the network specialization.
ii) Interaction evenness (IE): this estimates the heterogeneity of links between the two interacting groups within the bipartite network. It is calculated as the sum of the proportion of the ij interaction (Shannon-diversity) divided by the total number of interactions, as follows:
in which, pij is the proportion of interactions between i and j across all interactions that i is involved, and L is the number of realized links (Dormann et al., 2009). Its value increases as interaction links become more evenly distributed among species/individuals in both halves of the network. We associate higher IE as a proxy of higher niche partitioning as the links among nodes do not concentrate in a single and/or few nodes but are evenly spread across the network, therefore denoting different uses of resources by the distinct species/individuals.iii) Pollen niche overlap: this estimates the mean similarity of interactions of the pollen type level, calculated by the Horn’s index (Horn, 1966; Dormann et al., 2009). It’s value ranges from zero to one; a niche overlap of zero means that no interactions are shared between pollen types given the use of pollinators’ bodies for transport, or the stigmas for deposition (i.e., higher specialization in the choice of transport or deposition sources); a niche overlap of one means that all pollen species are transported around by the same pollinators’ bodies, or are deposited on the same stigmas (i.e., low specialization).
Statistical analysesTo test for intraspecific variation within the target pollinator and plant species (objective 1 and 2), we used the Jarque-Bera Normality Test (Jarque and Bera, 1980) within the ‘moments’ package (Komsta and Novomestky, 2015). It measures the shape of the individual data distribution for each species, testing whether the distribution is left or right skewed (i.e., skewness), and whether it differs from a normal distribution (i.e., kurtosis); values far from zero indicates the data do not follow a normal distribution. Here, we would expect a normal distribution if most of the individuals perform similarly within the network context, i.e., most pollinators carry similar numbers of balsam pollen grains and most plants have similar numbers of stigmal balsam pollen deposition while only a few individuals would have extremely high or extremely low number of pollen grains, therefore representing the upper and lower tails of a normal distribution. In turn, we would expect a right-skewed distribution if only a few individuals of plants or pollinators have a high number of balsam pollen grains while most of the population have a low number, including many zeros.
To understand whether node specialization (objective 3) varies between network type (transfer and transport) and level (individual- and species-based) we used General Least Square (GLS) and Linear Mixed Models (LMM) in the nlme package (Pinheiro et al., 2016). We fitted individual models for each proxy of node specialization (i.e., degree and d’ specialization) used as the response variable tested against the interaction between network type and network level. Degree was log-transformed and d’ logit-transformed, as it is bounded between 0 and 1, to fit normal distributions. We then selected the best fixed and random structures based on AIC model selection and corrected for boundary effects following Zuur et al. (2009). We started by choosing the best random structure among the following random model candidates: species nested within treatment - to control for pseudoreplication effects within each treatment as the same species could be counted more than once in each plot given the natural variation in the number of individuals; treatment only – to control for differences between invaded and non-invaded areas; and species only – to control for differences in species occurrences among plots. The best random structure model was then used to proceed with the selection of the best fixed effects.
Finally, we fitted individual models for each proxy of structural specialization (i.e., H’2, interaction evenness, niche overlap) that were used as the response variable tested against the interaction between network type and network level (objective 4). Treatment (invaded, non-invaded sites) were added as a random factor to account for potential effects of balsam invasion. Data were log- and/or logit transformed to fit normality assumptions where needed (Warton and Hui, 2011). Model selection based on the Akaike Information Criteria (AICc) follows the protocols suggested in Zuur et al. (2009). We fitted the best model structure to linear regressions with “maximum likelihood estimation” while applying the “dredge” function (Barton, 2013) to select the best-fixed structure according to Akaike Information Criteria (AIC). Then, we used the Akaike weight of evidence (wAICc) to obtain the relative importance of the different models (Burnham and Anderson, 2002). Network metrics were calculated using the bipartite package in R (Dormann et al., 2009); all analyses were run in R v 3.0.1 (R Core Team, 2014).
ResultsOverall, we studied 566 individuals from the five focal species (A. mellifera: 38 individuals; B. pascuorum: 52; C. sepium: 68; C. lutetiana: 111; E. hirsutum 297). Differences in the number of individuals within each species and between invaded and non-invaded areas were controlled by the random factors in our models (see methods).
