In this study, we compare the variations of Margalef K and Shannon H diversity indices obtained for a fish community that suffered changes as a consequence of the impoundment of the upper Tocantins River in Goiás, Brazil. The analysis of different sites along the study area showed that the Shannon index was not sensitive to the environmental changes, whereas the Margalef K index varied significantly following the impoundment. Therefore, the Margalef K index appears to be a good diversity indicator as well as a valuable parameter for the temporal data series analysis, from changing environments and for the conservation of natural environments. Although the Margalef K index can be used as a diversity index, it can also be used to consistently indicate the speed of ecosystem evolution.
The Shannon index (Shannon and Weaver, 1949) is one of the oldest and more widely used diversity indices, although it has been severely criticized due to its simplicity (May, 1975; Magurran, 2004; Leinster and Cobbold, 2012). According to Hutchinson (1978), diversity indices were introduced into ecology studies irrespectively of each other, by MacArthur (1955) and Margalef (1956). The notoriety of the authors of the seminal papers on diversity indices guaranteed the credibility of these indices. In addition, the diversity indices originated from information theory, have a distant link with thermodynamics through entropy, and include simple calculations. These facts were all important in the dissemination and maintenance in the use of these indices by ecology researchers.
Margalef (1991) created the “K Diversity Index”, a diversity evaluation tool based on the causes of diversity. The K Diversity Index is based on the amount of energy flowing through ecosystems, which relates to the ecosystem biomass renewal rate. This rate is calculated as the ratio between the primary production and the biomass (P/B). The inverse of P/B is expressed in time units; thus, the ecosystem biomass renewal rate is a measure of the speed of the ecosystem evolution (e.g. ecological succession – Margalef, 1968).
Ecological succession is a consistent empirical pattern that still lacks an adequate explanatory theory (Margalef, 1991). An increase in the amount of nutrients such as nitrogen and phosphorus in the ecosystems reduces the biomass renewal time. This phenomenon can be observed in the eutrophication of freshwater ecosystems (Margalef, 1963; Carpenter and Brock, 2006) or in marine areas where an upwelling of nutrient-rich water occurs (Margalef, 1968; Arnold et al., 2001; Folke et al., 2005).
The Margalef's K index is based on a comparison between theoretical extreme ecosystems. The first extreme can be exemplified by a culture of several species of protozoans in a microcosm with a constant nutrient flow. When this ecosystem becomes reduced to a single species, the individual species with the fastest reproduction rate will predominate after a certain time interval. This situation can be represented by the equation S=N0, where S is the number of species and N is the total number of individuals. This example represents an ecosystem with a very high biomass renewal rate and zero diversity. The second extreme can be exemplified by an ecosystem in which all of the species are represented by a single individual with an equitability equaling 1 and can be represented by the equation S=N1. Natural ecosystems most often exist between these two extremes and can be represented by the equation S=Nk.
In the present work, we empirically evaluate the value of the information provided by the Margalef K index to explain the changes occurred in the structure of the fish community from the upper Tocantins River that had undergone major environmental changes as a result of a dam construction. Although formal statistical comparisons between these indices should use analytical approaches or computer simulations, we understand that such empirical comparison can be useful to show how these two indices behave differently. Moreover, this comparison can reveal the advantages of using Margalef's K due to its more interesting biological properties and ability to detect clear ecosystem differences driving diversity patterns.
Materials and methodsWe used data from the fish community of the upper Tocantins River (14°31′27″ S/49°02′33″ O and 13°37′42″ S/48°07′08″ O) over a 180-km stretch of river. This river stretch suffered environmental changes as a consequence of water impoundment to generate hydroelectric energy (Serra da Mesa Hydroelectric Power Plant), which resulted in the gradual transformation of a lotic environment into a lentic one. Forty-six bimonthly sampling events were performed between December 1995 and October 2010 except for site PG where only 36 sampling events were done. Each sampling followed a standardized effort using 650m2 of exposed nets. In December 1995, sampling events began at the Tocantins River that had been diverted, since 1986, through 2 tunnels. Samplings were performed at 5 different sites distributed over two large areas: 2 sites downstream (FU and PG) and 3 sites upstream (ALR, BPE, and RUR) from the tunnels. The 46 sampling events were divided between the lotic and lentic periods according to the prevailing environmental conditions at the specific period of the study (Table S1).
