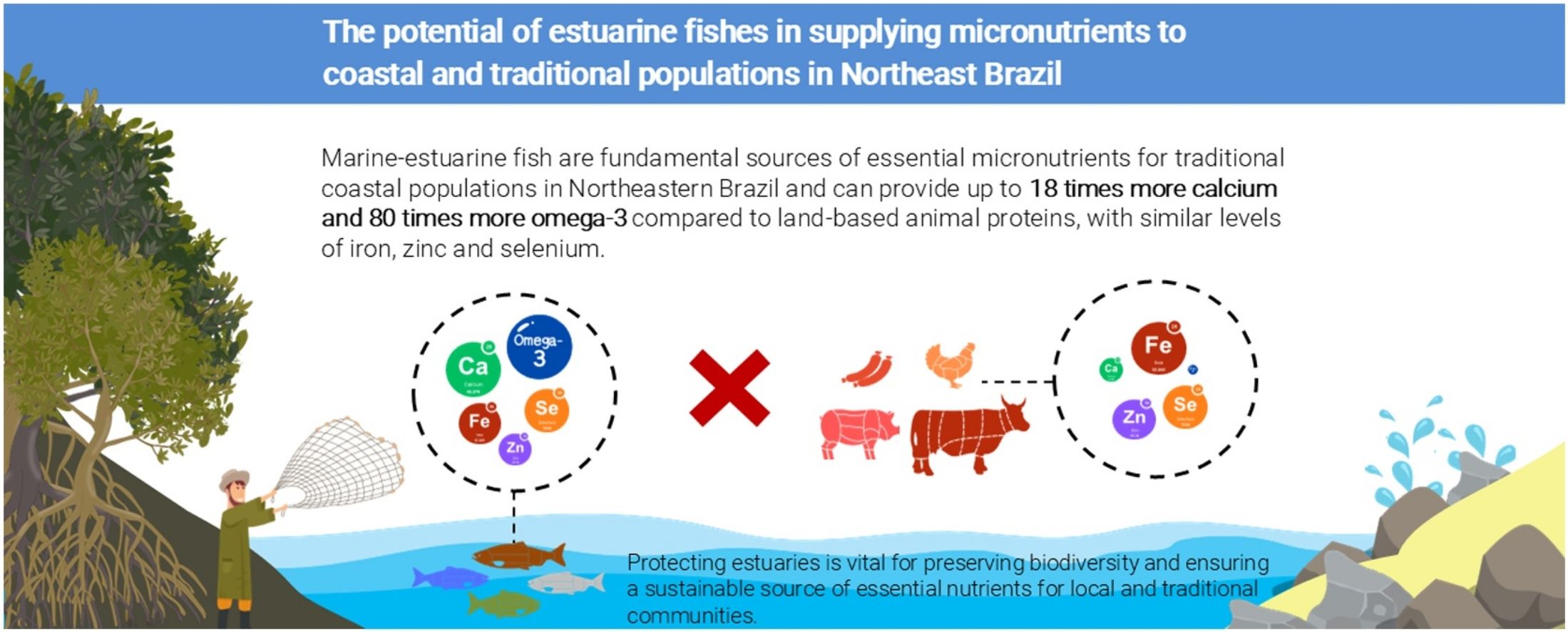
Tropical estuaries are among the most productive ecosystems on Earth, offering multiple ecological and socio-economic benefits for coastal communities. Although previous studies have investigated the value of estuarine resources as human food in Brazil, the potential of fish in providing essential nutrients is still a knowledge gap. Here we investigate the potential nutritional contributions of estuarine fisheries to the diet and food security of local communities. We combined the micronutrient content (calcium, iron, selenium, zinc, and omega-3 fatty acids) of 17 finfish species reported in scientific literature (1994–2025) as caught in Northeastern Brazil estuaries and compared their nutritional content to that of land-based animal proteins (beef, pork, chicken, and ultra-processed meat). We found that, despite species-specific variations in nutrient content, fish have high levels of essential micronutrients. Notably, fish have higher levels of calcium and omega-3 fatty acids, and similar levels of iron, zinc, and selenium compared to land-based animal proteins. These results reinforce the importance of fish in providing not just protein, but also indispensable micronutrients for coastal communities. Considering current threats to estuaries and the nutritional benefits of fish, efforts to protect and restore estuarine habitats are crucial to maintaining this valuable resource.