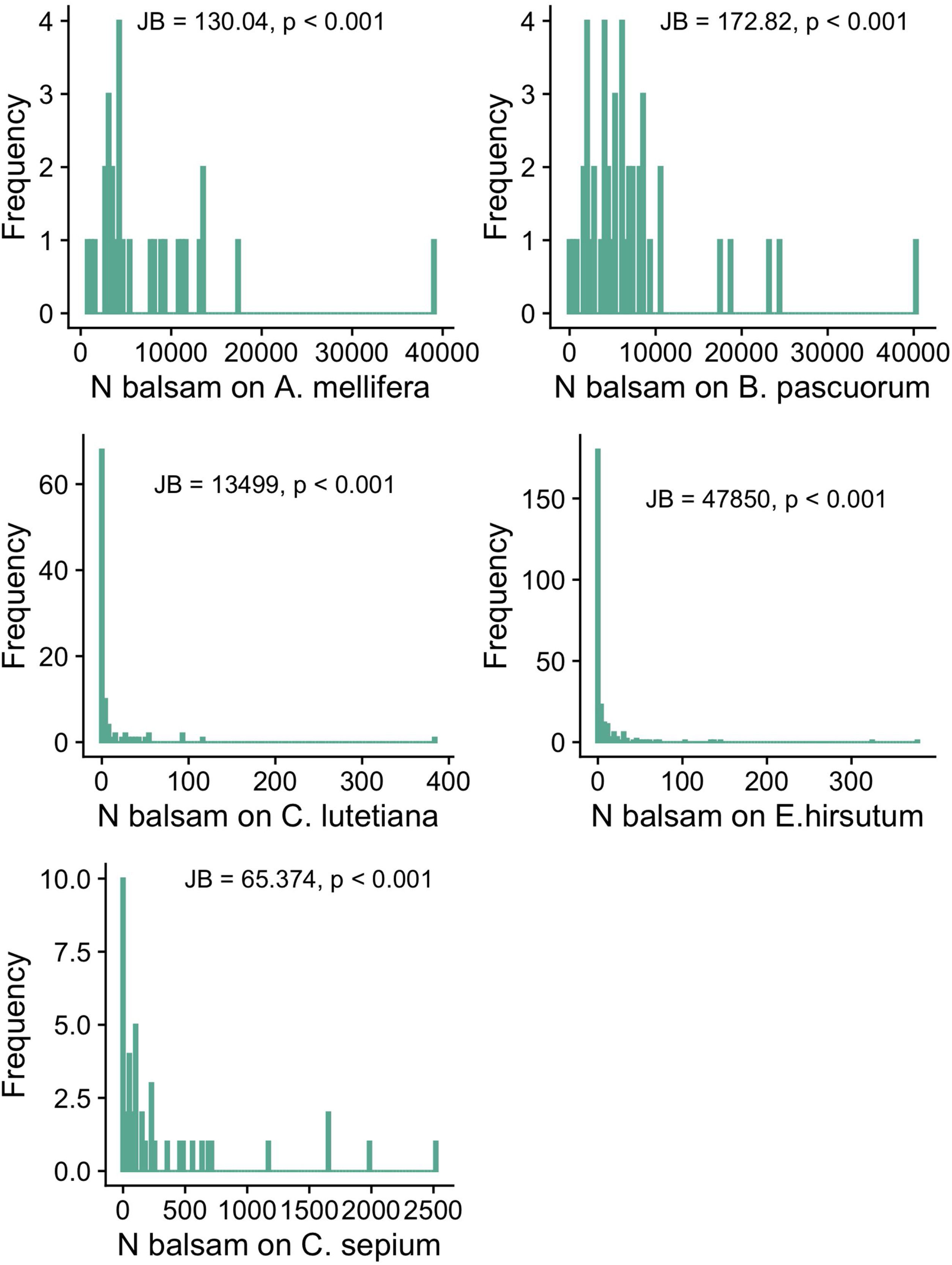
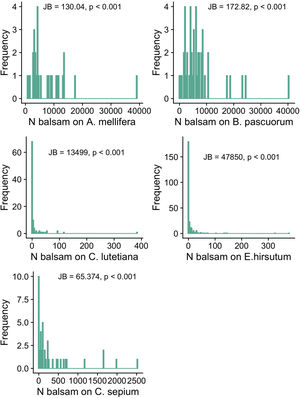
Objective 1 and 2. Large variation and right-skewed distribution in balsam pollen loads and stigmal depositionWe found large variation in the number of balsam pollen grains transported by different individuals within pollinator species: A. mellifera: 7623.14 ± 7475.71 (mean ± SD; range 807-39071); B. pascuorum: 7234.58 ± 7362.13 (mean ± SD; range 78–40333). The distribution of pollen grains on the pollinators’ bodies is right-skewed, i.e., a few individuals carry a large number of pollen grains while most individuals carry a few (Fig. 2a and b). Likewise, there was significant intraspecific variation in the number of balsam pollen grains deposited on stigmas: C. sepium: 360.98 ± 589.79 (mean ± SD; range 0–2519); E. hirsutum: 9.79 ± 35.96 (mean ± SD; range 0–378), C. lutetiana: 12.07 ± 42.94 (mean ± SD; range 0–384). Similar to the pollen load, the right-skewed distribution of data shows that a few stigmas received high numbers of pollen grains whilst the majority received very few, or none (Fig. 2c–e). In all cases, the shape of the data distribution in terms of skewness and kurtosis was significantly different from a normal distribution; thus, only a few individual plants and pollinators have high numbers of balsam pollen grains, while most of the population have just a few (Fig. 2).
The frequency distribution of the number of balsam pollen grains on the bodies of individuals of the commonest pollinators in the pollen-transport networks (Apis mellifera; Bombus pascuorum) and deposited on the stigmas of individuals of the commonest plant species in the pollen-transfer networks (Calystegia sepium; Epilobium hirsutum; Circaea lutetiana). Note the variation in the number of balsam pollen grains among individuals within each species. JB is the Jarque-Bera Normality Test showing the data is significantly right-skewed compared to a normal distribution. For visualization purposes, we present the original values without the log-transformation required for the applied normality test.
