The quality of the surrounding landscape matrix often determines the biodiversity pattern of the remaining natural habitat fragments. Dispersal of organisms depends mostly on species traits related to mobility and the contrast between the habitat fragment and the matrix. Therefore, variation in species composition among fragments, i.e. beta-diversity, can also be affected by matrix quality. In our study, we focused on structurally complex forest steppe fragments in Central Europe for revealing the effects of fragment size and matrix quality. We investigated 18 fragments along a gradient ranging in size from 0.2 to 6ha, and embedded in a gradient of changing matrix quality. We collected data on plants, spiders and ants in three different habitat types: natural forest and steppe parts of the forest steppe fragments and in the directly neighboring dominant element of the landscape matrix, being pine plantations. Species turnover (beta diversity) was higher for steppes than for forests indicating a higher degree of isolation for steppes. Increasing matrix quality decreased plant species richness in small fragments. The low dispersal between fragments prevents the displacement of poor competitors by stronger competitors and promotes the coexistence of species. Matrix quality positively affected spider richness independently of fragment size, but had no effect on ants. These differences among taxa highlight the complexity of the effects of landscape structure, and calls for a situation-specific optimization of landscape management in nature conservation. For our particular situation, the findings provide evidence that improving matrix quality would help preserving the threatened forest steppe biota.
Human-induced landscape changes are among the most important processes resulting in biodiversity loss and the deterioration of ecosystem functioning in terrestrial habitats (Fischer and Lindenmayer, 2007). The amount and spatial arrangement of natural habitat fragments have an important role in determining how organisms are distributed in highly modified landscapes (Fahrig, 2017). The majority of habitat fragmentation studies focus on habitat fragments vs. landscape matrix as a black and white dichotomy with habitat fragments being the only suitable area for the focal organisms (Fardila et al., 2017). The matrix is defined as an extensive land cover that has undergone intense anthropogenic perturbation. Matrix quality is the degree to which natural conditions are altered in the matrix. Matrix quality may encompass variation in the availability of resources that supplement those of the habitat patches (Shoffner et al., 2018; Yekwayo et al., 2016). Connectivity and thereby the dispersal of organisms between habitat fragments depend on matrix quality (Antongiovanni and Metzger, 2005; Watling et al., 2011; Freeman et al., 2018). A high quality matrix, which is structurally similar to the habitat fragment, may allow certain rates of dispersal and mitigate the effect of habitat loss and fragmentation by providing additional resources for species (Fischer and Lindenmayer, 2007). Some species are able to exploit a good quality matrix in a way that their populations may remain stable or even increase in abundance throughout the fragmentation process (Antongiovanni and Metzger, 2005; Pfeifer et al., 2017). However, a low quality matrix strongly contrasts to natural habitats and thereby may impede the movement of species across the landscape. Regular immigration due to higher dispersal rates can help to avoid stochastic extinctions of populations with low abundance in small fragments (Öckinger et al., 2012a), thus matrix quality may exert a stronger effect on small fragments than on large fragments.
The landscape scale parameters affect the dissimilarity between local communities, and this may determine landscape-wide biodiversity (Tscharntke et al., 2012). This may also have a key effect on the variation of the species composition of habitat fragments, i.e. beta-diversity (Legendre, 2008). Several frameworks exist to characterize patterns of species distribution by partitioning beta diversity into its two components (Baselga, 2012; Carvalho et al., 2013; Podani and Schmera, 2011). According to Baselga (2012), the two components of beta diversity are, (1) species turnover, and (2) nestedness. Turnover is related to the replacement of species between communities, whereas nestedness expresses to what extent one community is a subset of another one. Their relative importance may change according to the processes structuring meta-communities (Tonkin et al., 2016). Random extinctions may contribute to nestedness in moderately connected fragments, however, increasing dispersal rates between fragments reduce differences in species composition resulting in the homogenization of the meta-community (Gianuca et al., 2017; Samu et al., 2018). Differences between the species composition of highly isolated fragments are mainly due to species turnover. Stochastic drift in species composition and the colonization of matrix species are the two main drivers of this phenomenon (Logue et al., 2011; Collins et al., 2017).