The diversity indices of Margalef (K=logS/logN – where, S is the number of species and N is the total number of individuals in the sample) and Shannon (H′=−∑i=1kpi ln pi – where k is the number of categories and pi is the proportion of the observations found in category i) were calculated for each sample. In order to analyze the performance of the two indices regarding the transformation of the lotic environment into a lentic one, we calculated the mean values of these indices, for each site and sampling period. The comparison between the mean values of K and H′, between sites and periods, were tested through ANOVA when these diversity values followed a normal distribution (Shapiro–Wilks test W). The statistical significance of means was achieved by Tukey test (HSD) for unequal n. In two sites (RUR and ALR) the distribution of K values did not fit the normal distribution, in these cases we used the Kruskal–Wallis ANOVA test H and Median test. All analyses were performed with Statistica 11.0.
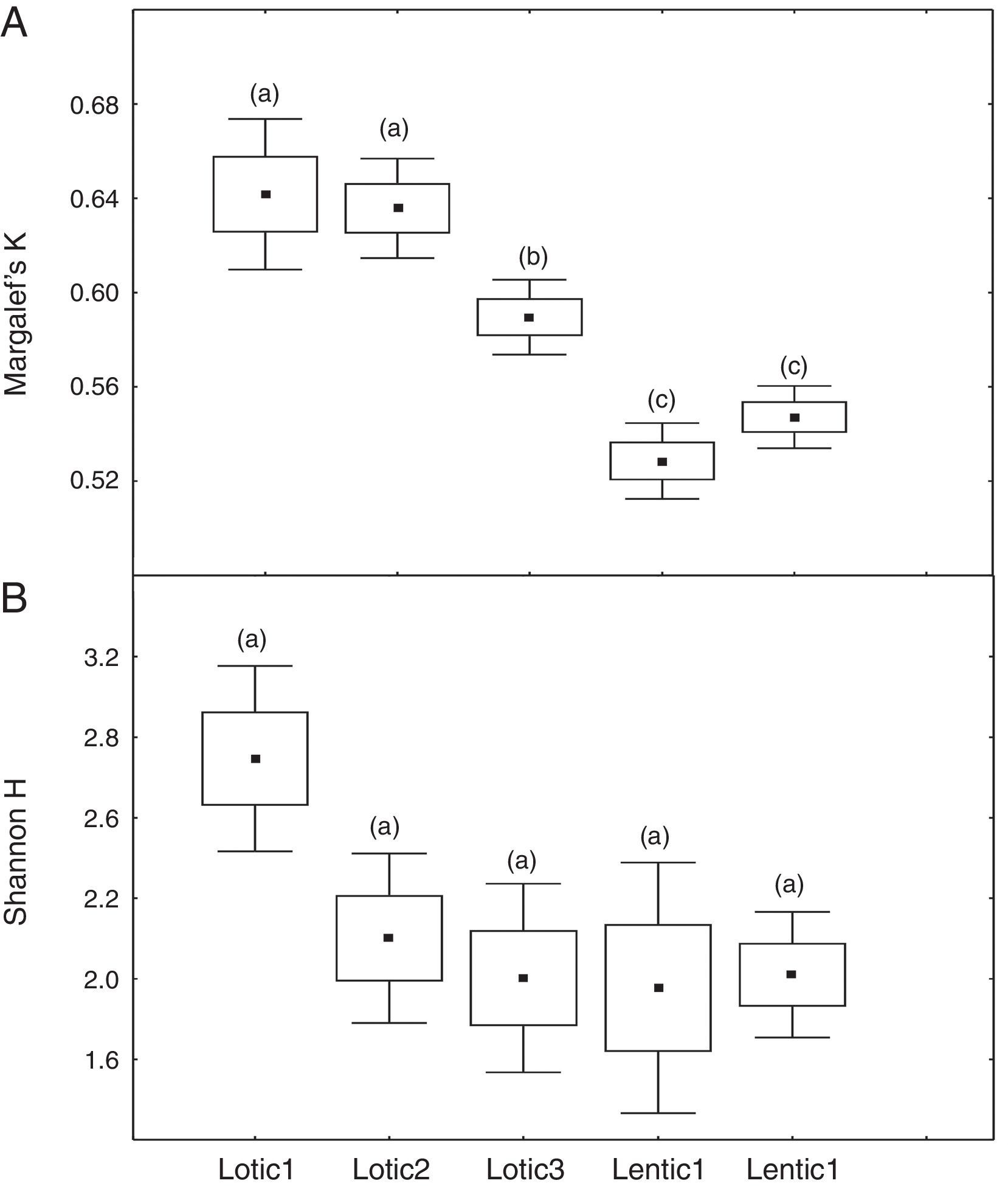
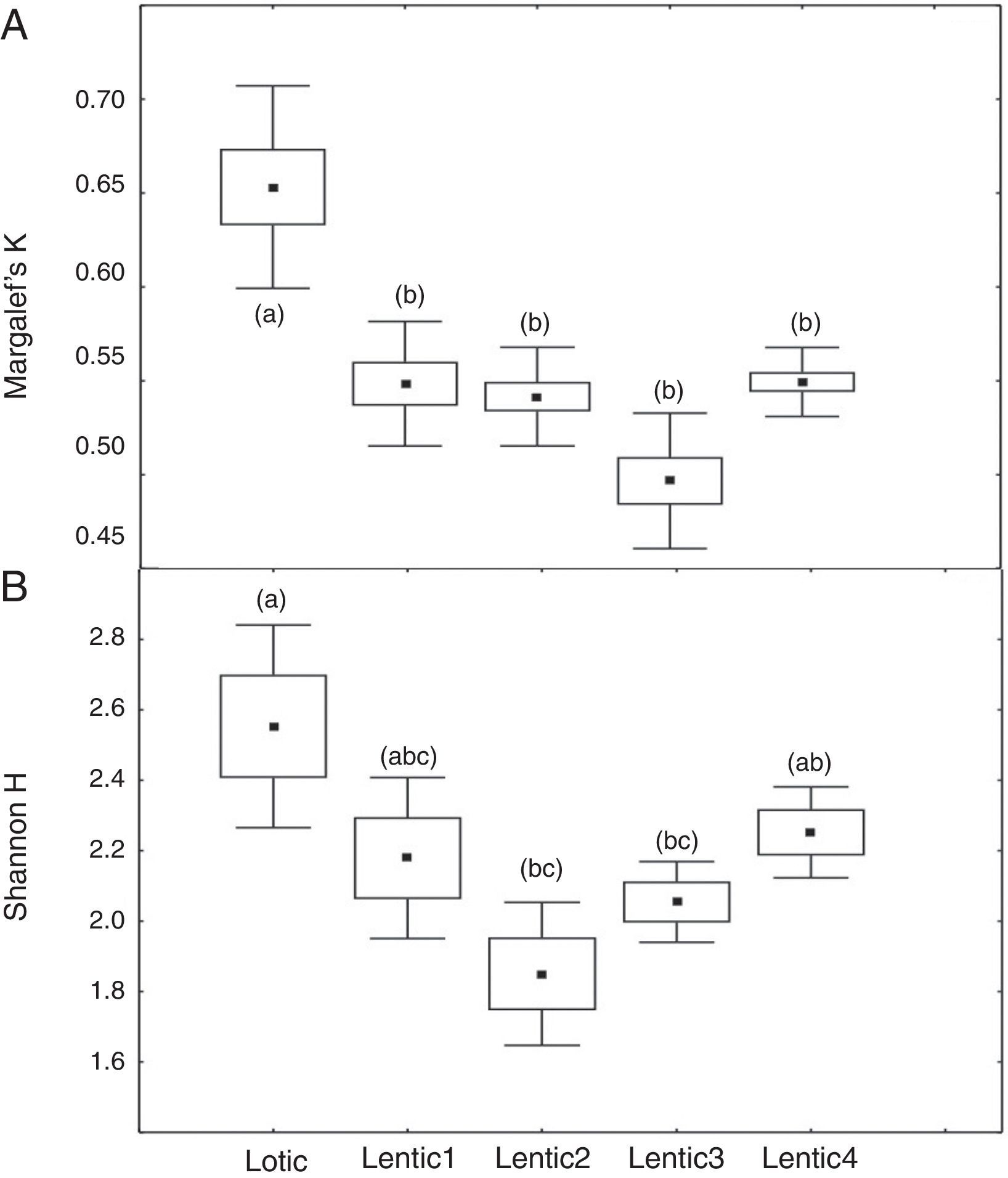
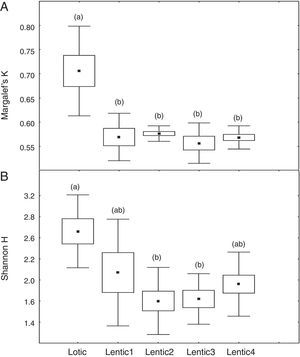
ResultsComparison of mean values of Margalef (K) and Shannon (H′) for the downstream sites (FU and PG) revealed marked different responses of these indices for the impoundment process. Mean values of K decreased significantly (ANOVA, F=21.70, p<0.001) from Lotic1 to Lentic1 periods at FU following the environmental impoundment. It was observed a significant decrease in the mean K during the Lotic3 period compared with Lotic1 and Lotic2, although the overall environment remained lotic (Fig. 1a). The H′ index was completely insensitive to the impoundment, so there were no statistically significant differences (ANOVA, F=2.34, p=0.07) between the mean H′ of these 5 periods (Fig. 1b). The mean values of K at site PG (Fig. 2a) were similar to those of K of site FU, except for the Lotic3 period that did not present statistically significant differences (ANOVA, F=4.79, p=0.007) in relation to the two previous lotic periods. Mean H′ values at site PG (Fig. 2b) did not show statistically significant differences between the Lotic1 and the remaining periods, and statistically significant differences were only observed between the Lotic2 and Lotic3 periods (ANOVA, F=5.36, p=0.004).
