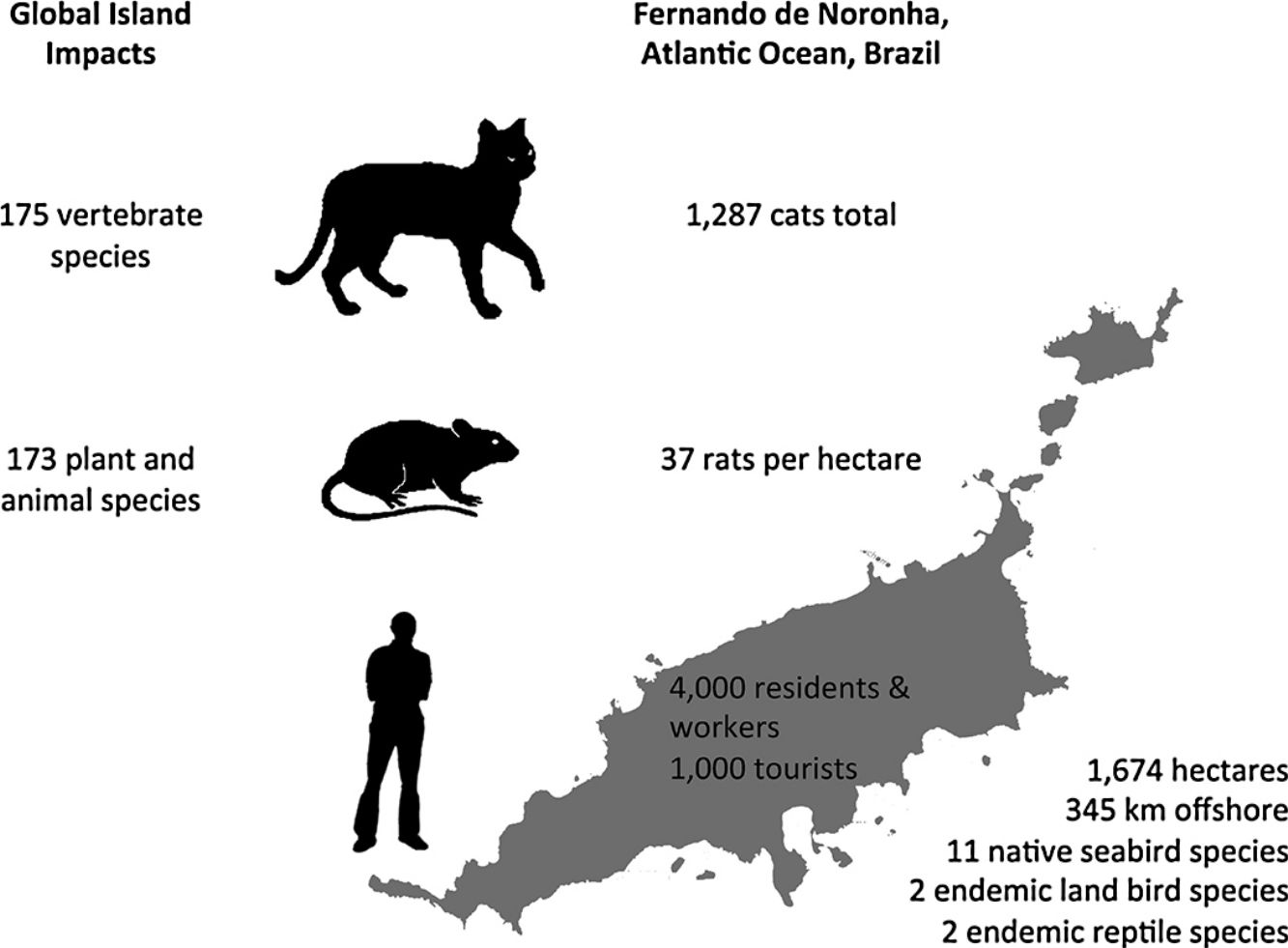
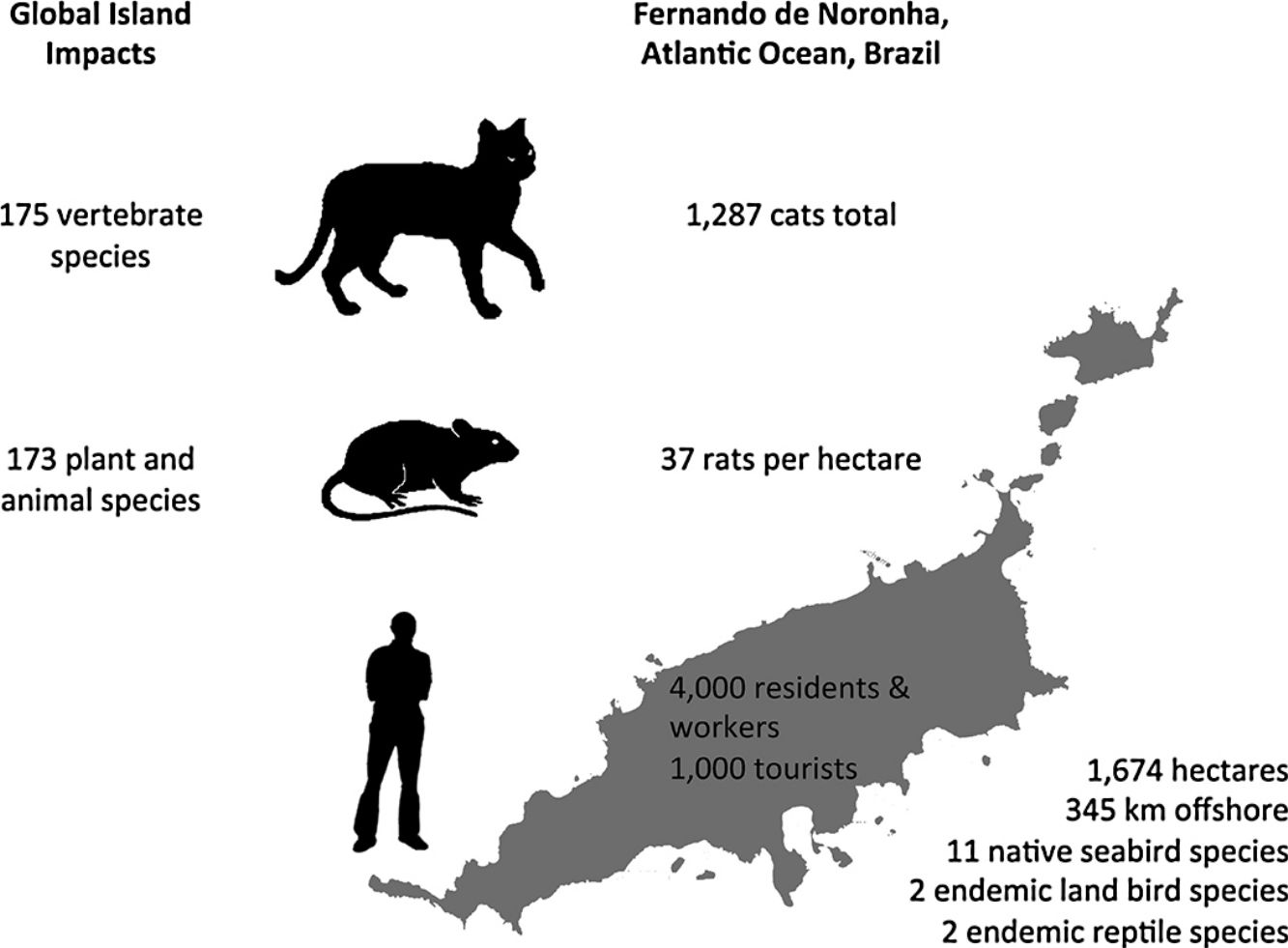
In this paper, an overview of introduced cat and rodent impacts on islands, and methods for their control and eradication, are presented. Fernando de Noronha, an inhabited oceanic island of Brazil, is used as a case study to illustrate the challenges of cat and rodent management on inhabited islands. Cat impacts have been recorded for 175 vertebrate species, and rat impacts for 173 plant and animal species. Eradication of cats and rodents for species conservation has been successful on small to medium- sized uninhabited or sparsely inhabited islands. However, examples of successful cat and rodent management programmes for biodiversity on inhabited islands are limited. On inhabited islands localised control of cats and rodents occurs, but historically with a focus on agriculture, human livelihoods and animal welfare, and only more recently on native species conservation. Control of cats and rodents on inhabited islands for species conservation lags behind uninhabited islands and the reasons for this are social and complex. Conservation managers often perceive a lack of support from island residents or administrators, which may or may not actually be the case. Where support does not exist, it may relate to the provisioning of control versus eradication, the techniques proposed, or wider socioeconomic issues. This ultimately translates to conservation inaction, and the ongoing decline and extinction of island fauna. Abundance estimates for cats and density estimates for rats on Fernando de Noronha are presented, along with documented biodiversity impacts, to support recommendations for future management on Fernando de Noronha.
Oceanic islands are reservoirs for biological diversity but have routinely been heavily negatively impacted following their discovery by humans (Tershy et al., 2015). Colonisation of oceanic islands by humans led to the introduction of new species, many ultimately invasive (Blackburn et al., 2004). These introductions in turn led to a wave of extinctions following each major human colonisation event, with the loss of endemic species from birds and many other taxa (Duncan et al., 2013). Cats (Felis silvestris catus) and commensal rodents have been the most widely introduced mammal species to islands (Doherty et al., 2016). Norway rats (Rattus norvegicus), black rats (Rattus rattus), Pacific rats (Rattus exulans), and mice (Mus musculus) were unintentional stowaways on ships (Atkinson, 1985; Jones et al., 2013) and had devastating impacts on island fauna and flora naïve to mammalian predation and herbivory (Towns et al., 2006). Following rodent invasion cats were typically introduced to control them, with further unintentional impacts on native prey species naïve to cat predation (Nogales et al., 2013).