Forest steppes offer the opportunity to test the effect of matrix quality on the biota of different natural habitat types within habitat fragments. Forest steppes are structurally and compositionally very complex ecosystems. Habitat types create a gradient of plant and presumably invertebrate species richness (Erdős et al., 2020). In general, forest and steppe patches provide different physical conditions (Anenkhonov et al., 2015; Tölgyesi et al., 2018a) resulting in different plant communities that harbor a high species diversity with numerous endemic plant (e.g. Dianthus diutinus Kit. ex Schult., Colchicum arenarium Waldst. & Kit.), rare insect (e.g. Myrmeleotettix antennatus (Fieber, 1853), Hyponephele lupine Costa, 1936) and bird species (e.g. Coracias garrulus L., 1758, Caprimulgus europaeus L., 1758) of community interest in the European Union. Thus, forest steppes are recognized as important biodiversity hotspots (Dengler et al., 2014) and listed in the Habitats Directive (European Union 1992, codes: 91I0, 91N0, 6260).
Nevertheless, forest steppes are also among the most threatened ecosystems in Central Europe due to habitat loss and fragmentation. Most forest steppe mosaics of the region have been transformed into commercial plantations of exotic (mainly Pinus sylvestris L. and P. nigra J.F. Arnold) and native tree species (Populus alba L.) with extremely low habitat heterogeneity (Erdős et al., 2018). The dense canopy of plantations decreases the light availability on the forest floor resulting in lower understory biomass, moist and sheltered microclimate and homogeneous habitat structure (Balandier et al., 2006). Contrarily, native forest steppes are characterized by small-scale habitat heterogeneity and high microhabitat diversity due to the mosaic structure of the two prevailing physiognomic units, the forest and the steppe.
In the present study we focused on plants, spiders and ants for several reasons. Herbaceous vegetation is an important component of terrestrial food webs and provides the primary structure of habitats for ground-dwelling arthropods. Spiders are among the most abundant arthropod predators in all terrestrial ecosystems (Wise, 1995; Nyffeler and Birkhofer, 2017), and their diversity pattern sensitively indicates small differences in local and landscape scale environmental changes (Ziesche and Roth, 2008). Ants are a diverse and locally very abundant group of omnivorous terrestrial insects. Besides interspecific biotic interactions, particularly competition, habitat and landscape parameters are also important in determining their diversity (Gibb, 2011).
We aimed to understand the community composition and species richness patterns of plants, spiders and ants in highly modified forest steppe regions by focusing on the three main habitat types, steppe and forest parts of the forest steppe fragments (hereafter ‘steppes’ and ‘forests’) and the most abundant element of the landscape matrix, pine plantations (hereafter ‘plantations’). More specifically, we tested the following hypotheses: (1) Plantations resemble forests more than steppes in terms of plant, spider and ant species composition. (2) Steppes are more isolated than forests amidst the plantation-dominated landscape matrix, thus species turnover is larger, and nestedness is smaller between steppe than between forest patches of isolated habitat fragments. (3) Increasing matrix quality (i.e. higher share of semi-natural habitats in the landscape) has a stronger positive effect on species richness of small than large fragments.
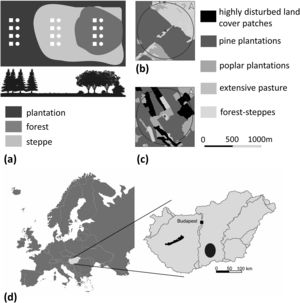
Material and methodsStudy region and sampling designThe study was carried out in the Great Hungarian Plain. This region has a continental climate with a mean annual temperature of 11°C and a mean annual precipitation of 550−600mm (Tölgyesi et al., 2015). The natural vegetation of the region was sandy forest steppe, which is a dynamic mosaic of forested patches, consisting of P. alba L., and Crataegus monogyna Jacq. (‘forests’) and dry bunchgrass steppes dominated by Festuca vaginata Waldst. & Kit. ex Willd and Stipa borysthenica Klokov ex Prokudin (‘steppes’) (Erdős et al., 2014, Appendix S1.).
In our study region, only small and isolated forest steppe fragments have been spared from large-scale land use change. The landscape matrix primarily consists of pine plantations, however, deciduous plantations, pastures, arable fields, and farmhouses also occur. We selected a total of 18 landscapes near the villages of Pirtó (n=9), Kéleshalom (n=4) and Bócsa (n=5), and selected small forest steppe fragments within each landscape, ranging from 0.2–6.0ha in size. We established a sampling site in the natural forest habitat and in the steppe habitat of each forest steppe fragment and another site in their close vicinity in the landscape matrix, which was in all cases a pine plantation (n=18×3=54 sampling sites) (Fig. 1a). We draw buffer circles with a radius of 500m around each sampling site with ArcGIS 10.1 software and measured the area of the habitat patches within the buffers using Google Earth satellite images. We used 500m radius following comparative studies (Gallé and Schwéger, 2014; Toivonen et al., 2015). We applied five land cover categories and gave the largest value to the highest quality patches: (1) highly disturbed land cover patches: arable fields, farm buildings, young plantations (less than 5 years old); (2) pine plantations; (3) intensively managed poplar plantations; (4) extensively grazed pastures and (5) intact forest steppes (Gallé et al., 2017, 2018; Ingle et al., 2019) (Fig. 1b,c). We expressed the matrix quality by weighting the proportion (percentage area) of each land cover type with the rank of the given habitat type (Tölgyesi et al., 2014). The matrix quality was thus measured on a continuous scale and the values ranged between 195.8 and 388.4.