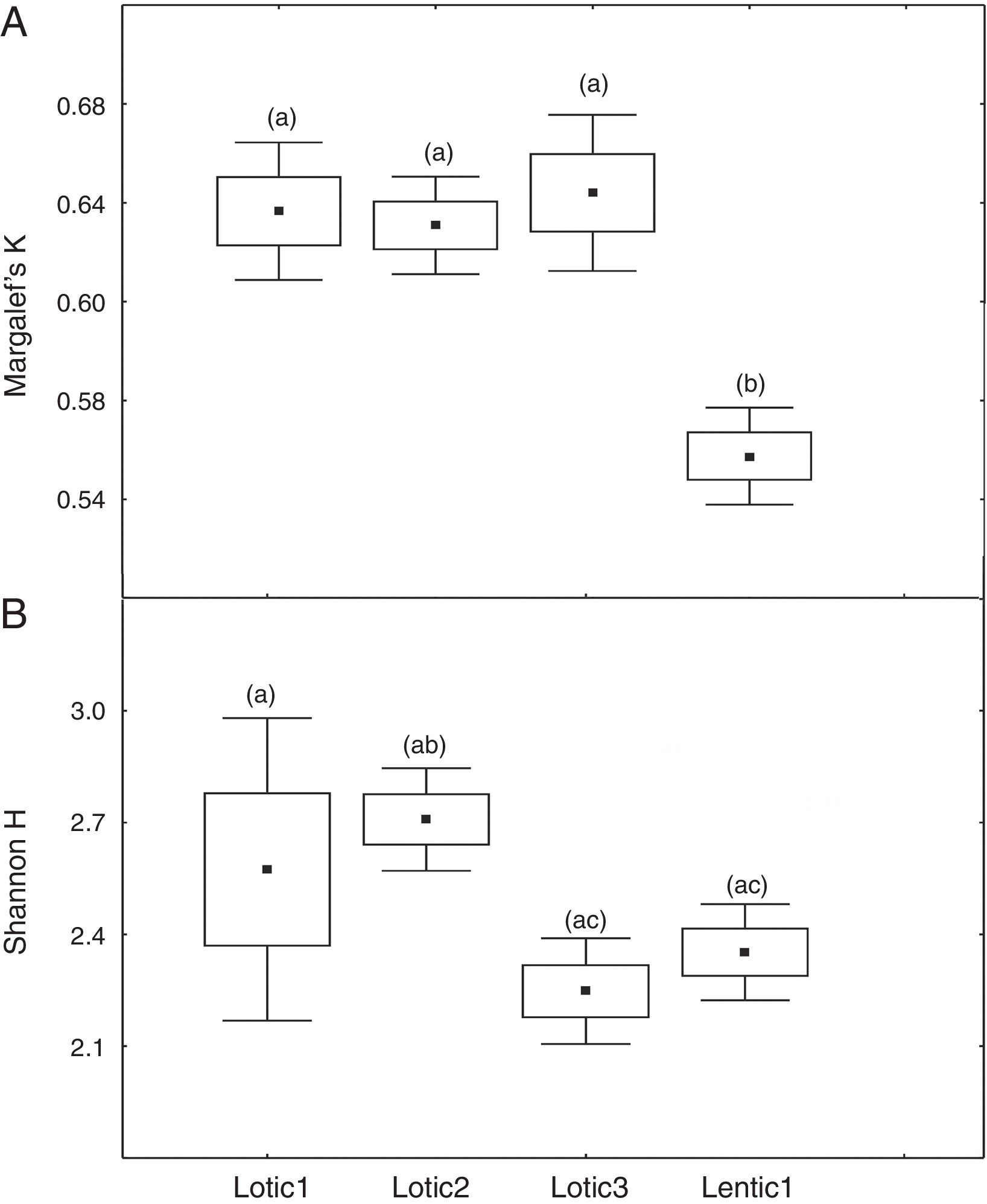
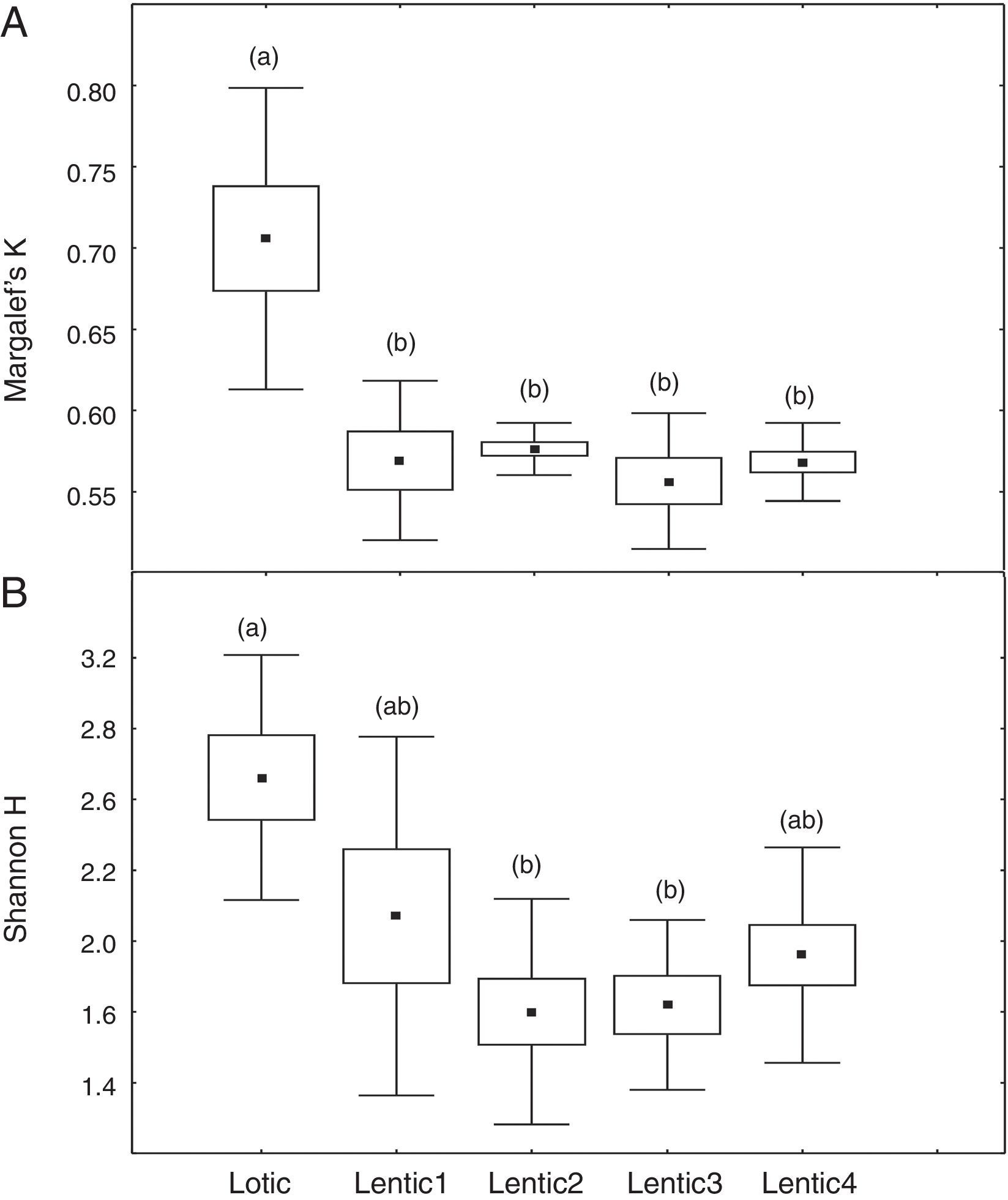
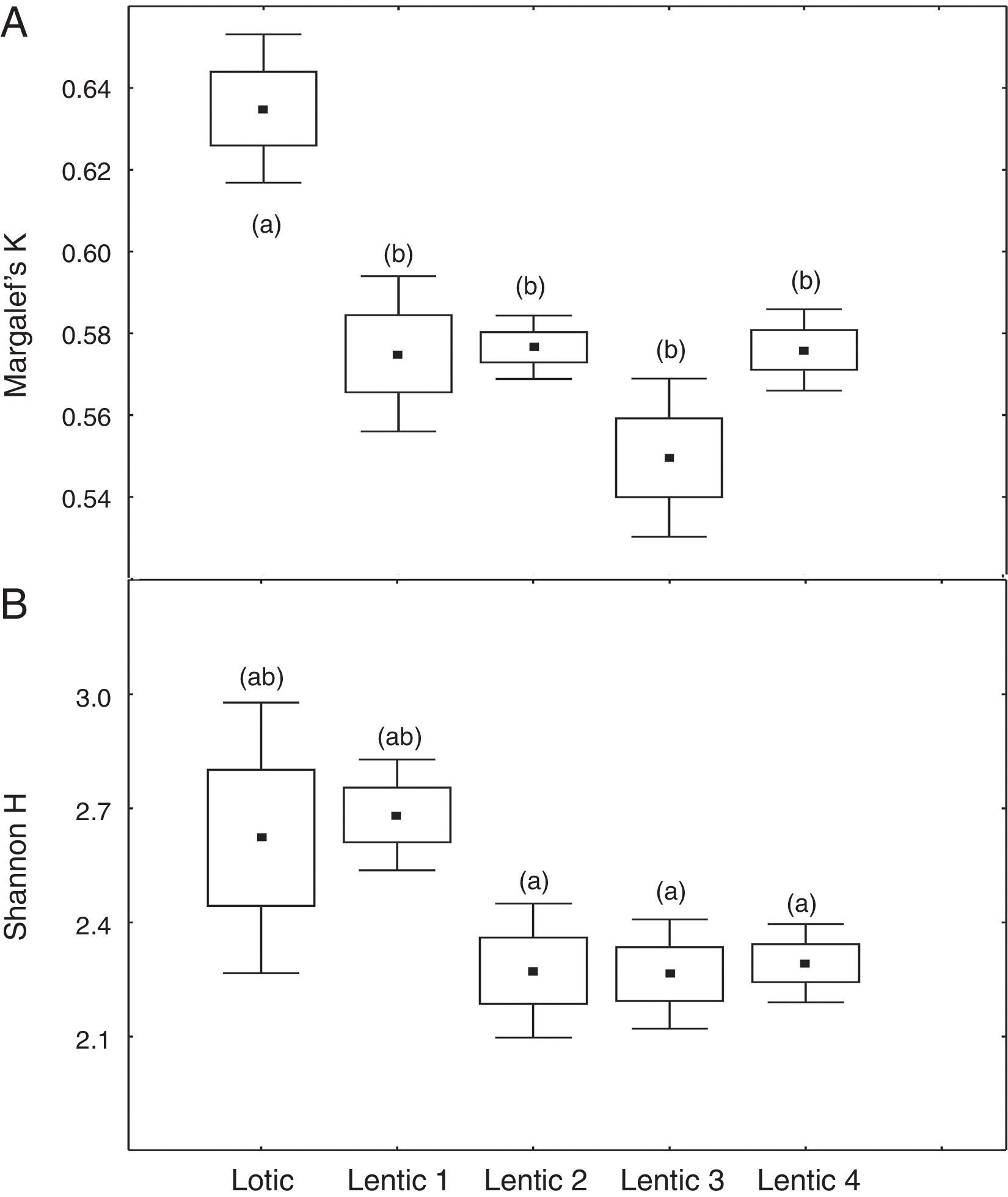
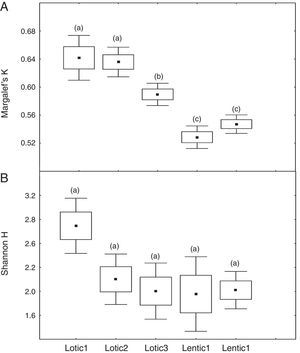
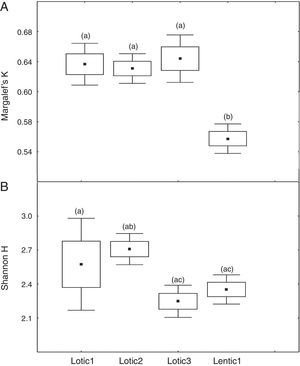
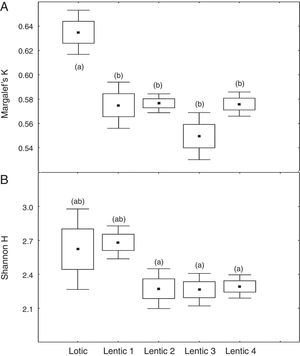
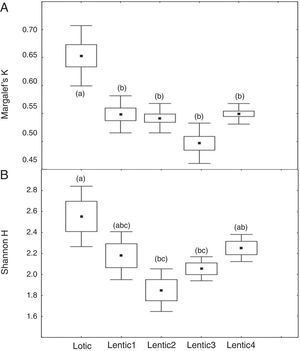
At the three sites located in the reservoir (RUR, BPE, and ALR) mean values of Margalef index varied according to exactly the same pattern. At site RUR, 100km distant from the dam, mean values of K significantly (Kruskal–Wallis, H=18.4, p<0.001) decreased from the moment when the environment changed from lotic (Lotic) to lentic (Lentic1, Lentic2, Lentic3, and Lentic4) (Fig. 3a). Variations in the H′ index did not follow these environmental changes. H′ values were significantly different (ANOVA, F=6.84, p<0.001, Fig. 3b) but they did not differ between the lentic and lotic environments. At site BPE, 60km distant from the dam, mean values of K showed the same pattern of