Estuaries are known for their importance to carbon storage, coastal protection and nurseries for marine biodiversity (Barbier et al., 2011). Despite their ecological and socio-economic contributions, estuaries are among the most threatened ecosystems globally. Urbanization, unsustainable aquaculture practices, and climate change have accelerated habitat degradation and biodiversity decline in these ecosystems (Kennish, 2022). Additionally, the overexploitation of aquatic species in estuaries can lead to depletion of fisheries stocks and impact food security and human well-being (Whitfield and Cowley, 2010; Ickowitz et al., 2023). This situation is particularly concerning in developing countries, where traditional communities often depend heavily on estuarine resources for their subsistence, while simultaneously exerting much of the exploitation pressure on aquatic species (Ickowitz et al., 2023; Reis-Filho et al., 2019).
In tropical regions, estuaries are predominantly composed of mangroves, highly productive and vital ecosystems for small-scale fisheries and gleaning of crustaceans and shellfish (Carrasquilla-Henao and Juanes, 2017; Middleton et al., 2024). Brazil harbors the second-largest global mangrove extension, stretching from the state of Amapá (∼5 °N) to Santa Catarina state (27 °S), covering an area of approximately 9900 km² (Diniz et al., 2019). Despite being legally protected as permanent preservation areas (Brasil, 2012) and included in national scale conservation policies (Ministry of Environment and Climate Change of Brazil (MMA), 2019) and the Presidential Decree No. 12,045 of June 5 2024, creating the ProManguezal Program, Brasil (2024), Brazilian mangroves and associated estuaries continue to face significant threats derived from urbanization and aquaculture (Ferreira and Lacerda, 2016). These threats compromise estuarine systems and jeopardize the livelihoods of local communities that depend on aquatic resources like fish, crustaceans, and mollusks for food and income (Santos et al., 2017; Owuor et al., 2024). While the role of estuaries in supporting local food systems has gained increasing attention (Analuddin et al., 2019; Ickowitz et al., 2023), the specific contribution of estuarine fishes to nutritional security remains poorly explored (Middleton et al., 2025), especially along the Brazilian coast (Awuku-Sowah et al., 2022). Advancing our understanding of the nutritional value of estuarine fishes to human diet can enhance the recognition of estuaries as essential for food security of tropical coastal regions.
In 2022, over 33 million people in Brazil (∼15.5% of the national population) were at risk of severe food insecurity (VIGISAN, 2022), underscoring the need to assess the value of biodiversity to human diets (Gomes et al., 2023). The country’s Northeast region is ranked second in hunger, with 21% of the population experiencing severe food insecurity, a situation even more concerning for groups like the Quilombolas (Afro-Brazilian traditional communities formed by descendants of enslaved Africans), Black, and Indigenous populations (VIGISAN, 2022). The Northeast region also exhibits some of the highest rates of mangrove deforestation in the country (Ferreira and Lacerda, 2016). In this troubling context, aquatic foods may be the only viable option for mitigating food insecurity in several coastal communities, given their high-quality protein, vitamins and minerals content (Bernhardt and O’Connor, 2021). Among “bluefoods" (food of aquatic source), fish constitute the primary catches of small-scale and traditional fisheries across Brazilian Northeastern estuaries, serving as a major resource for peoples' sustenance (Santos et al., 2017). Fish contribute to the local economy, self-sufficiency, and sustainability, and are integral to the food culture of these communities (Reis-Filho et al., 2019; Gamarra et al., 2023). Nutritionally, fishes are rich in essential micronutrients such as calcium, iron, and zinc—nutrients that are critical for human development (Hicks et al., 2019). Thus, ensuring that coastal populations continue to benefit from the resources offered by estuaries could be critical for their survival, particularly due to the widespread inadequate intake of micronutrients worldwide (Passarelli et al., 2024).
We investigated the potential of fishes caught by estuarine small-scale fisheries to contribute to the nutritional security of coastal communities in Northeast Brazil. We contrasted the calcium, iron, zinc, selenium, and omega-3 content of 17 finfish species targeted by fisheries in the region’s estuaries to those found in other animal protein sources (beef, chicken, pork, and ultra-processed meat). As observed in other model systems (e.g. coral reefs and the Amazon forest), fish can have higher micronutrients concentration than land-based animal foods, especially omega-3 and calcium (Robinson et al., 2022; Heilpern et al., 2021). We hypothesized that estuarine fish would have higher levels of calcium and omega-3, and similar levels of iron, zinc, and selenium compared to other animal protein sources and therefore a greater potential for promoting nutritional security of coastal populations. We aim to highlight the importance of aquatic resources for the diet and health of human populations that derive part of their food from estuaries.