Biodiversity samplingWe recorded all herbaceous plants and shrubs in four 2×2m plots in each sampling site and estimated their percentage cover in June 2018 (18 landscape×3 habitat type×4 replicates=216 plots, Fig. 1.a.). Plots were located in the center of the forest and in steppe habitat and at least 30m from the edge in the case of pine plantations and were approximately 8m apart from each other. We pooled the species data according to sampling sites resulting in 54 statistical samples.
We collected arthropods (spiders and ants) using pitfall traps (500-ml white plastic cups, 8.5cm in diameter). We fitted the traps with transparent plastic funnels to reduce vertebrate bycatches and increase trapping efficiency (Császár et al., 2018). We filled them with 50% ethylene–glycol and water solution containing a few drops of odorless detergent to preserve the sample and to reduce the chance of escaping. We placed a plastic roof above each trap to prevent the dilution of the preservative. At each sampling site, we employed four pitfall traps, spaced 8m apart, along a transect running parallel with the edge of the sampled habitat. Pitfall traps were placed circa. 1−2m from botanical plots (Fig. 1a). We installed a total of 216 traps (18 landscapes×3 habitat type×4 traps). We conducted sampling in three periods, from 4th to 18th May, from 14th to 26th June and from 20th September to 5th October in 2018. For further analysis, we pooled the species data according to sampling sites, resulting in 54 statistical samples. We identified plants using the key of Király (2009), the collected invertebrates were identified using the key of Nentwig et al. (2021) for spiders, and Czechowski et al. (2012) for ants. Voucher specimens of invertebrates are stored in the collection of the Department of Ecology, University of Szeged, Hungary.
Data analysisWe compared the composition of plant, spider and ant communities in the three habitats (i.e. forests, steppes and plantations) with permutational multivariate analysis of variance (perMANOVA) using 5000 permutations. We visualized the community composition of the sampling sites with non-metric multidimensional scaling (NMDS) with Bray–Curtis distance using the vegan package (Oksanen et al., 2013) in R environment (R Core Team, 2019). We applied Hellinger transformation on the data before the analysis (Legendre and Gallagher, 2001). We used indicator value analysis to identify the characteristic species of plants, spiders and ants in forests, steppes and plantations, with the ‘labdsv’ package in R (Roberts and Roberts, 2016).
We calculated beta diversity for the natural habitat types, between forests and between steppes, respectively, and decomposed it into turnover and nestedness components using two approaches. First, applying the incidence-based multiple-site dissimilarity approach, we calculated the replacement (related to turnover) and nestedness-resultant components for species compositional heterogeneity and overall beta diversity with Sørensen dissimilarity index. Second, with abundance-based dissimilarity we separated balanced variation in abundance (related to turnover) and abundance gradients (related to nestedness) with Bray-Curtis dissimilarity index (Baselga, 2017). We tested the differences in turnover components of forests and steppes with 500 random sampling procedures. We resampled the two incidence-based and the two abundance-based multiple site dissimilarities for a subset of ten steppe and forest sites of the original data frame using the R-package betapart (Baselga and Orme, 2012). We divided the turnover component with the total beta diversity for each random sample (Dobrovolski et al., 2012) and this turnover ratio was tested with one-way ANOVA to reveal differences between forests and steppes. We log-transformed turnover ratio values to meet the assumptions of normality. We ran the above analysis for plant, spider and ant data separately.