variation observed at RUR (ANOVA, F=15.33, p<0.001, Fig. 4a), and again H′ showed significant differences (ANOVA, F=4.57, p=0.003) but they did not differ consistently between the lotic and lentic environments (Fig. 4b). At site ALR, 40km distance from the dam, mean values of K and H′ showed the same variation pattern observed at the two other sites in the reservoir (Kruskal–Wallis, H=24.98, p<0.001, Fig. 5a and ANOVA, F=6.61, p<0.001, Fig. 5b).
Spatial variation of mean values of the Margalef K (a) and Shannon H (b) indices at the sites RUR located upstream the Serra da Mesa reservoir. The letters marking the different periods denote significant differences as determined by Kruskal–Wallis (to Margalef index, a) and ANOVA (Shannon index, b).
Spatial variation of mean values of the Margalef K (a) and Shannon H (b) indices at the sites ALR located upstream the Serra da Mesa reservoir. The letters marking the different periods denote significant differences as determined by Kruskal–Wallis (to Margalef index, a) and ANOVA (Shannon index, b).
The transition from a lotic to a lentic environment is characterized by the enrichment of the environment usually resulting in a biomass increase. This pattern was observed at all sites where the change in environment from lotic to lentic occurred. Table S2 presents the results of the site RUR as a representative pattern of our observations for all of the reservoir sites (BPE and ALR). In addition to an increase in the total biomass, an increase in piscivorous fish biomass was observed.
DiscussionOur goal was to call attention to the explanatory capacity of the Margalef K index, which was created in 1991 but has been seldom used. The construction of the K index follows the well-known pattern of acceleration of the ecosystem functioning when energy enters an ecosystem. An influx of energy and subsequent enrichment occurred at three studied sites from the reservoir (RUR, BPE, and ALR) during the transition from a lotic to a lentic environment. This enrichment could be due to the accumulation of nutrients as the reservoir was being filled, generating an important increase in primary production with the establishment of a phytoplankton community along with an increase in secondary production due to the establishment of a zooplankton community.
We expected the Margalef K index to decrease significantly along with these environmental changes. In fact, in response to the influx of energy, the K values decreased significantly in all cases where a lentic environment was established. At the site downstream from the reservoir (FU), the K value followed the same pattern observed at the reservoir sites (RUR, BPE, and ALR) decreasing significantly when the environment changed from lotic to lentic. However, the K value significantly decreased at the FU site when the Serra da Mesa power plant became operational (Lotic3).