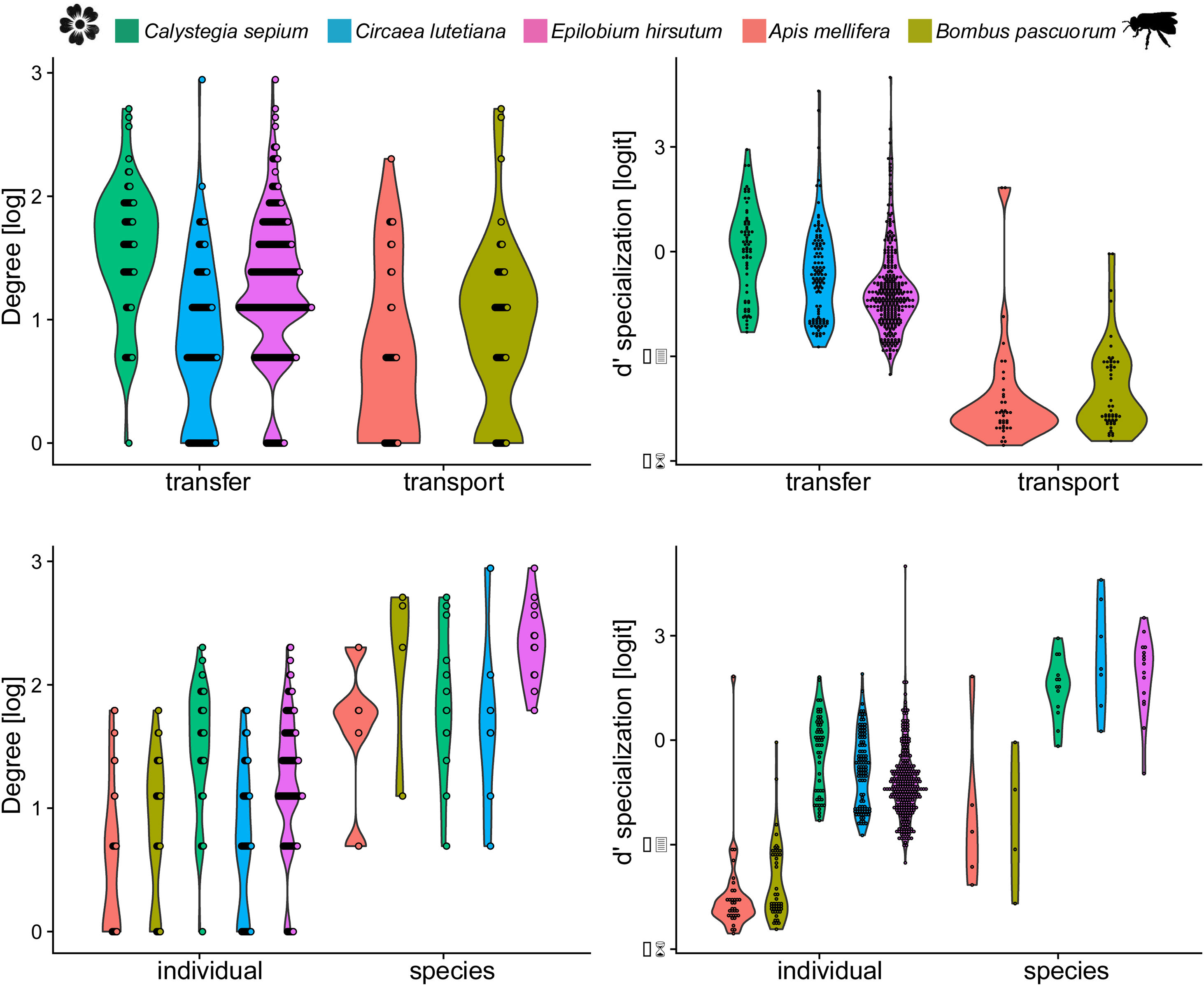
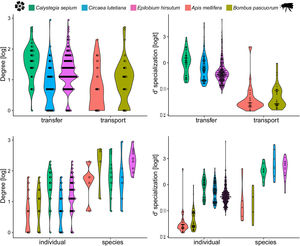
Three models were chosen as best explanations when testing differences in degree between network type and network level while one single model was selected for d’ specialization in the same context (Table 1). In both cases, species nested within treatments (i.e., invaded vs non-invaded areas) was selected as the best random structure, explaining 34.48% and 35.5.% of the variance for degree and d’ specialization models, respectively. In turn, treatment explained 0.01% and 34.13% of the variance for degree and d’ specialization, respectively. The intra-specific variation in the distribution of degree and d’ among species and between network type and level can be seen in Fig. 3.
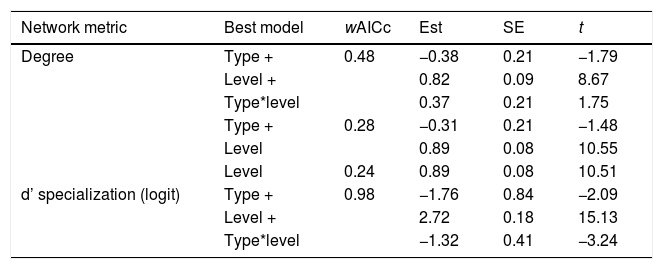
Results of the Linear Mixed Models testing the effects of network type (pollen-transport, pollen-transfer) and network level (individual-based, species-based) on the node specialization of pollination networks. Only models with delta AICc < 2 were selected as plausible explanations for the observed patterns. wAICc is a probability estimation of that given model to be the best choice under the AICc criteria. The coefficient t represents the importance of the corresponding parameter within the model (−2 < t > 2 indicates significance with > 95% confidence). “+” indicates that the following variable is co-varying to explain the changes on the corresponding network metric in each model.