We categorized all species according to their habitat affinity to habitat specialist (i.e. grassland species for steppes and forest species for forests and plantations) and generalists according to literature data. We used GLMMs to determine the effect of habitat type (i.e. forest, steppe and plantation), matrix quality (measured in 500m radius buffer), fragment size and their first order interaction on the species richness and specialist species richness of the herb layer and arthropods with “village” as random factor. Continuous fixed variables (i.e. matrix quality and forest steppe size) were measured on different scales, thus, prior to analysis, they were scaled between 0 and 1. We used Poisson distribution error term in the models. We ran GLMMs for all possible combinations of predictor variables, and calculated AICc values (Akaike’s Information Criterion with correction for small sample size). We performed model averaging on models with a delta AICc ≤6 of the best model using the R-package MuMIn (Barton, 2012). To control the potential effect of outliers we calculated Cook’s distance for each sampling points and AICc values were checked again after the removal of influential points (Zuur et al., 2009).
ResultsWe recorded 164 plant species in the 54 sampling sites of the 18 landscapes. The most abundant graminoids were F. vaginata, S. borysthenica and Carex liparocarpos Gaudin. Common forbs included Teucrium chamaedrys L., Potentilla arenaria Borkh. ex G.Gaertn., B.Mey. & Scherb. and Euphorbia seguieriana Neck (Appendix S2, S3). We collected 5595 adult spiders of 111 species. The most abundant ones were Pardosa alacris (C.L. Koch, 1833), Alopecosa sulzeri (Pavesi, 1872), and Zelotes apricorum (L. Koch, 1876), (Appendix S2, S3). Finally, we collected 8773 ants belonging to 32 species, with Lasius psammophilus Seifert, 1992, Plagiolepis taurica Santschi, 1920 and Myrmica sabuleti Meinert, 1861 being the most abundant species (Appendix S2, S3).
Community composition of plants, spiders and ants was influenced by habitat type (Fig. 2). We found significant differences in the species composition of the three habitat types according to the perMANOVA analyses, with the most expressed differences between steppe and the other habitat types (Table 1). We identified several significant indicator species of forest (plants:19; spiders: 7, ants: 2 species), steppes (plants:24, spiders: 9, ants: 5 species), and plantations (plants:0; spiders: 6, ants: 4 species) (Appendix S2).
Pairwise differences between species composition of forests, grasslands and pine plantations according to the perMANOVA analyses.
| Vegetation | Spiders | Ants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | F | p | R2 | F | p | R2 | F | p | |
| Steppe-forest | 0.388 | 21.59 | <0.001 | 0.542 | 40.38 | <0.001 | 0.434 | 26.12 | <0.001 |
| Forest-plantation | 0.148 | 5.79 | <0.001 | 0.119 | 4.61 | 0.002 | 0.107 | 4.07 | <0.001 |
| Plantation-steppe | 0.393 | 21.58 | <0.001 | 0.511 | 35.57 | <0.001 | 0. 370 | 20.05 | <0.001 |
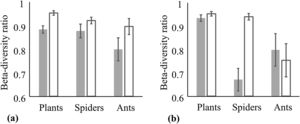
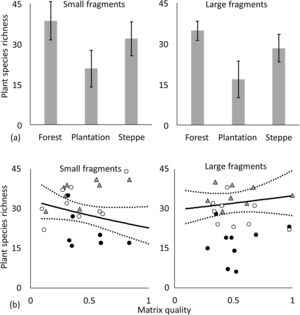
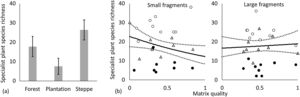
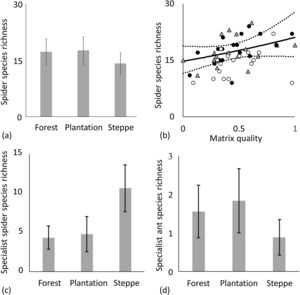
The turnover component of beta diversity was higher for steppes than for forests both in the case of incidence-based and abundance-based approaches, i.e. we found higher patchiness of the steppe habitat compared to forests (Fig. 3, Table 2.). Plant species richness was highest in forests followed by steppes and it was the lowest in plantations according to the GLMM models, furthermore, the difference between plantations and steppes was larger in small than in large fragments (Fig. 4a, Appendix S4). Matrix quality had a negative effect in small fragments, however this effect was positive in large fragments (Fig. 4b, Appendix S4). We found higher specialist plants species richness in steppes followed by forests and plantations (Fig. 5a, Appendix S4). We found similar interacting effect of matrix quality and fragment size for plants and specialist plats, matrix quality had a negative effect in small fragments, however this effect was positive in large fragments (Fig. 5b, Appendix S4). We found higher species richness of spiders in plantations and forests than in steppes and matrix quality had a positive effect on them (Fig. 6a, b, Appendix S4). However, specialist spider species richness was the highest in steppes followed by plantations and forests (Fig. 6c, Appendix S4). We found no significant effects on ant species richness, yet we detected more specialist ant species in plantations than in steppes (Fig. 6d, Appendix S4).