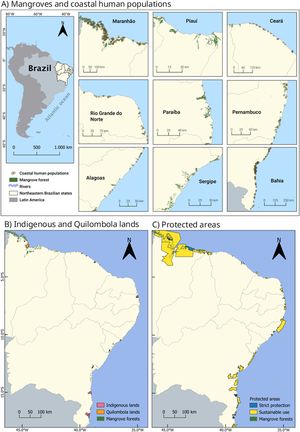
MethodsStudy ecosystemMangroves and estuaries stretch along Northeast Brazil, from the northern part of Maranhão (∼1 °S) to the south of Bahia state (∼18 °S), covering approximately 690 km² of forested area (Lacerda et al., 2021; Fig. 1). These mangroves are mainly composed of three species: Rhizophora mangle Linnaeus, Laguncularia racemosa Gaertn, and Avicennia schaueriana (Silva et al., 2003), which form complex structures that support hundreds of aquatic species (Maciel et al., 2024). Estuarine systems in Northeast Brazil have been historically occupied by hundreds of communities, and today, thousands of people rely on estuarine resources for their livelihoods (Netto and Reis-Neto, 2023). From small fishing villages, Indigenous and Quilombola communities to large urban areas, all are linked to mangroves and estuaries, and somehow influence their dynamics and preservation. In the studied region, 14 Indigenous lands and 22 Quilombola communities have mangroves within their boundaries (Fig. 1B). The mangroves of Northeastern Brazil are bounded within 46 protected areas, 8 of which are no-take areas (IUCN Categories I and II) and 38 are of sustainable use (IUCN Categories IVVI; Fig. 1C). Given the socio-ecological relevance and growing threats to estuaries in Northeast Brazil (Ferreira and Lacerda, 2016; Santos et al., 2017), we focused our research effort on this region as a case study to assess the potential nutritional benefits of estuarine finfish for coastal and traditional human populations.
Map showing mangroves and estuarine human populations along Northeast Brazil (Maranhão, Piauí, Ceará, Rio Grande do Norte, Paraíba, Pernambuco, Alagoas, Sergipe and Bahia states). A) Mangroves, major rivers, and human coastal populations living near estuaries in the region. B) Indigenous and quilombola lands that include mangroves within their boundaries. C) Protected areas that encompass mangroves, strictly protected and of sustainable use. Table S1 provides detailed information about these protected areas.
To obtain information on estuarine fish species that are important fishing targets for coastal and estuarine communities, we conducted a systematic review following the PRISMA protocol for systematic literature review procedures (Liberati et al., 2009). We searched the Web of Science in February 2025 for articles published between 1994 and 2025 in English, Portuguese and Spanish using the keywords: estuaries OR estuarine OR estuary OR magrove OR mangroves AND fisheries OR fishing OR fisheries OR “artisanal fisheries” OR “small-scale fisheries” AND Brazil OR Brasil. Our search produced 1161 titles, but we only selected articles that specifically studied the occurrence of fish and its relevance as fishing targets in Northeast Brazil. A total of 95 studies fit our inclusion criteria, with 50% published between 2012 and 2021 (Fig. S1). Then, we selected finfish that were reported in at least one-third of the studies (∼31 publications), resulting in a subset of 17 species. We selected species that appeared in at least a third of the studies to ensure that the analysis is based on widely reported species, reducing the influence of sporadic records and the bias caused by uneven sampling among studies. All nine states in the Northeast region have published studies on estuarine fish diversity, with the following distribution: Alagoas (13), Bahia (15), Ceará (6), Maranhão (6), Paraíba (24), Pernambuco (35), Piauí (2), Rio Grande do Norte (6), and Sergipe (7; Fig. S2).