On small uninhabited islands conservation managers can now routinely eradicate introduced mammalian predators to enhance island restoration (Keitt et al., 2011; Russell and Holmes, 2015). Such eradications lead to marked recoveries in island fauna and re-colonisation by previously extinguished species (Jones et al., 2016). However, due to minimum habitat requirements some critically endangered insular species only persist on large islands (e.g. Reuleaux et al., 2014). These islands are also more likely to be inhabited. Eradications on inhabited islands are more challenging, especially because of issues associated with social and political support (Oppel et al., 2011; Glen et al., 2013). Resolution of conflict arising from wildlife management on inhabited islands will require working with diverse stakeholders towards a win–win scenario for the environment, human and animal health and the economy (Crowley et al., 2017; Russell et al., 2018).
In this paper, an overview is given of the literature on the impacts and management of cats and rodents on islands, with a novel emphasis on highlighting the complexities around their management on inhabited islands. The case study is then given of cat and rodent management on Fernando de Noronha, an inhabited oceanic island of Brazil located in the tropical Atlantic Ocean. Finally, recommendations to develop social and administrative capacity in cat and rodent control and eradication on inhabited islands are made.
CatsNegative impacts of introduced cats have been recorded for 175 insular vertebrate species. They are implicated in 14% of species extinctions on islands and are the principal threat for 8% of critically endangered insular species (Medina et al., 2011). The distinction between feral and domestic cats on inhabited islands is constructed around their interactions with humans and can relate very little with their impact on wildlife, since both are able to prey upon native species (Farnworth et al., 2011). All cats on islands can impact wildlife, although cats resource subsidised (i.e. fed) by humans may, although not always, have a reduced impact on wildlife (Grant and Longnecker, 1999). However, the provisioning of resource subsidies to cats, whether from humans or alternative prey (e.g. introduced rodents) can induce prey-switching whereby the loss of that subsidy (e.g. cessation of feeding by humans or rat control) may cause cats to change diet, possibly to a more vulnerable insular species (Peck et al., 2008).
Eradication of cats is possible on very large islands (Campbell et al., 2011) and in the presence of similarly sized native mammals (Hanson et al., 2015). Cats have been successfully eradicated from over 90 islands up to 290km2 (Campbell et al., 2011). Eradication of cats uses complementary methods including leg-hold traps and hunting (spotlighting, and the use of trained dogs), possibly in combination with secondary poisoning following a rodenticide operation, or occasionally with the release of a biological control such as feline enteritis (Nogales et al., 2004; Parkes et al., 2014). Evidence of potential indirect effects following cat eradication should be investigated (e.g. Bergstrom et al., 2009) but must be weighed against the benefits of removing strong direct effects of cats, and not over-stated (e.g. Dowding et al., 2009).
Techniques for cat control in addition to those utilised for eradication might include live trapping followed by neutering and release, or relocation. This method is not suitable alone for eradication, however, it may be employed and require complementary methods that will allow the removal of all individuals (Castillo and Clarke, 2003). Relocation can be expensive and will be most applicable for human-conditioned cats in to domestic environments. Trap-neuter-release is an attractive method to pro-cat lobby groups and the general public (Longcore et al., 2009), however, it does not address the lethal impacts of cats on island fauna, and its efficiency as a control technique (i.e. actually reducing the population size) has not been demonstrated. For cat populations larger than a few dozen, trap-neuter-release is effectively an expensive equivalent to the ‘do nothing’ option (Loyd and DeVore, 2010), although when used in tandem with micro-chipping and stronger domestic cat regulations it can serve as a gentle introduction to the longer-term goal of cat eradication on inhabited islands (Algar et al., 2011).
RodentsIntroduced rats compete with one another on islands but are typically able to co-exist, especially on tropical islands (Russell et al., 2014). All three species of invasive rat are able to exist on islands even without human subsidy (i.e. commensalism), in contrast to continental environments where native mammalian competitors and predators limit their distribution. The distribution of all three rat species reflects the facts that R. rattus is the dominant rat on islands given its superior climbing ability (50% of the world's island groups), followed by R. norvegicus which thrives in wetter environments (36% of the world's island groups), followed by R. exulans which is only widespread on Pacific Ocean islands (24% of the world's island groups) (Atkinson, 1985). When alone on islands each species is able to invade most niches. All three rat species are competitively dominant to mice (Caut et al., 2007). Where rodents co-exist with cats a complex intra-guild predation relationship takes place (Russell et al., 2009).
Negative impacts of introduced rats have been recorded for 173 insular plant and animal species (Towns et al., 2006), including 75 seabird species (Jones et al., 2008). Their invasion has led to rapid extinctions of island endemic species (Bell et al., 2016). Mice impacts have only been well recorded on Southern Ocean islands (Angel et al., 2009). Rats and mice both have substantial impacts on native rodents (Harris, 2009) and invertebrates (St Clair, 2011). Invasive rats are distributed on over 80% of the world's oceanic island groups (Atkinson, 1985), and mice are equally widely distributed (Jones et al., 2013). Although rats are found more often on tropical islands than mice, their impact and management on tropical islands is under-addressed (Shiels et al., 2014; Harper and Bunbury, 2015; Russell and Holmes, 2015). Commensal rodents were probably never intentionally introduced to islands, but were exceedingly successful at hitchhiking with humans. Once an island was colonised, invasive rats, particularly R. norvegicus and R. rattus, were capable of swimming to invade neighbouring islands or secondary islets (Russell et al., 2008).
Eradication of rodents is also possible on very large islands (Howald et al., 2007) and in the presence of similarly sized native mammals (Howald et al., 2010). Rodent eradications have been attempted on over 600 islands up to 3903km2 (Elliott et al., 2015; Holmes et al., 2015; Martin and Richardson, in press). Eradication of rodents for islands larger than about 25ha uses aerial or ground distribution of second generation anticoagulants, usually brodifacoum, although in the future other techniques may be available (Campbell et al., 2015; Carter et al., 2016). Eradication of mice is more challenging because of their higher densities and comparatively higher resistance to anticoagulants (MacKay et al., 2007). With appropriate planning and execution by experienced eradication practitioners and a financial and motivational commitment to success (see Bomford and O’Brien, 1995), success rates are typically over 80%, and closer to 100% for small and relatively biologically and geographically simple islands (Howald et al., 2007). Eradications in tropical environments have proven more challenging (Holmes et al., 2015).
Techniques for rodent control usually focus on the use of toxins, particularly second generation anticoagulants (Duron et al., 2017). However, the methods used by commercial pest control services are not suitable for scaling to eradication. On small scales, trapping can also be an effective control method (Howald et al., 2007). Ongoing use of anticoagulant poisons for rodent control creates a strong risk of the target population developing anticoagulant resistance (Buckle, 2013), which not only compromises existing control but also prevents any future eradication. In contrast to cat control, most people are usually supportive of lethal rodent control. However, support for the methods of control, particularly the use of toxins or how they are distributed (e.g. aerially) may be more controversial (Russell, 2014). The methods of rodent control in any given location must therefore balance the desires of the community with the pest control goal, possibly utilising mixed methods of control simultaneously, keeping in mind that currently the only consistent method for rodent eradication on anything other than very small islands (<100ha) is aerially distributed toxin (Howald et al., 2007).
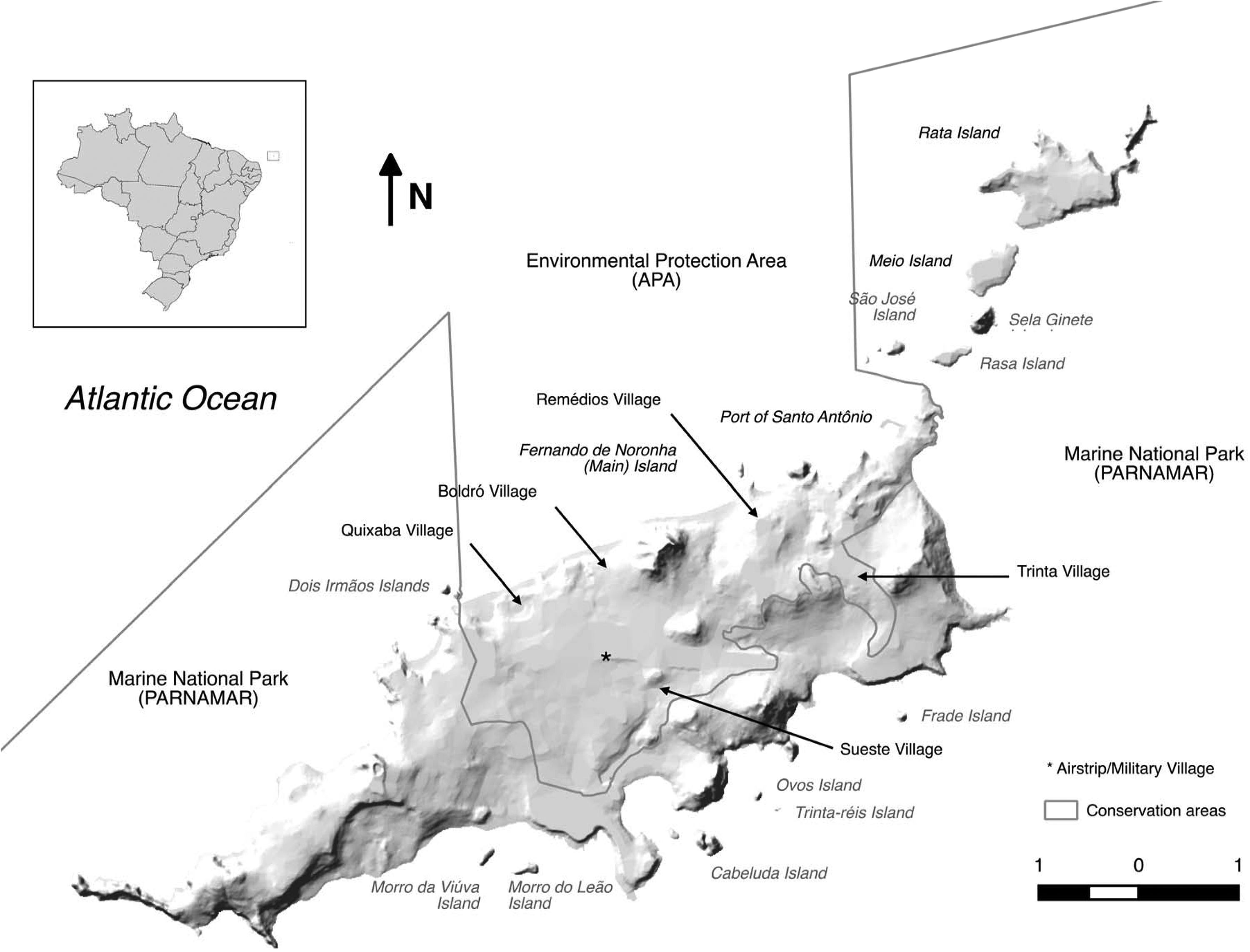
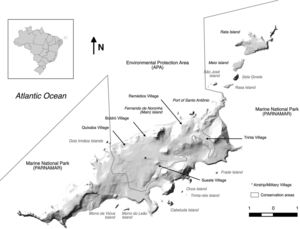
Fernando de NoronhaBackgroundFernando de Noronha is an inhabited oceanic island group lying just south of the equator 345km off the north-east coast of Brazil (3°50′S, 32°26′W). The main island is 1674ha with 21 secondary islets and rock stacks located around its coast (Fig. 1). The island was first discovered at the start of the 16th century and has served as a Portuguese fort, penal colony, and later Brazilian military base, with occasional American presence, in the 20th century. Since 1988 the island has been jointly managed as freehold land with about half of the island in uninhabited federally-managed national marine park and the remaining half as an inhabited federal and state-managed (Pernambuco, Brazil) environmental protection area, and from 2001 as a UNESCO World Natural Heritage site (de Oliveira, 2003). The governance structure is described as complex, autocratic and top-down (Reis and Hayward, 2013). The human population on Fernando de Noronha probably comprises about 4000 residents and non-resident workers, and up to 1000 tourists at any single time.
Cats, rats and mice have all been present since at least the late 19th century when they were first recorded (Branner, 1888), but likely arrived much earlier. The introduction of cats and rats likely led to the rapid extinction of the endemic Vespucci's rat (Noronhomys vespuccii) through predation, competition and disease (Carleton and Olson, 1999). Today, cats are widespread across the main island but are not found on secondary islands. In the forested national marine park area, feral cat sign is found on all tracks, but cats are rarely seen. In the inhabited environmental protection area, cats are commonly observed in association with humans. Although not specifically kept as pets, owned or explicitly fed, the animals exist in close association with humans and the population is subsidised by human feeding. Black rats of the alexandrinus colour morph are widespread across the main island. In the inhabited environmental protection area, rats are somewhat systematically controlled by industrial pest control, and the presence of cats may reduce signs of rat activity by altering rat behaviour through a landscape of fear (Themb’alilahlwa et al., 2017). Black rats are present on close islands of the northern chain (São José, Rasa, Meio, Rata), and absent from islands off the more exposed southern coast, where rat-vulnerable seabirds breed (e.g. Chapéu, Viúva, Leão) (Soto, 2009). Norway rats are less common on the island, but are found in both inhabited and natural areas of the main island. Mice are scarce but known from around inhabited areas. Other widespread introduced species on Fernando de Noronha, although native to continental Brazil, include the tegu lizard (Salvator merianae), mocó rodent (Kerodon rupestris), cururu toad (Rhinella jimi), snouted treefrog (Scinax x-signatus) (Oren, 1984) and little fire ant (Wasmannia auropuntata) (J. Russell unpubl. data), none of which are currently known from offshore islands except for a small population of tegu on Rata Island (Abrahão et al., in press).
On Fernando de Noronha cats have been recorded preying upon native mabuya skinks (Trachylepis atlantica), introduced mocós, rats and mice, and chasing seabirds on the beach. Norway rats have been recorded preying on turtle nests. Mice are difficult to detect and little is known about their distribution and impacts. The rodent population historically reached an irruptive peak during invasion (Ridley, 1890) but today natural resources have been reduced and rodent irruptions of the magnitude seen historically no longer occur. Toxoplasma gondii and Leptospira spp. are pathogenic agents prevalent in cats and rats, among other domestic and wild animals on the archipelago (Costa et al., 2012).
The seabird population collapsed following human discovery of Fernando de Noronha and seabird breeding is now almost entirely restricted to small cat and rat-free secondary islets, except for red footed boobies (Sula sula) and noddies (Anous spp.), able to nest in trees and escape cat predation, and white-tailed tropic birds (Phaethon lepturus) and white terns (Gygis alba) able to nest on the sheer cliffs of Pico peak (323m). A small remnant population of fewer than 20 masked booby pairs at Capim Açu point, where cat sign is abundant, is the only remaining breeding population on the main island. The larger red-billed tropic bird (Phaethon aethereus) is almost extinct on the island and now fewer than 10 individuals are found around the sheer cliffs of Caracas point. A relict population of about 15 Audubon's shearwater pairs (Puffinus lherminieri) is found on two small offshore islets (Mestre et al., 2009).
Currently, cat management on Fernando de Noronha is a function of norms from the Brazilian continent. On continental Brazil cats are mostly found in association with humans in urbanised areas, and hence cat management is undertaken in a population health context, particularly around zoonoses, and reflecting norms relating to private ownership, animal rights and a perception of little impact of cats on the natural environment. Cat control programmes generally focus on sterilisation (Mendes-de-Almeida et al., 2007). Five out of 26 states in Brazil forbid lethal control of cats, including Pernambuco, to which Fernando de Noronha has been administratively attached as a state territory since 1988. Although the national marine park is federally administered by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) of the Brazilian Ministry of Environment, the land itself remains governed by the State of Pernambuco.
Veterinarians stationed on the archipelago are only permitted to euthanize cats when they are ill, in agony or carrying zoonosis, according to the Pernambuco State law. An adoption programme did exist to re-locate cats from the island to the Brazilian continent, but the initiative has so far had limited uptake with only 10–20 cats re-located. Hence overall, cat management on Fernando de Noronha today reflects continental urban norms around cat management, with an emphasis on veterinarian treatment and sterilisation programmes. The impacts of cats on island fauna, particularly in the marine national park area, are not addressed, although there is some desire from the governing agencies of ICMBio and the island administration to address the issue.
Rat management on Fernando de Noronha is also a function of norms around the rat as an urban pest in a population health context, and control is limited to poison bait stations around infrastructure checked and re-stocked at regular intervals. There is some awareness that reinvasion of rats from forested areas to the urbanised area is taking place, but the distribution and impact of rats in the forested marine national park area is generally under-appreciated. No rat surveillance in the port is in place, and invasion from shipped cargo is neglected. Hence, for both cats and rats on Fernando de Noronha, the prevailing paradigm of management currently reflects continental norms around the cat and rat as urban population health problems with limited impacts in the natural environment. Such a paradigm is common on other tropical islands that are politically part of larger continental nations.
DensityPopulation studies of cats on Fernando de Noronha were undertaken in November 2015. The results of this study are described in full elsewhere (Dias et al., 2017). Seven line transects across the main island were established and surveyed twice using nocturnal spot lighting by two observers. Cat density was estimated using distance sampling for cats observed up to 30m either side of the transects, and extrapolated to an island-wide abundance estimate. The status of cats in the population was estimated using household surveys of cat ownership and management. Cats were distinguished as either supervised or unsupervised, given that residents of Fernando de Noronha did not directly feel a sense of ownership over any individual cat, but nonetheless felt a sense of protection, although without responsibility. Most of the cats on the island were supervised and subsidised and found around the inhabited areas. Only few supervised cats were restricted in their movements. The total cat population on the main island was estimated at 1287 animals. Population modelling was then used to simulate different management scenarios on the population size of the resident cat population. Modelling both reproductive control and removing cats from the archipelago had the greatest impact on reducing cat population size, although removal of cats was far cheaper than reproductive control.
Population studies of rats on Fernando de Noronha were undertaken by the authors of this paper in February (transition from dry to wet season) 2015 and 2016, and October (transition from wet to dry season) 2016 and 2017. In addition, in February 2015, six tracking tunnels ran for six nights in the Capim-Açu trail recorded 100% rat presence and rats were regularly observed during daylight around the island. In February 2016 three tracking tunnels were run for two nights on each of Chapéu Island, São José Island and Rasa Island. No rats were detected on Chapéu Island while black rats were confirmed by tracks and visually on São José Island and Rasa Island. Black rats were previously also confirmed by tracks and visually on Rata Island in October 2015.
In February 2015 and 2016 and October 2016 and 2017 black rat density was estimated in forested habitat opposite the Atalaia bus parking garage near Quixaba Village. The habitat consisted of secondary regenerating native forest of Sapium argutum canopy with understory of Capparis flexuosa, Capparis frondosa and Croton hirtus invaded by jasmine (Jasminum fluminense) and lantana (Lantana canescens). The endemic reptiles mabuya skink and Noronha worm lizard (Amphisbaena ridleyi) were both present on the site, as were introduced cats, tegu and mocó all observed. Live trapping followed the methods of Russell et al. (2011). Twelve cage live-traps at 20m spacing (3×4) were baited with pineapple pieces and run for six nights and checked each morning. All rats captured were transferred to a plastic bag, sexed and marked with a unique numbered ear-tag prior to release. Rats captured on the final two nights were euthanized. Density was estimated using spatially-explicit capture–recapture (SECR) with a half-normal detection curve (Borchers and Efford, 2008). Preliminary investigations revealed sex was not an important predictor of g0 or σ so the full likelihood testing for differences among sessions, season (February or October) and years (2015–2017) on density, probability of capture at the animals activity centre (g0) and distance from activity centre (σ) was fitted (Borchers and Efford, 2008).
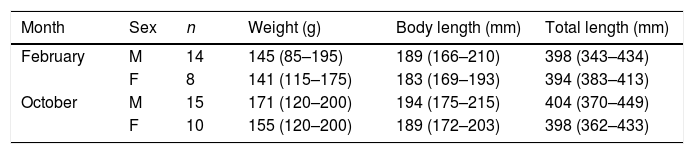
A total of 86 unique rats were captured 144 times over the three years of our study. Model selection using AIC indicated the preferred model allowed g0 to vary by session while density and σ were constant across our study. Detection parameter estimates were typical for black rats with g0 varying from 0.16 to 0.39. Density was estimated at 37 rats per hectare (95% CI 29–49) with σ equal to 10.25m (95% CI 8.79–11.95). Rats euthanized on the final two nights of live trapping (n=47) were examined in the laboratory and morphological measurements taken (body weight to nearest 5g, body length and total length to nearest millimetre). Differences in rat weight and morphology by sex, season and year were tested for using analyses of variance (α=0.05). Only body weight differed significantly by season with rats heavier at the end of the wet season (p=0.02) and so measurements were pooled across years (Table 1). Black rats were of a body size typical for rats in tropical environments co-existing with introduced predators (Russell et al., 2011; Harper and Bunbury, 2015).
Morphological measurements of adult black rats trapped on Fernando de Noronha from 2015 to 2017 in February and October. Mean (range in brackets).
| Month | Sex | n | Weight (g) | Body length (mm) | Total length (mm) |
|---|---|---|---|---|---|
| February | M | 14 | 145 (85–195) | 189 (166–210) | 398 (343–434) |
| F | 8 | 141 (115–175) | 183 (169–193) | 394 (383–413) | |
| October | M | 15 | 171 (120–200) | 194 (175–215) | 404 (370–449) |
| F | 10 | 155 (120–200) | 189 (172–203) | 398 (362–433) |
In order to identify the appropriate management actions for invasive cats and rats on islands the first step is to determine the severity of their impacts on fauna and in natural environments. This requires conservation managers to take an insular perspective on wildlife management, recognising the severity of invasive species as a primary threat to island biodiversity (Tershy et al., 2015). Islands tend to be bottom-up driven trophic fountain systems where species such as invasive rats are not predator-limited, but instead food-limited (Russell, 2011). Once this is recognised, conservation managers can then consider appropriate methods to manage invasive cats and rats on islands. The ‘do nothing’ option for invasive cat and rodent management will ultimately result in the loss of all vulnerable island fauna (Nogales et al., 2013; Towns et al., 2006).
Where complete eradication of either cats or rats is not possible because of island size, eradication cost, or lack of sufficient social or administrative support, then the only alternative to preserve biodiversity is to consider control (Duron et al., 2017). Cat and rat control in natural areas must be focused on biodiversity outcomes, i.e. to a level that achieves stated biodiversity management goals, e.g. recovery of certain populations of endemic species. To that end, prioritisation of target species for conservation is essential. The format of pest control will be context-dependent, but might include upper trigger harvest where target pest populations are only controlled once they exceed a certain threshold (Baxter et al., 2008). It is important to remember that the price of ongoing pest control eventually exceeds the one-off cost of eradication (Pascal et al., 2008).
Cats have long been believed to control rodent populations. Instances of cats consuming rodents at the individual level (i.e. an observation) do not necessarily translate to numerical rodent population control (Russell, 2011). The removal of cats cannot lead to an explosion of rodents when rodents are food-limited i.e. bottom-up system (Russell et al., 2009). This is typically the case on islands, particularly seasonally trophic islands such as tropical (wet/dry season) islands (Russell and Holmes, 2015). The presence of cats on islands more likely alters the behaviour and reduces the activity (hence perceived abundance) of rats, as well as their body-size (Russell et al., 2011). The removal of cats may thus lead to rats increasing in body-size, and becoming more visible on islands, despite not significantly increasing in population size. Even if the rat population should increase, from a conservation perspective any such increase in rat impacts is likely offset by a larger reduction in cat impacts (Russell, 2011).
A major obstacle for management may be limited availability of legally permitted control techniques, such as cat euthanasia or rat poison distribution. Conservation management may require legislative change, which can be potentially challenging on islands with multiple management agencies and complex governance structures and relationships (Reis and Hayward, 2013). It may be possible to have a special legal protection of an island and designation of an island-specific management plan which incorporates pest control and appropriate exceptions to existing laws, even if temporary.
Ultimately, successful cat or rat eradication or control for conservation on inhabited islands requires empowering the local communities and stakeholders to value their unique island biodiversity and work together towards its protection. This includes educating visitors to islands about how such biodiversity protection adds to their overall unique island experience, whether it be tourism or otherwise. Most people usually support control of cats and rats in uninhabited natural areas for conservation purposes (e.g. Russell, 2014) and these areas should be a primary focus for suppression to zero density. Although complete public support for management interventions is aspirational, it is not likely, nor is it necessary (Caplat and Coutts, 2011). Nonetheless understanding, and where possible incorporating, the environmental attitudes of stakeholder groups is essential (Crowley et al., 2017).
RecommendationsThe first important step for terrestrial biodiversity management on Fernando de Noronha will be for the two managing agencies, the federal ICMBio and Pernambuco state government, to take a joint whole-island perspective to island management and biosecurity (Reis and Hayward, 2013). This would best be achieved through a joint agency legally enforceable island management plan covering both terrestrial and marine environments and all of species conservation, pest management and biosecurity. The Environmental Preservation Tax (TPA) levied upon all tourists upon their arrival at Fernando de Noronha would provide a rich source of funds for enacting such an island management and biosecurity plan, and the overseas experience is that such spending provides a positive return-on-investment for tourism (Russell et al., 2015). On Fernando de Noronha development of terrestrial conservation experiences such as hiking, birdwatching, and visits to secondary islands, which are currently not encouraged, would diversify the existing tourism economy whose current environmental component is almost entirely based around marine activities.
In the short-term, cat and rat management in the national marine park is a priority, as well as control of the introduced tegu lizard whose predatory impacts are also likely to be high (Abrahão et al., in press). Although its exact trophic relationship with cats and rats remains unknown, it is likely to be a symmetrical intraguild predator with cats (Russell, 2011). Given their completely feral status and negative impacts on island biodiversity, all three species should be lethally controlled in the national marine park area (Russell et al., 2016). Re-homing of unsupervised cats and tegu from the national park would not be logistically feasible and would pose an unacceptable risk to human and wildlife health. A Toxoplasma gondii type exotic to continental Brazil and potentially pathogenic to humans has been detected in cats and rats on Fernando de Noronha (Silva et al., 2017) and Salmonella strains have been detected in tegu lizards of Fernando de Noronha (Abrahão et al., in press). The impact of cururu toads and little fire ants should also be investigated as they are currently unknown but potentially severe, although the population of cururu toads suffers from severe deformities (Tolledo and Toledo, 2015). The mocó appears to be having little impact on the island ecosystem, perhaps because it acts as an ecological surrogate, albeit not the most appropriate, for the extinct endemic Vespucci's rat. It does, however, act as an alternative food source for cats on the island.
Eradication of either cats or rodents is not currently feasible on Fernando de Noronha due to legal restrictions, limited conservation funding, and issues around social acceptability of methods, but these barriers are surmountable with the appropriate mechanisms. In the interim, around inhabited areas, management of cats should focus on encouraging ownership, individual identification and movement restriction (such as achieved for dogs since 2012), neutering supervised cats, and euthanasia for unsupervised (i.e. feral) cats (Dias et al., 2017). For rodents, interim management should focus on a sustained systematic control grid suppressing rodent to zero detectability on the main island, and eradication and biosecurity prevention on offshore islands. Ongoing monitoring of pest status and biosecurity to prevent invasions on the secondary islands is also a priority, and where necessary eradicating any introduced pests. All secondary islands are within the swimming range of both species of rat (Russell et al., 2008). The northern chain of islands, particularly the large Rata Island (88ha), warrants consideration as experimental sites for pest eradication to protect and restore breeding seabird colonies upon them. Such a conservation action would be defendable along the stepping-stone series of islands leading to Rata Island, as well as for diversifying the eco-tourism industry on the island through managed guided trips to a pest-free secondary island to visit seabird breeding colonies, particularly during the rainy low tourist season. Biosecurity for the entire archipelago also needs to be invested in immediately, to prevent new pest species arrivals on cargo ships and flights, and to set up social norms for a future, when extant pest species might be eradicated.
ConclusionsEvidence of the widespread negative impact of cats and rodents introduced to islands is clear. Management of cat and rodent populations on islands is required to halt and where possible reverse these impacts. Permanent eradication of cats and rodents from islands is the most powerful management intervention currently available for island conservation and restoration, but as well as technical limitations to island size, is limited by conservation funds and community acceptability on inhabited islands. On inhabited islands, management of introduced cats and rodents has historically tended to focus on agricultural and population health and zoonotic issues associated with their presence. In order to successfully control or eradicate introduced cats or rodents on inhabited islands, it is necessary to assess environmental attitudes of the island inhabitants and stakeholders towards biodiversity management on the island. Changes or exceptions to legislation may be required in order to enact the most appropriate control methods. Control of cats and rodents in natural environments coupled with appropriate management in inhabited areas may be seen as a pathway to ultimately eradicating these species from islands and allowing the complete benefits of islands restoration to be realised. Fernando de Noronha provides a case study illustrating the complexities of managing invasive cats and rodents on inhabited islands, but also the potential for eradicating them to bring about significant benefits to all of biodiversity, human health and livelihoods.
This work was undertaken while James Russell was visiting the University of São Paulo funded by PVE/CAPES (Proposal 235453) from the Ministério da Educação, Brazil with Licence 43589-4 from ICMBio to research in Fernando de Noronha. Thanks to Thayná Mello from ICMBio and Tatiane Micheletti and Paulo Rogério Mangini from the Brazilian Institute for Conservation Medicine – TRIADE for field support. Jean C. R. Silva and Ricardo A. Dias are recipients of scholarships from CNPq. Feedback was provided by three anonymous referees.