Effect of habitat type, matrix quality and fragment size on the species richness of plants and specialist plants. Species richness of plants: (a) interaction of fragment size and habitat type (mean±sd), light grey bars: small fragment, dark grey bars: large fragments, (b) interaction of matrix quality and fragment size. Open circles: steppes, grey triangles: forests, black dots.
Species richness of specialist plants: (a) habitat type (mean±sd), (b) interaction of matrix quality and fragment size. Small fragments: size between 0.24 and 0.75ha; Large fragments: size between 1.1 and 6.0ha. Values for matrix quality index are ranged between 0 and 1. Open circles: steppes, grey triangles: forests, black dots: plantations. Dotted lines represent the 95% confidence intervals.
Effect of habitat type and matrix quality on the species richness of spiders and ants. Species richness of spiders: (a) habitat type (mean±sd), (b) matrix quality, open circles: steppes, grey triangles: forests, black dots. Species richness of specialist spiders: (c) habitat type (mean±sd). Species richness of specialist ants: (d) habitat type (mean±sd). Values for matrix quality index are ranged between 0 and 1. Open circles: steppes, grey triangles: forests, black dots: plantations. Dotted lines represent the 95% confidence intervals.
Species composition of plantations was more similar to that of the forests than to the steppes. Along this line, we also found higher species turnover ratios for steppes than for forests for all taxa indicating that steppes are more isolated than forests. Matrix quality affected both plant and spider species richness of all habitat types; this effect was positive for spiders, however, matrix quality effect was modified by fragment size for plants. It negatively affected plant species richness of small fragments, but this effect was positive for large fragments.
We found a clear separation of the biota of focal habitat types (Hypothesis 1). The plants of the three habitat types had no overlap according to the NMDS ordination. Numerous studies showed significant differences between the vegetation structure and plant species composition of forests and steppes in forest steppe ecosystems (e.g. Bátori et al., 2018; Erdős et al., 2014, 2018). However, there is virtually no information on the role of the main matrix habitat, i.e. plantations, as a potential secondary habitat for forest steppe vegetation. We found that the species richness of plantations was less than half of the species richness of the forests and this was also true for the specialist species richness, presumably due to the thick and allelopathic litter layer of pine needles, and the low water and light availability in the forest floor of the plantations (Selvi et al., 2017). The dominant plant species of these plantations were mainly generalist, disturbance tolerant (e.g. Calamagrostis epigejos and Poa angustifolia) or even invasive species (e.g. Asclepias syriaca) indicating that pine plantations are very low quality secondary habitats for natural vegetation.
Arthropod species composition of the three habitat types was also significantly different. However, plantations and forests overlapped according to the NMDS ordination plots for both spiders and ants. Interestingly, we found higher spider species richness in plantations than in steppes. Steppes formed on sandy soils are characterized by harsh microclimatic conditions with high and fluctuating ground temperature (maximum summer temperature of bare ground can exceed 60°C) and low air humidity (Erdős et al., 2014). The hot and dry conditions favor invertebrate species with certain trait state combinations (e.g. xerotolerant and thermophilous species), and serve as a strong environmental filter (Gallé et al., 2018). A relatively small number of specialist invertebrate species are able to colonize such habitats (Gallé and Torma, 2009). In line with Gallé et al. (2018) and Ingle et al. (2019) the present study also suggests that forest generalist species constitute a large part of the fauna of plantations, and these species are unable to colonize forest steppes.
We found that the turnover component had a high contribution (>0.6) to beta diversity of forests, and even higher (>0.9) for steppes for both incidence-based and abundance-based multiple-site dissimilarity approaches, confirming our Hypothesis (2). Such remarkably high contribution of species replacement was observed for island butterflies (Yong et al., 2012) and spiders (Wu et al., 2017) suggesting a higher level of isolation for steppes than for forests. A low-quality matrix with high habitat-matrix contrast may decrease functional connectivity by preventing movement of organisms between fragments (Reider et al., 2018; Tölgyesi et al., 2018a,b). Community composition of plantations was more similar to forests than to steppes according to the NMDS ordinations, suggesting a higher habitat-matrix contrast and a less permeable landscape for the biota of grasslands than that of the forests. Communities in less permeable landscapes are more exposed to random shifts in community composition due to higher extinction rates and random colonization of species (Watling et al., 2011). Therefore, these isolated communities have more divergent species compositions than communities for which the matrix is more permeable (Watling et al., 2011). Another possible explanation for the high turnover ratio between steppes could be the micro-habitat heterogeneity across the sampled grasslands. Small-scale differences in soil properties are known to affect vegetation in dry grasslands (Maestre and Cortina, 2002), and microhabitat structure affects spider (Gallé et al., 2010; Heneberg and Řezáč, 2014) and ants (Castracani et al., 2010) of arid grasslands. Presumably, low matrix permeability together with high microhabitat diversity resulted in the high turnover component of the beta diversity of steppes.
Matrix quality may have an interacting effect with fragment size, as increasing matrix quality may have a weaker effect on large fragments (Öckinger et al., 2012b). Increasing matrix quality had a positive effect on both the total and specialist plant species richness of all habitat types in case of landscapes with large habitat fragments, however, this effect was negative for landscapes with small fragment size (Hypothesis 3). High quality matrix may exert its positive effect via improved immigration rates of forest steppe specialists to the habitat fragments (Rand et al., 2006 Low quality matrix diminishes dispersal and regular immigration of specialist species into habitat fragments (Driscoll et al., 2013). The low dispersal between fragments prevents the displacement of poor competitors by locally strong competitors and promotes the coexistence of specialist species (Levine and Rees, 2002; Büchi and Vuilleumier, 2014). However, this phenomenon is not pronounced in large fragments where stronger competitors are present in higher numbers. This suggests the type of matrix in which natural habitat fragments are situated can explain a substantial amount of the widely reported variability in biodiversity responses to fragmentation (Hatfield et al., 2020).
We found that increasing matrix quality had a positive effect on the species richness of spiders. Several studies also concluded that increasing matrix quality has a positive effect on the species richness of a habitat fragment, and this effect is the most expressed if the amount of suitable habitats and high quality matrix elements are low (Ruffell et al., 2017; Reider et al., 2018). This emphasizes the positive effect of increasing matrix quality for spiders, which are generally moderately good dispersers, even in high quality landscapes.
According to our findings, ant species richness was unaffected by habitat type, matrix quality and fragment size. Matrix may also reduce fragment size effects, i.e. small and large fragments will harbor a similar number of species. This could be because high landscape heterogeneity results in high resilience and stability of ecological processes (Freeman et al., 2018). This is in line with Dauber et al. (2003), who found that matrix quality explains relatively low variance in ant species richness, suggesting the importance of other factors, such as competition, in shaping ant community structure (Hölldobler and Wilson, 1990). Interference competition has an increasing importance in advanced successional stages (Gallé et al., 1998; Gibb, 2011), such as in natural forest steppe patches. However, habitat type had an effect on the species richness of specialist ants. In contrast to plants, we found higher specialist ant species richness in the plantations than in steppe habitats, since numerous forest species colonized plantations presumably due to the thick litter layer and closed canopy.
Several landscape management strategies based on a binary partitioning of land cover types have been suggested to enhance connectivity between habitat patches in fragmented landscapes, such as stepping stones (Saura et al., 2014) and habitat corridors (Gilbert-Norton et al., 2010). Our study emphasizes that matrix quality metrics may provide more insight into the distribution of organisms than binary metrics (Fischer and Lindenmayer, 2007). Landscape perception is species specific and depends on numerous trait states (Rieder et al., 2018), however, detailed databases do not exist for all species groups of conservation importance. We argue that matrix quality assessment and evaluation for several taxonomic groups could help in developing landscape-wide conservation strategies.
The main implication of our study is that enhancing matrix quality by changing land use (i.e. land cover) type and increasing the share of the fragmented natural habitat in the landscape would help to maintain habitat specialist species. Although, it may not be economically feasible to restore large forest steppe areas in Central and Eastern Europe due to the growing demand for timber production, substituting the relatively poor preforming pine plantations with forest plantations of native tree species, such as silver poplar and establishing extensive pastures would support the native biota of forest steppes. Furthermore, isolation of steppes could be mitigated by substituting the neighboring high contrast plantations with low contrast pastures.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the Hungarian National Research, Development and Innovation Office (NKFIH FK 131379 for RG, NKFIH KKP 133839 for RG, GSN, AT and PB; NKFIH K 124796 for ZB and NKFIH PD 132131 for CT). RG, CT and ZB were supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. We are also grateful for the support of the New National Excellence Programme of the Ministry for Innovation and Technology, Hungary (CT: ÚNKP-21-5-SZTE-591, ZB: ÚNKP-21-5-SZTE-581).