Because there was no information on the nutritional composition of the 17 fish species in Brazil's main food composition database (Tabela Brasileira de Composição de Alimentos – TACO; https://nepa.unicamp.br/publicacoes/), we compiled data on calcium, iron, zinc, selenium, and omega-3 content available from FishBase (Froese and Pauly, 2024; https://www.fishbase.se/Nutrients/NutrientSearch.php) to assess their nutritional content compared to beef, chicken, pork, and ultra-processed meat. Nutrient concentration values (median and 95% CI) are not based on direct analytical measurements for the focal species, but are predictions derived from hierarchical Bayesian models (Hicks et al., 2019). These models integrate nutrient empirical data of species with life history traits and phylogenetic relationships, thus allowing estimates for species that do not have direct composition analyses. Our focus on fish as a food resource from estuaries stems from the higher precision in identifying captured species compared to crustaceans and bivalves, which are generally grouped into broader taxonomic levels. Additionally, nutritional information on invertebrates is more limited, especially for estuarine species, which could affect the interpretation of results. We obtained nutrient data on beef, chicken, pork, and ultra-processed meat (sausage) from the United States Department of Agriculture/Agricultural Research Service (USDA/ARS) (2020). Nutrient concentrations in livestock were measured for different edible parts. All nutrient concentrations are provided per 100 g of raw edible portion, with selenium measured in micrograms, calcium, iron, and zinc in milligrams, and omega-3 in grams.
Data analysisTo assess differences in nutritional diversity between fish species and livestock, we performed a principal component analysis (PCA) based on the five nutrients. Prior to the analysis, all nutrient values were standardized (z-transformed), which is appropriate for data with different measurement scales. We used the first two axes of the PCA to visualize and interpret the contribution of individual nutrients to the overall nutritional variation among food sources. Sausage was excluded from the analysis due to the lack of data on selenium.
To compare the micronutrient concentrations (response variable) between different animal-based foods (explanatory variable), we used a permutation-based analysis of variance (permutation ANOVA, 5000 iterations), which does not require assumptions of normality or homogeneity of variances. For the estuarine fish species, only the median nutrient values were used for comparisons, and for livestock we used the median nutrient concentrations in the different edible parts. We then applied a post-hoc permutational test to investigate pairwise differences. Sausage was not considered in pairwise comparisons for selenium due to missing data for this mineral in this food type. A p-value threshold of < 0.05 was considered significant for determining differences between groups.
We also assessed the contribution of a standard 100 g portion of each food type to the daily recommended nutrient intake (RNI) of children under five years of age. This age group was chosen because ensuring adequate nutrition in early childhood is critical for growth and development, and improvements at this stage can produce long-term benefits across the life course (Bogard et al., 2015; Xu et al., 2025). For each animal-based food, we used average nutrient concentrations. RNI reference values were obtained from the Institute of Medicine (2006): 600 mg calcium, 4.7 mg iron, 20 μg selenium, 4.8 mg zinc, and 0.8 g omega-3 fatty acids. We calculated the percentage of RNI provided by dividing the nutrient content into a 100 g food portion by the corresponding reference value and multiplying by 100. Each nutrient’s contribution was capped at 100% to reflect daily requirements, while the combined contribution across all nutrients could reach up to 500% if a food fully met the RNI for every nutrient considered. All analyses were performed using the R software (R Core Team, 2025), with the packages ‘FactoMineR’ (Lê et al., 2008), ‘factoextra’ (Kassambara and Mundt, 2020), ‘lmPerm’ (Wheeler, 2016), and ‘rcompanion’ (Mangiafico, 2024).
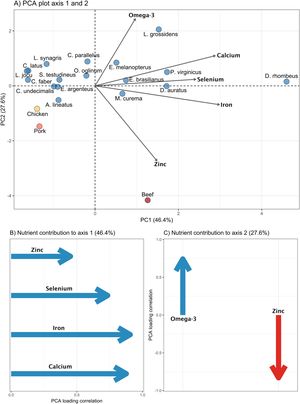
ResultsMarine-estuarine fish exhibit a wide range of nutritional variability compared to livestock (Fig. 2A). The first two PCA axes combined explain 74% of the variation in species' nutritional content. The first axis (PC1) is strongly influenced by zinc, selenium, iron, and calcium, while the second axis (PC2) is mainly driven by zinc and omega-3 (Fig. 2B, C; Table 1). Fish species such as Diapterus rhombeus, Lycengraulis grossidens, Polydactylus virginicus, Lutjanus jocu, Diapterus auratus, and Caranx latus, are associated with nutrient content along the first axis, which shows high concentrations of calcium and selenium. Meanwhile, species such as Lycengraulis grossidens, Centropomus parallelus, and Eucinostomus melanopterus are more closely associated with the second axis due to the high influence of omega-3, along with pork and beef linked to high zinc concentrations. Additionally, when comparing different nutrients across animal-based foods, we found that fish have higher concentrations of calcium and omega-3 (P < 0.001) but similar levels of iron, zinc, and selenium to those of livestock (Fig. 3, Table S3). The average calcium concentration in fish was 78.55 mg (CI ±35.56 mg), about three times higher than sausage and nearly 18 times higher than beef, chicken, and pork (Fig. 3A). For omega-3, fish had an average content of 0.24 g (CI ±0.07 g), 80 times higher than other food sources (Fig. 3B).
Principal component analysis (PCA) of the nutritional content of calcium, omega-3, selenium, iron, and zinc on food sources. A) Nutritional variation across 17 finfish species (blue dots: Caranx latus, Centropomus undecimalis, Mugil curema, Eucinostomus argenteus, Diapterus rhombeus, Lutjanus jocu, Opisthonema oglinum, Sphoeroides testudineus, Eugerres brasilianus, Lutjanus synagris, Achirus lineatus, Chaetodipterus faber, Centropomus parallelus, Citharichthys spilopterus, Atherinella brasiliensis, Eucinostomus melanopterus, Diapterus auratus, Lycengraulis grossidens, Polydactylus virginicus), beef (red dot), pork (orange dot), and chicken (yellow dot). B) Contribution of nutrients to axis 1 and C) to axis 2; blue arrows indicate positive correlations and red arrows negative correlations. Sausage was excluded from this analysis due to a lack of data on selenium content.
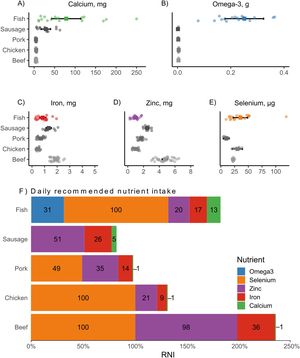
Micronutrient concentrations in different animal-based foods. A) calcium (fish = 17, sausage = 13, pork = 16, chicken = 16, beef = 32), B) omega-3 (fish = 17, sausage = 6, pork = 5, chicken = 8, beef = 6), C) iron (fish = 17, sausage = 13, pork = 16, chicken = 16, beef = 32), D) zinc (fish = 17, sausage = 13, pork = 16, chicken = 16, beef = 32), E) selenium (fish = 17, pork = 5, chicken = 8, beef = 6). Each point represents a micronutrient sample, the squares represent average values for each group, the lines indicate the 95% confidence interval, and the letters show differences between groups. All micronutrient concentrations are per 100 g of raw edible portion, and their units are indicated in the panel titles. Sausage was not evaluated for selenium due to a lack of data for this mineral. F) Percent contribution of a standard 100 g portion of animal-based foods to the daily recommended nutrient intake (RNI) for children under five years of age. Each point represents a micronutrient sample, the squares represent average values for each group, the lines indicate the 95% confidence interval, and the letters show differences between groups. All micronutrient concentrations are per 100 g of raw edible portion, and their units are indicated in the panel titles. Values inside the bars indicate the percentage of the RNI supplied by each food for a specific nutrient, capped at a maximum of 100%. Sausage was not evaluated for selenium due to a lack of data for this mineral.
Regarding iron, fish had an average concentration of 0.86 mg (CI ±0.24 mg), 1.8 times higher than chicken and similar to pork, but lower than sausage and beef (Fig. 3C). The average zinc concentration in fish was 0.98 mg (CI ±0.23 mg), similar to chicken and lower than other foods (Fig. 3D). In terms of selenium, fish had an average concentration of 37.36 μg (CI ± 14 μg), about four times higher compared to pork, but similar to levels found in chicken and beef (Fig. 3E; Table S3). Considering these average values, a 100 g serving of fish may supply about 31% of the recommended daily intake of omega-3, 100% of selenium, 20% of zinc, 17% of iron, and 13% of calcium. In contrast, sausage may contribute mainly to zinc (51%) and iron (26%), while pork could provide selenium (49%), zinc (35%), and iron (14%). Chicken appears particularly relevant for the intake of selenium (100%) and zinc (21%), whereas beef may supply substantial amounts of selenium (100%), zinc (98%), and iron (36%) (Fig. 3F).
DiscussionWe evaluated how fish caught in estuaries have the potential to provide essential micronutrients and therefore contribute to the nutritional security of coastal communities in Northeastern Brazil. We observed that estuarine fish species largely vary in their nutritional content while offering higher amounts of calcium and omega-3, as well as similar concentrations of iron, zinc, and selenium to that associated with other livestock-based foods. This indicates that estuarine fish can be considered essential nutrient sources for maintaining the diet and health of human populations that rely on estuaries resources.
The high bioavailability of calcium and omega-3 in fish is related to their metabolism and diet (Tocher, 2003; Lall and Kaushik, 2021). Calcium is essential for fish, playing a key role in skeletal development and osmoregulation. Fish absorb calcium directly from their environment through the gills, gastrointestinal tract, and skin (Lall and Kaushik, 2021). While most calcium is stored in the skeleton, it is also present in muscles, scales, and the intestines, where it can be deposited as calcium carbonate (Takvam et al., 2021). In marine-influenced estuarine environments, high calcium concentrations in soil and water contribute to elevated calcium levels in resident fish. As a result, marine-estuarine species, particularly smaller ones that are often consumed whole, may serve as excellent dietary calcium sources for coastal communities (Islam et al., 2023). Omega-3 is incorporated into fish mainly through the consumption of organisms such as microalgae and small crustaceans, accumulating along the food chain (Deckelbaum and Torrejon, 2012). Fish can convert alpha-linolenic acid (ALA, 18:3n-3), commonly found at lower trophic levels, into eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), the two main types of fatty acids recommended for human consumption by health agencies (Tocher, 2003; Arbex et al., 2015). The high concentrations of omega-3 found in estuarine fishes highlight their fundamental role as nutrient sources to human populations living near estuaries.
Recognizing the importance of local, nutrient-rich foods in the diet is critical in combating malnutrition, particularly in underdeveloped countries with a significant part of the population under food insecurity (Ahern et al., 2021; Simmance et al., 2022). Although our study does not account for the seasonality of fishing yields or the temporal variability in species composition—factors that can influence consumption patterns among human populations (Middleton et al., 2025)—it provides an important initial step toward understanding the potential contribution of estuarine fisheries for food security of coastal communities in Northeast Brazil. Moreover, we recognize that, although we used 17 fish species widely reported in scientific literature, our results may be missing regional and local variations in food intake for the various traditional communities located on the Northeast coast. Despite these limitations, our assessment is especially relevant for low-income groups, including Indigenous, Quilombola, and Afro-Brazilian communities, who often rely on biodiverse, locally sourced foods in the Northeast region (Gomes et al., 2023). For these groups, estuarine fish may offer an accessible and, in some cases, low-cost options that provide a nutritionally balanced diet (Islam et al., 2023; Viana et al., 2023). Thus, considering the nutritional heterogeneity among fish species, it is crucial to ensure the accessibility of these coastal populations to diverse fish resources provided by estuaries.
Nutrient concentrations in wild-caught fish vary with trophic levels, diet, and environmental variability (Hicks et al., 2019), which can lead to larger inter- and intraspecific variation compared to farmed animals with controlled diets. Such ecological complexities help explain the broader nutrient profiles observed among estuarine fish species. Because of their high amounts of calcium, iron, and selenium, fish can contribute to the recommended nutrient intake in Northeast Brazil and help mitigate malnutrition-related childhood stunting and anemia (de Oliveira et al., 2021). Species such as Mugil curema, Eucinostomus argenteus, Eucinostomus melanopterus, Eugerres brasilianusm, Diapterus rhombeus and Diapterus auratus—which are commonly abundant or frequently caught in estuaries (Silva-Júnior et al., 2017; Araújo et al., 2018)—are particularly important, as they provide substantial amounts of calcium, iron, and zinc. These nutrients are often consumed at inadequate levels, with deficiency rates exceeding 25% across different age groups in the region (André et al., 2018; Verly et al., 2021). Also, with the global increase in the consumption of ultra-processed foods and the resulting weight gain in the population (Popkin and Ng, 2022), which contributes to higher cholesterol levels and heart diseases (Juul et al., 2021), fish can serve as valuable resources in helping to reduce these health problems (Arbex et al., 2015). Although ultra-processed foods can be fortified with nutrients (Capozzi et al., 2021), they generally contain high levels of saturated fat and sodium, which are associated with cardiovascular diseases (Khalili Tilami and Sampels, 2018). On the other hand, the high concentrations of omega-3 in fish can help reduce the accumulation of low-density lipoproteins (LDL cholesterol), which are harmful to human health, thereby lowering the risk of heart diseases (Deckelbaum and Torrejon, 2012; Khalili Tilami and Sampels, 2018). Given that 21% of the population in the Northeast facing severe food insecurity represents at least 11.4 million people (IBGE – INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA, 2022), many of whom live in coastal areas, estuarine fish have the potential to make important contributions to alleviating nutritional deficiencies in vulnerable communities.
Equally important as recognizing resources that provide food and nutrition to local communities is conserving the habitats that allow species to persist in the ecosystem. In Brazil, it is estimated that between 3.6% and 5.2% of mangroves have been degraded over the last three decades (Ferreira and Lacerda, 2016). Despite the low deforestation rate compared to other countries, such as Indonesia, which has lost around 30% of its mangroves since 1980 (Quevedo et al., 2023), the expansion of urban areas and the establishment of aquaculture farms have caused serious damage to Brazilian estuaries (Ferreira and Lacerda, 2016; Bernardino et al., 2021). Mangrove degradation directly affects the feeding and shelter areas of many fish species (Hutchison et al., 2014; Vila-Nova et al., 2011), therefore impacting fish stocks (Malik et al., 2017). In Northeastern Brazil, shrimp farming has been the primary driver of mangrove deforestation, resulting in a loss of over 10% of mangrove area in the past two decades (Ferreira and Lacerda, 2016; Lacerda et al., 2021). Beyond vegetation removal, mangrove conversion into shrimp farms exacerbates soil erosion and increases effluent release into surrounding ecosystems (Lacerda et al., 2021). Such discharge significantly alters environmental conditions and ecosystem functions, often leading to estuarine eutrophication, bacterial contamination, and the mortality of various organisms (Sousa et al., 2006; Barcellos et al., 2019). These impacts may also compromise the availability of estuarine-derived food to local populations, often forcing dietary changes that result in increased consumption of less nutritional foods (Garcia et al., 2020). Thus, conserving estuaries goes beyond climate maintenance and coastal protection, the food resources provided by these ecosystems are also essential for the nutritional security of coastal communities and the preservation of local cultures.
ConclusionsEstuarine fishes can provide high micronutrient quantities to human coastal populations in Northeast Brazil. In a future where population growth and climate change are likely to increase inequality and hamper the access of marginalized populations to quality food (Wheeler and Von Braun, 2013), mangroves-estuarine systems may be critical to the survival of local communities. Mangrove conservation will not only maintain biodiversity but also sustain a valuable nutrient source for vulnerable communities. Additionally, conserving mangroves will support the livelihoods and traditions of local communities, ensuring food and cultural sovereignty for traditional populations on the Northeast coast of Brazil and potentially in other areas with similar configurations worldwide. Ultimately, this connection between the health of estuarine ecosystems and human well-being underscores the need for conservation policies and sustainable fishing practices to ensure long-term benefits for both the environment and local communities.
CRediT authorship contribution statementFabricio C. Albuquerque: Conceptualization; Data curation; Formal analysis
Investigation; Methodology; Validation; Visualization; Writing original draft; Writing - review & editing; Mariana G. Bender: Conceptualization; Funding acquisition; Investigation; Project administration; Resources; Validation; Writing - review & editing; Guilherme O. Longo: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing - review & editing.
Declaration of Generative AI and AI-assisted technologies in the writing processThe authors declare that they do not use generative AI or AI-assisted technologies in the writing process of this manuscript.
Data availability statementAll data and codes used in this manuscript are available at: https://github.com/Fcl95/estuarine_fishes_nutrient.git.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by Serrapilheira Institute (Grant No. Serra-1708-15364, awarded to GOL), the National Council for Scientific and Technological Development (CNPQ) through the INTEGRA-Mar Research Network (Chamada PPBio/CNPq/MCTI/FNDCT Nº 07/2023; Grant #441226/2023-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)– Code 001. GOL is also grateful for a research productivity scholarship provided by CNPq (308072/2022-7).