| Network metric | Best model | wAICc | Est | SE | t |
|---|---|---|---|---|---|
| Degree | Type + | 0.48 | −0.38 | 0.21 | −1.79 |
| Level + | 0.82 | 0.09 | 8.67 | ||
| Type*level | 0.37 | 0.21 | 1.75 | ||
| Type + | 0.28 | −0.31 | 0.21 | −1.48 | |
| Level | 0.89 | 0.08 | 10.55 | ||
| Level | 0.24 | 0.89 | 0.08 | 10.51 | |
| d’ specialization (logit) | Type + | 0.98 | −1.76 | 0.84 | −2.09 |
| Level + | 2.72 | 0.18 | 15.13 | ||
| Type*level | −1.32 | 0.41 | −3.24 |
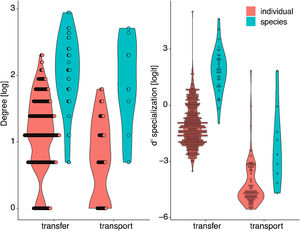
The inter- and intra-specific variation of node specialization of individual and species-based pollen-transport and pollen-transfer networks, considering the most common pollinator and plant species in the study sites invaded by Impatiens glandulifera (balsam). Differences on degree and d’ specialization between network types and individual- and species-level networks. For simplicity, data from invaded and non-invaded sites are not shown, either because the treatment was non-significant in the model, or it explained very little of the total variance.
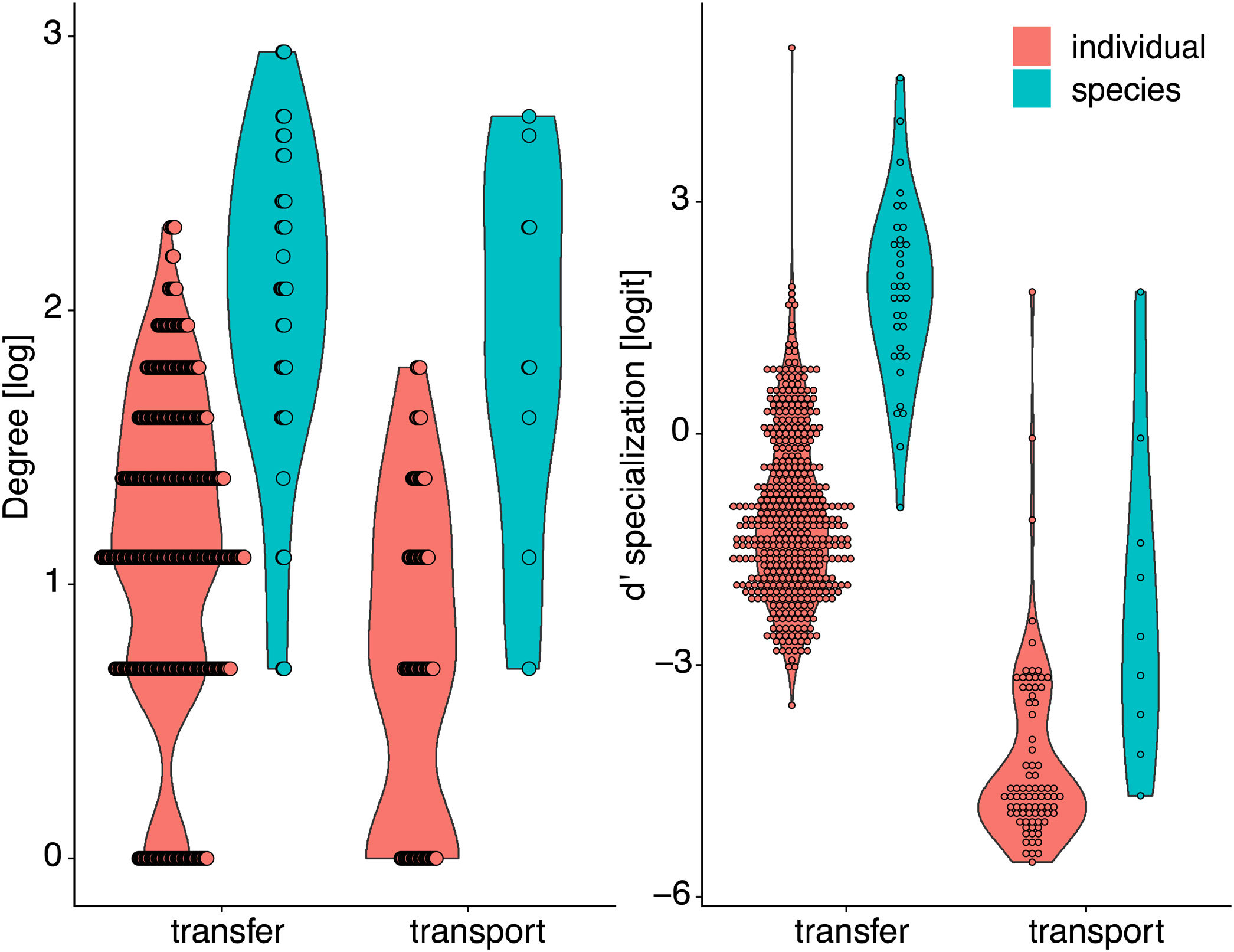
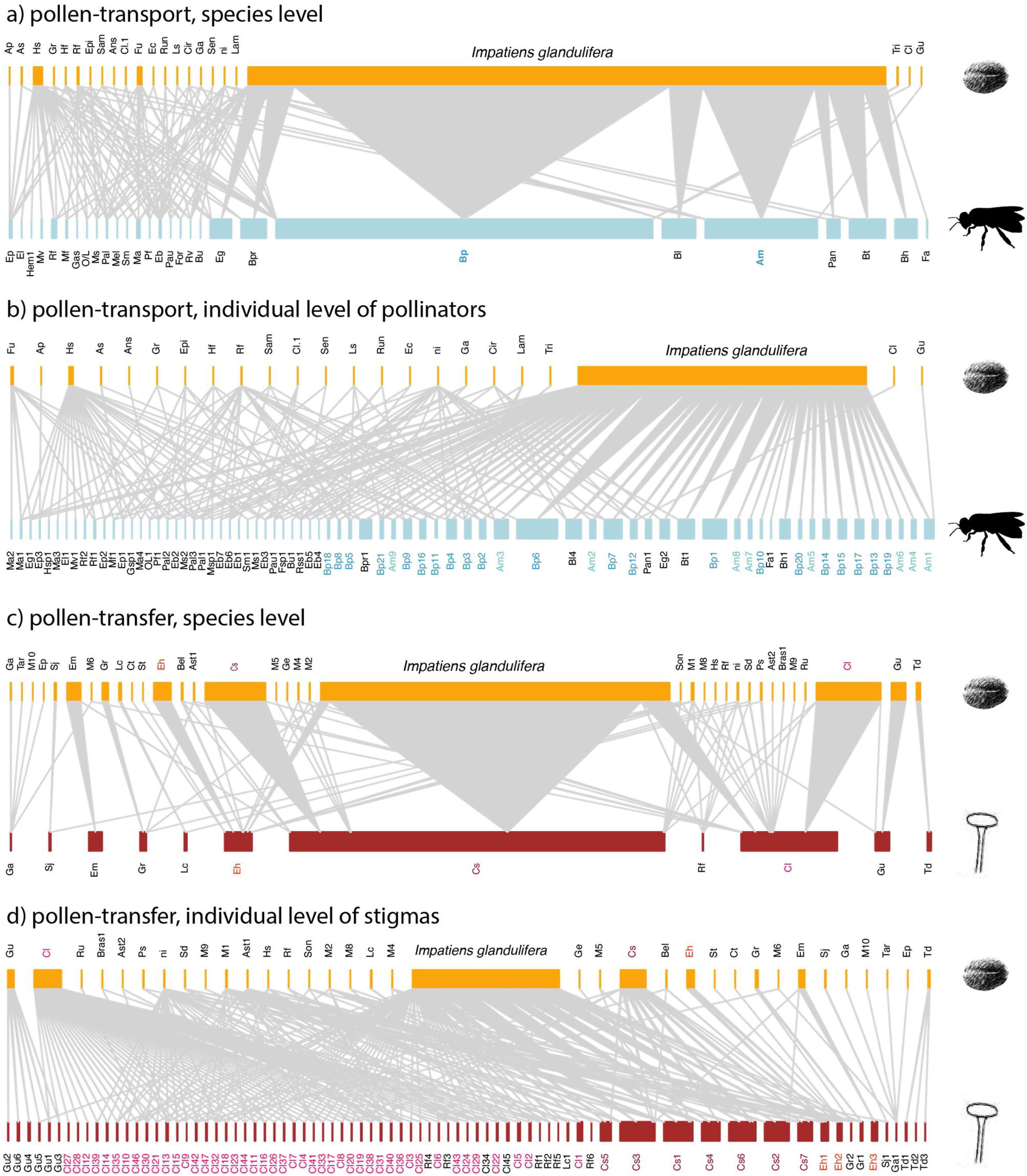
Degree was always higher at the species level than at the individual level as expected given that it is the aggregation of all individuals within each species (Fig. 4, Table 1). Among the selected models, the first ranked one showed a significant interaction between network type and level, showing that degree was higher for nodes within pollen-transfer networks but only at the individual level (Table 1, Fig. S1). This apparently counterintuitive result is related to a higher number of distinct pollen types recorded at the stigmas’ surface in comparison with those recorded at the pollinators' bodies when individuals are considered, a finding masked when data is pooled in species-based networks. Indeed, there were significant differences between individual-based and species-based networks in all models, including when level was selected as the only response variable. In comparison, network type did not significantly affect the degree of the target species.
Differences in node specialization (degree, d’ specialization) between network types (pollen-transport, pollen-transfer) and levels (individual-based, species-based) of invaded pollination networks. For simplicity, data from invaded and non-invaded sites are removed because treatment was non-significant in the models or explained very little of the total variance.
The full model was chosen as the explanation for differences in d’ specialization across the different levels of biological organization (Table 1, Fig. S2). Like degree, a significant interaction between network type and level was detected when analyzing d’ specialization, i.e., nodes showed higher selectiveness in the interactions they perform in the pollen-transfer networks, but only at the species level. Such a pattern was driven by differences between levels, in which individual-based networks are significantly more specialized than species-based networks (see results in the next section; Fig S3).
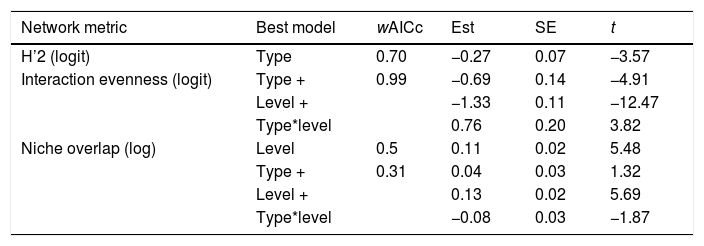
Objective 4. Specialization is higher for pollen-transfer networks at the individual levelOverall, the three complementary metrics used here as proxies for specialization showed significant differences for network type and level (Table 2, Fig. 5, Fig. S3). Specifically, H’2 specialization index only differed between network types, in which pollen-transfer networks were significantly more specialized than pollen-transport networks while there were no differences between individual- and species-based networks. The models for interaction evenness showed a significant interaction between type and level, in which individual-based networks showed higher uniformity in the distribution of links within the pollen-transfer networks but not for pollen-transport networks (Table 2, Fig. S2). When considered independently, the difference in the magnitude of interaction evenness was greater for individual-based networks and there were also significant differences between network types, in which greater evenness was observed in the transfer of pollen to stigmas compared to the movement of pollen on pollinators’ bodies.
Results of the General Least Square Models testing the effects of network type (pollen-transport, pollen-transfer) and network level (individual-based, species-based) on the structural specialization of pollination networks. Only models with delta AICc < 2 were selected as plausible explanations for the observed patterns. wAICc is a probability estimation of that given model to be the best choice under the AICc criteria. The coefficient t represents the importance of the corresponding parameter within the model (−2 < t > 2 indicates significance with > 95% confidence). “+” indicates that the following variable is co-varying to explain the changes on the corresponding network metric in a given model.
| Network metric | Best model | wAICc | Est | SE | t |
|---|---|---|---|---|---|
| H’2 (logit) | Type | 0.70 | −0.27 | 0.07 | −3.57 |
| Interaction evenness (logit) | Type + | 0.99 | −0.69 | 0.14 | −4.91 |
| Level + | −1.33 | 0.11 | −12.47 | ||
| Type*level | 0.76 | 0.20 | 3.82 | ||
| Niche overlap (log) | Level | 0.5 | 0.11 | 0.02 | 5.48 |
| Type + | 0.31 | 0.04 | 0.03 | 1.32 | |
| Level + | 0.13 | 0.02 | 5.69 | ||
| Type*level | −0.08 | 0.03 | −1.87 |
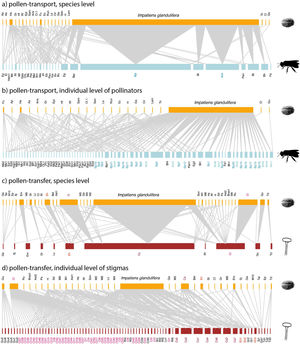
Empirical examples of pollen-transport networks (a, b) and pollen-transfer networks (c, d) at the species (a, c) and individual level (b, d) in areas invaded by Impatiens glandulifera (balsam). Blue bars represent the insect pollinators at both (a) species- and (b) individual-based networks; red bars represent the plant stigmas at (c) species- and (d) individual-based networks; yellow bars are the pollen types recorded on (a, b) pollinators’ bodies and (c, d) deposited on stigmas. Species are connected by weighted links whose width corresponds to the frequency of interactions. Note that the number of species in the upper side of the networks remains constant within network types because we are evaluating intraspecific variation in just one side of the networks, i.e. at the pollinator or stigma level. The target pollinators (Bombus pascuorum - Bp, Apis mellifera - Am) have their names highlighted in shades of blue (a, b) and the target stigmal plant species (Calystegia sepium - Cs, Circaea lutetiana - Cl, Epilobium hirsutum - Eh) in red (c, d). See Suplementary Material for species abbreviation names (Table S1, S2).
Finally, niche overlap of pollen types in the ‘use’ of pollinator’s bodies and stigmas was significantly lower in the individual-based networks but did not differ between network types (Table 2, Fig. 5, Fig. S3). Yet, there is a tendency of lower niche overlap of individual-based, pollen-transfer networks in comparison to individual-based, pollen-transport networks while no difference seems to occur at the species-based networks, as indicated by the marginally significant interaction between network type and level. Treatment (invaded, non-invaded areas) as a random factor was not selected as the best model structure in any of the above models. These results suggest that pollen-transfer networks are more specialized than pollen-transport networks, at least at the individual level, and that niche partitioning tends to be higher given the pollen transfer to stigmas in comparison with pollen transport data from pollinators` bodies when intraspecific variation is considered.
DiscussionOverall, intraspecific variation, node and structural specialization of invaded pollination networks varied according to the ecological process and the level of biological organization analyzed. We found considerable intraspecific variation in the amount of balsam pollen load on pollinators' bodies and deposition on plant stigmas. Plus, we uncovered a higher node and structural specialization for pollen-transfer networks in comparison to pollen-transport networks at the individual level, with significant modelling interactions between network type and level contingent to the metrics analyzed. Our findings suggest a lack of dominant, highly generalist links when downscaling from pollen-transport to pollen-transfer networks, and from species to individual-based networks. We also found higher niche partitioning at the individual level regarding the use of pollinators’ bodies as a vector of pollen transport and the use of stigmas for pollen deposition. These findings highlight the importance of considering the multiple facets of biological organization when analyzing ecological processes. In this section, we discuss the limitations of our study, along with the implications of our findings for the specific case of balsam invasion and more generally for current and future research on pollination networks.
LimitationsAlthough the networks studied here are from the same study system, i.e., they both study the invasion of Impatiens glandulifera in urban and semi-natural areas around the city of Bristol in the UK, the pollen-transport and pollen-transfer networks were built independently, in different years, by different ecologists, with a different design and different number of replicates. Thus, caution is needed when comparing both networks due to possible fluctuation in species composition between years and methodological differences (see Arceo-Gómez et al., 2018). That said, samples were collected from the same regional pool of species and while rare species in networks vary year to year, common species and network topology tend to remain the same (Alarcón et al., 2008; Petanidou et al., 2008; Dupont et al., 2009). Thus, Forup et al. (2008) found that, when abundance is taken into account, up to 94% of the species in networks remained the same between samples with three years intervals (2001 and 2004). Plus, the analyses estimating intraspecific variation and node specialization were run with the five most abundant plant and pollinator species in the area, therefore minimizing the possible effects of abrupt temporal fluctuations that could influence our results. Ideally, sampling the four types of networks should take place concurrently (a huge task though), but our results provide some intriguing pointers to what would be expected. Moreover, our results are largely in agreement with what would be expected given our understanding of pollinator behavior. It is also important to note that, as any other study without temporal dimensions, our findings are the results of metrics and models based on a static picture of the community and we cannot capture fluctuations over time. Thus, some of the uncertainties on the underlaying causes of the patterns observed here can only be addressed in future studies that use experiments designed to address the relationship between different types of networks and built on different levels of biological organization over time.
Implications of intraspecific variation and individual-based analyses for network ecologyDarwin (1859) pointed out that intraspecific variation in traits is the basic material of natural selection, which allows individuals to co-exist and evolve. This is recognized in other fields of biology such as on studies of niche partitioning (e.g., Bolnick et al., 2003; Pauw, 2013), foraging behaviour (e.g., Jakobsson et al., 2008; Song and Feldman, 2014) and predator – prey interactions (e.g., Araújo et al., 2011; Tinker et al., 2012). Intraspecific variation has been explored rather little in pollination networks though (but see Dupont et al., 2011; Tur et al., 2014 for exceptions). Here we showed that individuals of two common pollinator species, A. mellifera and B. pascuorum, show considerable variation in the amounts and diversity of pollen they carry on their bodies. The same pattern was observed in pollen deposition on stigmas of three common plant species, C. sepium, E. hirsutum and C. lutetiana. Our findings suggest that the two patterns could be linked: the intraspecific variation of pollen types transported by individual pollinators translating into the intraspecific variation observed in pollen deposition. If this is the case, one can expect a cascading effect of intraspecific outcomes through the different levels of biological organization when considering the movement of pollen grains from plant anthers to plant stigmas.
Pollen-transfer networks were more specialized than pollen-transport networks. Both types of networks are poorly explored in pollination studies, which traditionally use plant-flower visitor interactions as a proxy of pollination. Pollen-transfer networks are less well understood and to date, as far as we know, very few studies have used this approach to understand pollen movement at a community level (see Fang and Huang, 2013; Emer et al., 2015; Ballantyne et al., 2015; Parra-Tabla et al., 2020). Because pollen deposition on a conspecific receptive stigma is a key step for pollination success, an interplay of mechanisms of competition and facilitation are expected to be in place to optimize the probability that the right pollen reaches the right stigma in multi-species communities which share pollinators (Ashman et al., 2020). Indeed, our analyses of niche overlap showed that pollen types overlap less in deposition on stigmas, either at the species or individual level, in comparison to the use of pollinators` bodies as transport vectors, which suggest the existence of pollen filtering mechanisms. It is beyond the scope of this study to identify these mechanisms, but we advocate that further studies should incorporate pollen-stigma chemical and physiological interactions (reviewed in Ashman et al., 2020). These could provide an explanation for the structure of the pollen-transfer networks as our findings suggests that individual variation in the morphological (e.g., Montgomery and Rathcke, 2012), chemical and/or physiological (e.g., Hiscock et al., 2002; Allen et al., 2011) traits of the interacting pollen and stigma are in place to avoid heterospecific pollen adherence to stigmatic surfaces (Arceo-Gómez et al., 2016, 2019). Electrostatic forces between pollinators and plants also play important roles in pollen transfer (Clarke et al., 2013 and references therein). Thus, mechanisms such as chemical reactions and electrostatic fields that occur at the individual level could explain, or be part of the explanation, concerning the species-specificity of pollen load on plant stigmas identified here. Plus, factors observed to influence plant fitness such as plant pollinators’ attractiveness (Chittka and Schurkens, 2001), abundance and morphological traits (Willmer and Finlayson, 2014; Lázaro et al., 2019) of both interacting players might influence pollen movement at the community level and could explain the observed selectiveness of interactions regarding pollen movement from anthers to stigmas.
Implications for our understanding of the impacts of balsam invasionAlthough many studies have investigated balsam invasion, there is still no consensus on its impact on native communities. For instance, while Chittka and Schurkens (2001) experimentally report negative effects of balsam on the seed set of a native species, the observational studies of Cawoy et al. (2012) and Bartomeus et al. (2010) found no evidence of balsam affecting the seed set of native species or outcompeting for pollinators, respectively. In turn, Vilà et al. (2009) compiled community-wide effects of alien species across Europe, including balsam, reporting that its presence did not affect network structure. Our results shed some light on the observed variation in balsam impact. Thus, when revisiting Lopezaraiza-Mikel et al.’s (2007) dataset we found considerable intraspecific variation in the quantity of balsam pollen transported on the bodies of the two commonest pollinator species, A. mellifera and B. pascuorum. A. mellifera and B. pascuorum are well known as generalist species in plant-visitor networks (Goulson, 2003; Valido et al., 2019; Arroyo-Correa et al., 2020), and they visit many plant species. However, our results show that this generalization is not constant among individuals of the same species, rather there is substantial variation. One of the reasons for this intraspecific variability could be that different individuals show different levels of floral fidelity, depending on competition, niche partitioning and the resources available (Gegear and Thomson, 2004; Brosi and Briggs, 2013; Pauw, 2013; Huang et al., 2015). Pollinators which show a high fidelity to balsam would also explain the low rates of balsam pollen transfer to stigmas of other species. If this is the case, balsam would be unlikely to negatively impact the overall native plant reproduction. Why some plant species consistently received more balsam pollen deposition than others though, as observed by Emer et al. (2015), remains an open question that requires further investigation.
Balsam pollen transfer to interspecific stigmas is likely to be affected by the patterns of pollinators’ grooming. Grooming by foraging bees is common after flower visitation, varies in frequency and timing (Rademaker et al., 1997; Holmquist et al., 2012), and it is known to influence pollen transfer from donor to recipient flowers (Harder and Aizen, 2010; Willmer and Finlayson, 2014). One of the reasons pollinators’ groom is to remove excessive pollen grains attached to their bodies that can affect foraging ability (Harder and Aizen, 2010). Thus, we can hypothesize that higher frequency and/or intensity of pollinator’s grooming after visiting high pollen-producers such as balsam (Baude et al., 2016) could explain the low transfer of alien pollen to the stigmas of native plants.
Implications for our understanding on invasive species integration into ecological networksThe intraspecific variation of invaded pollen-transport and pollen-transfer networks are a double-edged sword with respect not only to the impact of balsam invasion but also to solve the puzzle in understanding how invasive species integrate into ecological networks (Emer and Timóteo, 2020). On one hand, the few individuals of pollinators with very high numbers of alien pollen grains on their bodies could function as “super-spreaders” of invasive species in a network context (Fu et al., 2015). Super-spreaders are usually rare and characterized by nodes within the network population that are particularly effective in spreading information, such as an infected organism which transmits a disease to a high number of individuals or a politician that strongly influences their many voters (Zhang et al., 2019). Here, the very few pollinators that carry large amounts of alien balsam pollen grains on their bodies could potentially act as super-spreaders of invasive species, therefore strongly affecting the dynamics of biological invasions. That said, most stigmas had very low numbers of alien balsam deposition which limits the impact of the invasive species. Overall, our results suggest that, even if a highly invasive plant species produces vast quantities of pollen and shares many pollinators with native species, most alien pollen-transport is mediated by relatively few pollinator individuals and transferred to only a few plant individuals in the community. Therefore, the negative effects, if any, on native plant reproduction due to alien pollen interference (e.g., stigma clogging), are likely to only affect a few plants, buffering the impact of the plant at the population and community level. In a broader context, one can speculate that the integration of invasive plant species in ecological networks may be mediated by a few individuals that function as super-spreaders at different levels of biological organization. In the case of pollination, super-spreaders could be present from the flower-visitor to the pollen-transfer level, and their effects may be different depending on the scale in which they act.
Finally, our findings showing that only a few individuals within a population can drive the patterns observed at the species level support the idea the that intraspecific effects can be comparable to, and sometimes stronger than, species effects (Des Roches et al., 2018). This reinforces the importance of quantifying the magnitude of intraspecific variation. In the specific case of invasion biology, knowing whether individual pollinators within a species vary in their transport of heterospecific pollen and whether individual plants vary in their stigmal pollen load capacity, may help to explain why the impact of invasive species can be positive (Molina-Montenegro et al., 2008), neutral (Bartomeus et al., 2008a, b), or negative (Chittka and Schurkens, 2001, Richardson and Traveset, 2020).
ConclusionOur results begin to explain why many studies on alien plant species fail to detect negative effects on native plants’ reproduction at the species level, despite disrupting pollinator communities. Understanding the mechanisms behind patterns of intraspecific variation on niche partitioning and specialization, which act on the different stages of the pollination process and span across levels of biological organization, is a promising avenue towards a more comprehensive understanding of the dynamics of invasion biology on mutualistic systems. Moreover, the processes determining individuals' performance within a given system may also provide a key to understanding the emergent complexity of interacting species, from populations to communities and ecosystems. Looking forward, what is needed are studies which look at multiple types of networks simultaneously, as these would provide a considerable boost to our understanding of how alien species affect native networks of interactions and of the impact of pollinator behavior on network structure and function more generally.
Author’s contributionCE and JM conceived the ideas and designed the analytical approach. CE collected the data on pollen-transfer networks, ran the analyses and wrote the first draft of the study. JM provided the data on pollen-transport networks. Both authors contributed critically to the drafts and gave final approval for publication.
Data availabilityData will be made available on the author’s GitHub upon the acceptance for publication.
Conflict of interestThe authors declare no conflict of interest regarding the ms entitled: Intraspecific variation of invaded pollination networks – the role of pollen-transport, pollen-transfer and different levels of biological organization submitted to Pecon by Carine Emer and Jane Memmott.
CE was granted a PhD scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), a fellowship from the São Paulo State Research Foundation (FAPESP, Grant n 2015/15172-7) and Carlos Chagas Filho Foundation for Supporting Research in the Rio de Janeiro State (FAPERJ, Grant n E-26/200.610/2022). We are grateful to Sergio Timóteo, the editors and the anonymous referees for their comments on the previous version of this study.