If a decrease in K occurs when the environment is enriched with energy, then the following question arises: what type of enrichment could have taken place at that Lotic3 environment site? The enrichment in energy can be attributed to the presence of anoxic water, which was collected at a depth of 60m at the reservoir. Anoxic environments generally harbor a large amount of organic matter, which is preserved due to oxidation (Carpenter and Brock, 2006). Therefore, the overflowing water not only is anoxic but also contains large amounts of dissolved organic matter. In the absence of oxygen, this condition favors the proliferation of methanogenic bacteria, which in turn favors the growth of methanotrophic bacteria, which serve as food for zooplankton species (Utsumi et al., 1998; Bastviken et al., 2003; Burkepile and Hay, 2006; Jones et al., 2008; van Hardenbroek et al., 2010). These events resulted in the energy enrichment of the environment at the FU site, which led to a significant decrease in K value even though the environment was lotic (Lotic 3 in Fig. 1a).
At the PG site, we observed no statistically significant differences in the average K values between the three lotic periods, but the K values significantly decreased when the environment changed to lentic (Lentic1). In contrast with our observations for the FU site, no decrease in the average K value was observed during the Lotic3 time period. The water at the PG site contained dissolved oxygen, which had been absorbed along the 40-km course between the FU and PG sites. This increase in oxygen may have reduced the presence of methanogenic bacteria resulting in a decrease in the enrichment of the environment.
Careful observation revealed a small increase in the number of species during the filling of the reservoir (during the transition to a lentic environment), but the total number of individuals and their total biomass increased to a much greater degree. In ecosystems accelerated by the influx of nutrients, natural selection favors small-sized species, which are generally represented by a larger number of individuals than larger-sized species. At the 3 sites upstream from the reservoir, we observed a significant increase in total biomass and a significant increase in the piscivorous biomass. A great increase in small-sized fish species during the lentic periods was visually confirmed based on the presence of large schools of Astyanax and Creagrutus species near the reservoir margins (personal observation). This increase in small-sized fish species was expected due to the enrichment of the environment.
The Shannon index was unable to detect these pronounced changes to the environment. Creating ad hoc explanations to justify variations in the H value is possible; however, we consider this exercise futile. The results of the Simpson index (not shown), which is considered to be superior to the Shannon index (May, 1975; Magurran, 2004), demonstrated even less sensitivity than the H value.
Recently, Leinster and Cobbold (2012) proposed a new family of diversity evaluation tools for natural communities. Similarly to the traditional Shannon and many other diversity indices, this new approach uses the relative frequency of individuals of each species in an ecosystem (pi). However, this approach also includes a measure of similarity between the species, but, as stated by the authors, introduces a degree of subjectivity. In addition, these evaluation tools present other two problems: (1) in community samplings, the relative frequency of individuals of the same species is frequently overestimated or underestimated due to complex sampling problems (Pielou, 1974); (2) the introduction of similarity calculations represents an upgrade to the evaluation of diversity (Warvick and Clarke, 2001). However, for the vast majority of the tropical communities, no information exists on the functional characteristics of the species or their phylogeny, and their genome has not been sequenced, resulting in limitations to the use of these new indices. The traditional diversity indices are even worse because they contain theoretical inconsistencies such as dependency on the number of individuals and are very difficult to interpret because these models are not based on principles from physical or biological theories. The Margalef K index was proposed in 1991 but has been largely ignored by the scientific community. The Margalef K index does not use the pi value but is instead based on a very concrete aspect of ecological theory – the ecosystem biomass renewal rate. The ecosystem biomass renewal rate is in turn connected to the theory of evolution because the amount of energy flowing through the ecosystems fuels the process of natural selection.
We have shown that the Margalef K index is very sensitive to changes in the ichthyofauna structure and is therefore a good indicator of biodiversity. More importantly, the Margalef K index appears to be a valuable tool for the analysis of time series data from changing environments and for the natural environment conservation. The Margalef K index may incidentally function as a diversity index, but the Margalef K index transcends this function because this index consistently indicates the speed of ecosystem evolution.
Conflicts of interestThe authors declare no conflicts of interest.
We thank FURNAS Centrais Elétricas, Departamentos de Ecologia from Universidade Federal do Rio de Janeiro and Universidade do Estado do Rio de Janeiro for the opportunity to conduct this project. Several students from both institutions collaborated on the fieldwork. We thank the anonymous referees for the important suggestions that improved the final form of the manuscript and Erica Pellegrini Caramaschi and Miriam Pilz Albrecht for all of their help during the 15 years of study for the present project.
The following are the supplementary data to this article: