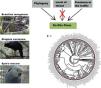
Species have been lost at unprecedented rates. Because only a small fraction of the threatened taxa have been managed under human care, contrasting the characteristics of taxa that have, and have not been targeted to ex situ conservation can reveal the reach of this conservation strategy, and can indicate its main challenges. Here we investigated whether the level of threat, diet, body mass, phylogeny, and previous presence in captivity due to non-conservation purposes could be potential parameters accounting for the occurrence of Brazilian threatened avian species and subspecies in ex situ conservation facilities and for their eligibility to organized ex situ conservation plans. Using Bayesian phylogenetic comparative models we found positive effects of body mass and phylogeny, and a negative effect of insectivorous diet in the occurrence of the taxa in non-conservation facilities. The previous presence in non-conservation facilities, together with phylogeny, diet, and body mass were the main parameters accounting for the occurrence of the threatened taxa in ex situ conservation facilities, and the previous presence in non-conservation facilities and phylogeny explained the existence of organized ex situ conservation plans. This is evidence that conservation breeding facilities have mostly harbored threatened confiscated birds than choosing them based on scientific criteria. We suggest that investing in the development of husbandry techniques, especially for insectivorous passerines, and choosing taxa based on scientific criteria are important challenges that should be on the agenda of conservation managers.
As a consequence of anthropogenic actions, species have been lost at rates maybe higher and faster than those recorded for the five big mass extinction episodes that occurred across the Earth’s geological history (Barnosky et al., 2011). More than 400 vertebrate species became extinct in the past 100 years and many more had their populations drastically reduced and are currently on the verge of extinction (Ceballos et al., 2020). Species loss is irreversible, brings consequences to ecosystem services and to the own human existence (Dirzo et al., 2014; Ceballos et al., 2020), and is morally unacceptable (Corlett, 2015). Because anthropogenic impacts on species and ecosystems were dramatic and are still increasing, and because there is little hope for the biodiversity erosion process to be reverted soon, conservation actions are recommended to impede at least part of the extinctions that will likely occur. For this reason, the Aichi Biodiversity Target 2.2 proposed by the parties of the Convention on Biological Diversity (CBD) during the conference of Nagoya in 2010, set the goal of avoiding extinctions and reverting the conservation status of the most critically endangered taxa until 2020, which for many species means integrating in situ and ex situ management (Bolam et al., 2020). CBD has defined ex situ conservation as the “conservation of components of the biological diversity outside their natural habitats”, and in Article 9, CBD describes the goals of ex situ conservation and emphasizes its importance as a complement to in situ strategies.
However, like all conservation approaches, ex situ management has important constraints, including the lack of space in institutions to develop programs for an unprecedented number of threatened taxa; financial limitations; the difficulties to deal with species presenting extreme body sizes; the lack of knowledge on husbandry techniques for certain organisms; adaptation to the environment under human care; diseases, and inbreeding depression (Soulé et al., 1986; Snyder et al., 1996; Pritchard et al., 2011; Conde et al., 2013). Because of these constraints, substantial attention has been given to the development of guidelines for determining when ex situ conservation should be used (IUCN/SSC, 2014; McGowan et al., 2017), which is believed to be a way to maximize the benefits obtained with the available investments.
Although the impossibilities for ex situ plan implementation for some animal groups are obvious (e.g. whales, dolphins, and marine turtles), many taxa with not-so-obvious limitations have become extinct in recent decades without ex situ conservation attempts (Lees et al., 2014). It poses a question of whether ex situ conservation has, in general, focused on taxa that are easier to be managed and acquired to the detriment of others that are more endangered, but for which ex situ management would require greater efforts in terms of technological and human resources investments. Then, contrasting the characteristics of endangered animal species that have, and have not been targeted to ex situ conservation plans can be an important way to reveal the real reach of ex situ conservation, and can indicate the main technological challenges that ex situ conservation should tackle to achieve its main purpose which should be avoiding the extinction of the most critically endangered taxa.
Here we used the Brazilian avifauna to evaluate potential parameters accounting for the existence of ex situ breeding plans for certain threatened birds, and not for others. We considered that the Brazilian avifauna is an ideal study model for addressing the challenges of ex situ conservation because: (i) information on bird conservation status is relatively good compared, for instance, to invertebrates and other groups of vertebrates (Verdade et al., 2012); (ii) Brazil is the richest country on earth in the number of bird species, and at the same time it is the country with the greatest number of threatened avian taxa (BirdLife International, 2021; Pacheco et al., 2021), and (iii) some of the threatened taxa are known to be a target to ex situ breeding plans, while many others are not (Hammer and Watson, 2012; Oliveira-Jr et al., 2016; Francisco et al., 2021). First, we addressed whether levels of threat, diet, body size, and phylogeny could influence in the choice of the endangered taxa by illegal and legal bird keepers not involved in conservation actions. Then, we evaluated whether the above parameters, as well as the previous availability of individuals in captivity due to reasons not related to conservation could influence in the simple presence of the taxa in ex situ conservation facilities, and in the eligibility of organisms for the creation of organized ex situ conservation plans. We predicted that the confiscation of individuals from the traffic by Brazilian authorities is what has supplied most of the conservation facilities with animals, and that ex situ conservation managers have been reluctant to implement captive programs for taxa that have not been traditionally maintained in captivity for non-conservation purposes. Although our work has focused on the Brazilian avifauna, we raise issues that are of global interest.
Material and methodsStudied taxa and levels of threatIn this study, we addressed the birds included in the most recent Brazilian Red List (ICMBio, 2018). This list contains 234 described taxa distributed across the following threat categories: Critically Endangered (CR); Endangered (EN), and Vulnerable (VU). Three species that were extinct long ago (Numenius borealis, Anodorhynchus glaucus, and Sturnella defilippi, being the latter not globally extinct), and other three species that were recently recognized as Globally Extinct (EX) (Pereira et al., 2014) were not included in our analyses (Cichlocolaptes mazarbarnetti, Philydor novaesi, and Glaucidium mooreorum). The taxa Paraclaravis geoffroyi (CR/PEX; Critically Endangered/Probably Extinct; see Lees et al., 2021), Neomorphus geoffroyi geoffroyi (CR/PEX), Myrmotherula fluminensis (CR/PEX), Calyptura cristata (CR/PEX), and Cyanopsitta spixii (CR/PEW; Critically Endangered/Probably Extinct in the Wild) were considered only as CR.
Presence in non-conservation facilitiesWe predicted that the previous availability of captive individuals derived from illegal bird trade and from legal amateur or commercial bird breeding activities, all not related to conservation, could be a source of threatened taxa for ex situ conservation facilities, and consequently for organized ex situ conservation plans. Then, we carried out different lines of investigations to list the taxa that could be already present in captivity independently of conservation purposes. First, we conducted literature searches for articles, theses, dissertations, and technical documents publishing lists of birds that have been targeted for the illegal pet trade. These works are useful because they often derive from police records of animals confiscated from poachers and illegal bird dealers (e.g.Borges et al., 2006); lists of animals present in rehabilitation facilities, in Brazil known as CETAS (e.g.Pagano et al., 2009; Souza et al., 2014), or they can derive from bird surveys elaborated during researcher’s visits to illegal animal keepers and to illegal markets (e.g.Oliveira et al., 2020; Silva et al., 2021). To achieve this purpose we carried out searches in Google Scholar and in the indexing base Web of Science, using combinations of the keywords: Aves; Birds, CETAS, traffic, Illegal trade, Brazil, and South America. Second, we checked for many Brazilian governmental reports listing the taxa present in legal amateur and commercial aviaries (e.g.Tavares et al., 2013). Although these facilities are legal, their main objective is not conservation and founder populations originally derived from illegal trapping. Third, we checked the texts available for each taxon in the own Brazilian Red List of endangered birds, and in BirdLife International Data Zone to see if trapping was among the described causes of threat. On the web, we carried out an exhaustive search using the popular names of each taxon together with the words trapping, cage, aviary, and captivity, both in English and Portuguese, in an attempt to find images or videos of the studied taxa in captivity. Both legal bird keepers and illegal bird traders often post pictures and videos of the animals, frequently without the owner’s identification. Finally, for this search, we also counted on long-termed (30+ years) authors’ observations (LFS and MRF) during their visits to animal rehabilitation centers and other facilities, once they are often invited to identify the species after police confiscations.
Simple presence in ex situ conservation facilitiesWe analyzed how the addressed variables (level of threat, diet, body mass, phylogeny, and the previous presence in non-conservation facilities) could influence in the simple presence of the threatened Brazilian avian taxa in official ex situ conservation facilities, currently or in the past, independently of the number of individuals and of reproductive success. This is because organized national or international ex situ conservation programs, i.e. those associated with studbooks and/or Conservation Action Plans (see below), are too few for Brazilian birds, but a greater number of endangered taxa is known to have been managed under human care in ex situ conservation facilities (Silveira et al., 2008; Soares et al., 2008; Schunck et al., 2011). For ex situ conservation facilities, we considered the two types of institutions recognized by the Brazilian legislation for captive conservation management (Conservation and Scientific breeding facilities), as well as Zoos and Conservation institutions all over the world.
To identify the taxa from the Brazilian Red List that have been managed in ex situ conservation facilities, from Brazil and other countries, we carried out literature searches for articles and technical documents in Google Scholar and the indexing base Web of Science using each species popular and scientific names combined with the keywords: Studbook, Action Plan, Zoo, Ex Situ, and Captive Breeding, with AND for the species name and OR for the other words as Boolean operators up to August 2022. We also reviewed all of the Brazilian National Action Plans (PANs) for endangered species conservation, and the studbooks’ list from AZAB (Brazilian Zoos and Aquariums Association). Finally, we analyzed the global taxa inventory of ISIS (International Species Information System) zoos, as reported by Conde et al. (2011), as well as the zootierliste website (https://www.zootierliste.de/en/), which is a database on the current and past vertebrate inventories from EAZA (European Association of Zoos and Aquaria) zoos. Because not always ex situ conservation managers publish their experiences, for this survey we also considered the personal observations of the authors (MRF and LFS), obtained during their frequent visits to zoos and conservation breeding facilities from Brazil and abroad, and during their participation on multiple PANs since 2006.
Taxa under organized ex situ conservation plansFor the taxa considered as a target for organized ex situ conservation plans, we selected only those with national or international studbooks, and those for which the national or international Action Plans for species conservation have reported the existence of legal captive populations and/or have indicated the captive reproduction as one of the conservation goals (e.g., Silveira et al., 2008; Soares et al., 2008; Schunck et al., 2011), independently of the existence of successful reproduction.
Diet and body sizeFor diet classification, we used the species-level global compilation of Wilman et al. (2014). These authors proposed a semiquantitative approach based on the relative importance of the consumed items, with the final classification representing the most frequently used diet component. Here we used this dataset with modifications. Specifically, for the representatives of the families Cracidae, Odontophoridae, Psophiidae, Capitonidae, Ramphastidae, Psittacidae, Pipridae, Tityridae, Cotingidae, Icteridae, Thraupidae, and Cardinalidae, that were classified by Wilman et al. (2014) as “Fruit/Nectar”, we used only “Fruit”, because nectar consumption is rarely reported for these taxa (Sick, 1997). For the hummingbirds (family Trochilidae), classified by Wilman et al. (2014) as Fruit/Nectar, we used only “Nectar”. For the Anatidae (Mergus octosetaceus), Phaetontidae, Fragatidae, and Sternidae, classified by Wilman et al. (2014) in the category “VertFishScav” (vertebrates; fish, and carrion) we used only “VertFish” because all of the representatives present in the Brazilian Red List were piscivores. For the representatives of the families Accipitridae and Strigidae, also classified as “VertFishScav”, we used only “Vert”, as none of the listed species were fishing or scavenger birds. Diet information was not available for Coryphaspiza melanotis (Thraupidae), then we arbitrarily classified it as “Seed”, following the diet of other closely related Thraupidae. Omnivorous were those taxa for which the relevance of at least two different diet items was similar (e.g. fruit/seed, fruit/invertebrate, seed/invertebrate, or vertebrate/invertebrate) (see Wilman et al., 2014). Body size information was also obtained from the dataset of Wilman et al. (2014), and when not available in this reference, we used Cornell’s Lab Birds of the World (https://birdsoftheworld.org).
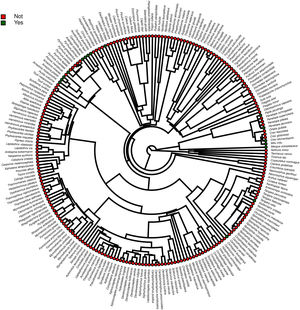
Modeling proceduresFirst, we aimed to investigate the parameters explaining the presence of threatened Brazilian bird taxa in facilities not involved in ex situ conservation: in illegal conditions; in amateur or commercial legal breeding facilities, and in animal recovery centers. To achieve this objective we used a generalized linear model with a binomial distribution, being the taxa present in at least one of these types of facilities coded as 1 and the absence in our searches coded as 0. For categorical explanatory variables, we used the level of threat in the Brazilian Red List (CR, EN, and VU) and diet (Fruit; Invertebrate; Nectar; Omnivore; Seed; Vertebrate, and Vertebrate/Fish), and as a continuous explanatory variable, we used log-transformed body mass. To address the potential effect of phylogeny on the presence/absence of certain taxa in these facilities, we carried out our analysis using a Markov chain Monte Carlo (MCMC) Bayesian Phylogenetic Mixed Model (BPMM) procedure, as available in MCMCglmm v2.20 R package (Hadfield, 2010). In this analysis, a phylogeny was included as a random effect term because it could reveal groups that are preferable because of characteristics that have not been pre-defined in the above parameters set, such as color patterns or song complexity. We obtained the phylogenetic information from the Mega Tree of birdtree.org (Jetz et al., 2012). Because BirdTree does not provide data for subspecies, and phylogenetic information cannot be duplicated between subspecies due to branches collapsing, when more than one subspecies occurred for a listed species we maintained only one in the analysis, which occurred for 10 species of the Brazilian Red List. For five of these taxa (Thamnophilus caerulescens, Phlegopsis nigromaculata, Gralaria varia, Sclerurus caudacutus, and Lepidothrix iris) the data available for all of the subspecies were the same (body mass, diet, level of threat), and none of them were recorded in captivity, then we chose one subspecies randomly to represent the taxon. In three cases (Phaetornis margarettae, Celeus torquatus, Iodopleura pipra) the subspecies differed only in the levels of threat, then we maintained the subspecies with the most critical threat level. For Crypturellus noctivagus, the two subspecies differed in that C. n. noctivagus was recorded in both non-conservation and in ex situ conservation facilities, while C. n. zabele was recorded only in ex situ conservation facilities (Table S1), then, we maintained C. n. noctivagus. For Neomorphus geoffroyi, we maintained N. g. dulcis because it is the only one with a confirmed record in captivity. For the modeling, we obtained a sample of 2500 trees from the Mega Tree and we used them to generate a consensus phylogeny (maximum credibility tree) with Phangorn R-Package (Schliep, 2011). Then, we carried out the modeling using the presence/absence in captivity as the response variable (1 and 0), and the consensus phylogeny as a random factor. We programmed MCMC to 1,000,000 iterations, with a burn-in of 10,000 and a thinning interval of 1000 iterations, resulting in a posterior distribution of 1000 samples. To facilitate model convergence, we used the inverse Wishart-prior (V = 1, v = 0.02) (see also Sayol et al., 2020). We checked for model convergence using the function gelman.diag of the R package Coda (Plummer et al., 2006), adopting as a threshold a scaling reduction factor (Rc) below 1.1 (Gelman and Rubin, 1992).
We used the same statistical procedures to address the simple presence of the threatened taxa in ex situ conservation facilities, as well as in organized ex situ breeding plans, but we included “presence in non-conservation facilities” as a further categorical explanatory variable (yes/not). To address whether phylogeny could influence in the presence of certain threatened taxa in captivity, for each of the above models we calculated the posterior probability (posterior mean) and the 95% credible interval of the phylogenetic signal (lambda) (Hadfield, 2010). We constructed Phylogeny images using the contMap function of the R-package Phytools (Revell, 2012).
ResultsOf the 234 threatened taxa present in the Brazilian Red List, we confirmed the presence of 59 of them (25.2%) in facilities not related to conservation (Table S1). The MCMCglmm modeling revealed a significant positive effect of body mass, and a significant negative effect of insectivorous diet accounting for the presence of animals in these types of facilities (Table 1). Among the threatened taxa, we recorded 64 (26.9%) in ex situ conservation facilities, and 40 of these 64 taxa (62.5%) were also present in non-conservation facilities (Table S1). The MCMCglmm modeling indicated positive effects of the previous presence in captivity for non-conservation reasons, body size, and a vertebrate diet to account for their simple presence in conservation breeding facilities, while the insectivorous diet was again negatively correlated (Table 1). Only 16 (6.8%) of the threatened Brazilian bird taxa had organized ex situ conservation plans, and of these taxa, 15 (93.7%) were also recorded in captivity for non-conservation purposes, being the Brazilian Merganser (Mergus octosetaceus) the only exception (Table S1). The presence in facilities not related to conservation was the only parameter significantly associated with the creation of organized ex situ conservation plans (Table 1). The Rc values were below 1.1 in all of the models, indicating that they have converged.
Posterior means (Post. mean), lower (l) and upper (u) 95% credible intervals (CI), and posterior probabilities (pMCMC) of predictive variables considered in the Bayesian Phylogenetic Mixed Models used to account for the presence of Brazilian endangered bird taxa in captive facilities not related to conservation (Presence in non-conservation facilities); in official ex situ conservation facilities (Presence in ex situ facilities), and in organized ex situ conservation plans (Existence of organized ex situ plans). Variables were diet, body mass, level of threat, and for the two last models also previous presence in non-conservation facilities (Non-conservation facilities).
| Post. mean | l CI | u CI | pMCMC | |
|---|---|---|---|---|
| Presence in non-conservation facilities | ||||
| (Intercept) | −3.09 | −5.89 | −0.24 | 0.030 |
| Diet_invertebrate | −1.41 | −2.68 | −0.22 | 0.012 |
| Diet_nectar | −1.61 | −5.54 | 2.18 | 0.432 |
| Diet_omnivore | −1.07 | −2.55 | 0.13 | 0.094 |
| Diet_seed | 0.68 | −1.01 | 2.15 | 0.398 |
| Diet_vert | −1.80 | −4.62 | 0.59 | 0.198 |
| Diet_vertfish | −1.42 | −3.57 | 1.02 | 0.216 |
| Log (body mass) | 0.52 | 0.15 | 0.91 | 0.008 |
| Level of threat (EN) | 0.23 | −0.84 | 1.22 | 0.626 |
| Level of threat (VU) | 0.47 | −0.44 | 1.36 | 0.298 |
| Presence in ex situ facilities | ||||
| (Intercept) | −2.22 | −4.67 | 0.09 | 0.058 |
| Non-conservation facilities | 1.05 | 0.26 | 1.90 | 0.004 |
| Diet_invertebrate | −1.19 | −2.33 | −0.11 | 0.048 |
| Diet_nectar | −1.03 | −4.59 | 2.78 | 0.562 |
| Diet_omnivore | 0.29 | −0.92 | 1.42 | 0.624 |
| Diet_seed | 0.45 | −0.76 | 1.66 | 0.448 |
| Diet_vert | 3.01 | 0.04 | 5.89 | 0.040 |
| Diet_vertfish | −0.31 | −2.08 | 1.63 | 0.728 |
| Log (body mass) | 0.44 | 0.11 | 0.79 | 0.002 |
| Level of threat (EN) | −0.48 | −1.44 | 0.42 | 0.296 |
| Level of threat (VU) | −0.04 | −0.95 | 0.73 | 0.930 |
| Existence of organized ex situ plans | ||||
| (Intercept) | −3.11 | −5.51 | −1.07 | 0.014 |
| Non-conservation facilities | 1.66 | 0.68 | 2.71 | 0.004 |
| Diet_invertebrate | −0.78 | −2.23 | 0.58 | 0.296 |
| Diet_nectar | 0.10 | −3.36 | 3.11 | 0.954 |
| Diet_omnivore | 0.28 | −0.95 | 1.34 | 0.664 |
| Diet_seed | −0.54 | −1.82 | 0.49 | 0.374 |
| Diet_vert | 0.08 | −2.18 | 2.51 | 0.966 |
| Diet_vertfish | 0.46 | −1.57 | 2.56 | 0.634 |
| Log (body mass) | 0.18 | −0.13 | 0.51 | 0.258 |
| Level of threat (EN) | −0.79 | −1.79 | 0.33 | 0.160 |
| Level of threat (VU) | −0.68 | −1.67 | 0.28 | 0.180 |
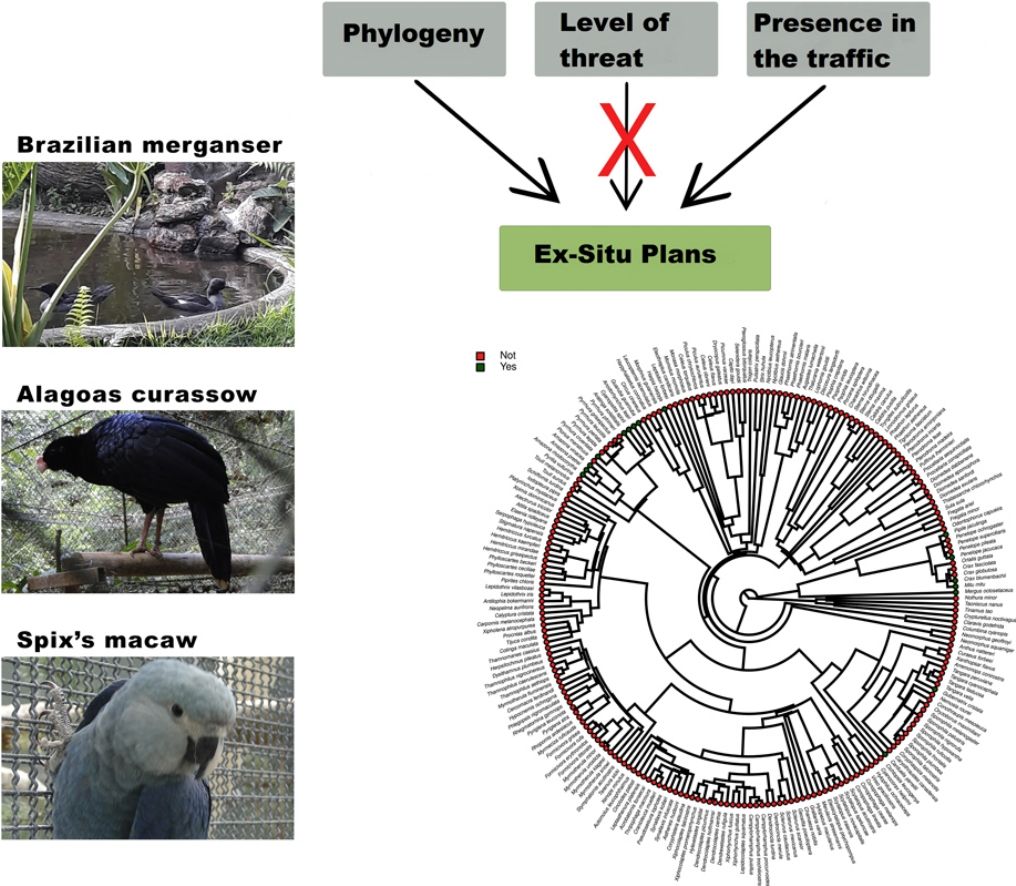
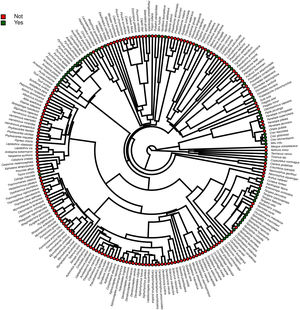
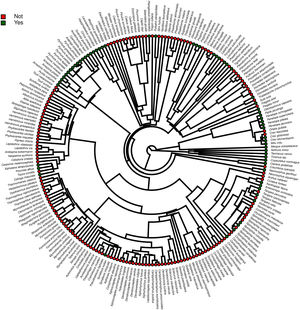
Furthermore, phylogenetic signals were high and 95% credibility intervals never overlapped zero in the three models, indicating their significances: 0.75 (0.57–0.88) for the model accounting for presence in facilities not related to conservation (Fig. 1); 0.61 (0.29–0.85) for the model accounting for the simple presence in ex situ conservation facilities (Fig. 2), and 0.43 (0.003–0.69) for the model accounting for the existence of organized ex situ breeding plans (Fig. 3). In Fig. S1 we presented the numbers of threatened taxa in Brazilian Red List; the numbers of taxa present in non-conservation facilities; the numbers of taxa present in ex situ conservation facilities, and the numbers of taxa with organized ex situ conservation plans for each family, and in Fig. S2 we provided an overview of the above groups of taxa based on their diets.
Our main finding was that the previous presence in captivity due to non-conservation purposes was an important parameter accounting for the eligibility of endangered Brazilian avian taxa for the creation of organized ex situ conservation plans. Because body mass, diet, and phylogeny also influenced in the presence of the species and subspecies in legal and illegal non-conservation aviaries, and consequently in the simple presence of these animals in ex situ conservation facilities, these parameters also may have played an indirect rule in the eligibility of the taxa for the organized ex situ conservation plans. The positive body size effects to explain the presence of the taxa in non-conservation and in ex situ conservation facilities were likely influenced by some families such as Tinamidae, Cracidae, Accipitridae, and Psittacidae, which are relatively large animals that are targeted by the traffic and as depicted by our phylogeny, are the predominant families in conservation breeding facilities and in organized ex situ breeding plans. They are among the preferred groups of birds in zoo collections, in the detriment of other groups for which representatives are smaller, e.g. Sporophila seedeaters, hummingbirds, and small insectivorous birds, likely because they are more visible by visitors or because they are easier to manage. This result is consistent with the previous findings that conservation breeding programs ruled by American and European zoo associations have focused on birds and mammals that were, overall, the largest among the species present on IUCN Red List, certainly because the larger animals were also the most charismatic (see Pritchard et al., 2011).
The significant effects of diet in the MCMCglmm modeling used to account for the simple presence of the threatened taxa in both non-conservation and in conservation ex situ facilities reflected the avoidance of insectivorous birds. It suggested that taxa for which diet can be easily replicated in captivity were preferred. Birds with other types of diets also may have been avoided by bird keepers, such as the nectarivorous, but the non-significant effect of this type of diet in the modeling may have resulted from the lower representativeness of this group of birds in the dataset (Red List) when compared to the insectivorous taxa.
The strong and significant phylogenetic effects suggested that other characteristics not predicted in our models also could have influenced in the eligibility of taxa for captivity. Phylogeny, diet, and body mass can be correlated parameters because whole families can be characterized by a specific type of diet and/or body mass patterns. However, our phylogenies were useful to show that even within certain diet categories, specific clades were more likely to occur in captivity. For instance, endangered members of the family Psittacidae, which are predominantly frugivorous, were virtually all recorded in captivity, while many frugivorous taxa belonging to the families Pipridae, Cotingidae, and Thaupidae were not present in our surveys. This was likely attributed to some characteristics presented by the psittacines, such as the capacity to imitate the human voice, plumage color, charismatic appearance, longevity, easy adaptation to captivity, or ease of capturing.
Our prediction that most of the endangered Brazilian avian taxa with records in ex situ conservation breeding facilities and in organized ex situ conservation plans could be the same that have been long target to the illegal pet trade was corroborated. Of the 59 taxa present in non-conservation facilities, the vast majority were confirmed to be derived from poaching, as their records were associated with legal or illegal bird dealing. Exceptions may include only five species of marine birds (families Diomedeidae, Procellaridae, Phaethontidae, and Sternidae) that were recorded in rehabilitation centers and may have been rescued after accidents. Because conservation programs that are initiated with the capture of animals in the wild for ex situ breeding purposes are extremely rare in Brazil (see below), these results suggest that conservation breeding facilities have, overall, harbored the endangered birds derived from police actions or from the centers of animal rehabilitation, instead of choosing taxa based on scientific criteria. This is also the most probable explanation for the lack of significance of the levels of threat in the eligibility of the animals occurring in aviaries where conservation actions are expected to occur and in the official organized ex situ conservation plans. Our results evidenced that planned ex situ conservation initiatives involving the detection of critical cases, development or adaptation of husbandry technologies, capture in nature, improvement of husbandry technologies, and captive breeding are too scarce in Brazil, the country that holds the richest avifauna and concentrates the largest number of endangered taxa on Earth. These findings were alarming because only a small portion of the endangered taxa present on the Brazilian Red List was the target to poaching and to non-conservation breeding, and by acting as sinks of animals derived from non-conservation practices, ex situ conservation managers have totally ignored the bird families that concentrate the greatest numbers of endangered taxa, such as the antbirds or flycatchers. The number of Brazilian endangered avian taxa with records in conservation breeding facilities and in organized ex situ conservation plans were very small and we see six main reasons for the low reach of ex situ conservation: (i) the available institutions and their spaces have been easily filled with the taxa derived from the traffic, many of which are also endangered; (ii) the ex situ conservation facilities have limited personnel, with limited time availability to work on the development of new husbandry techniques; (iii) the risks of failure with taxa that have not been traditionally raised in captivity are higher and managers could be inhibited by the idea that potential deaths of endangered organisms could occur during adaptation phases, compromising their images to the society and exposing them to critics; (iv) feeding insectivorous organisms, for instance, could be more time-demanding and requires a constant and predictable amount of insects and other invertebrates; (v) no incentive and legal support from Federal and State governmental agencies to start experiments and captive breeding of these “non-conventional” taxa, and (vi) the lack of interaction between captive and field conservation practitioners. Despite the quite high number of papers published about basic requirements in the wild for many taxa, and the information available for captivity, there is a generalized lack of information exchange between “people in the field” with the “people working with ex situ” populations.
Ex situ conservation has impeded the extinction of two Brazilian endemic bird species that were once extinct in the wild, the Alagoas Curassow (Pauxi mitu) and the Spix’s Macaw (Cyanopsitta spixii) (Hammer and Watson, 2012; Francisco et al., 2021), and important ex situ conservation actions have been done, led by Brazilian breeding centers, for other endangered taxa such as the Red-billed Curassow (Crax blumenbachii), the Black-fronted Piping Guan (Aburria jacutinga), the Brazilian Merganser, the Yellow Cardinal (Gubernatrix cristata), and many psittacines, to mention some. Managing and breeding “non-usual” birds are feasible and could bring relevant results in a very short time. The Brazilian Merganser conservation program is certainly one of the most successful in the country (ICMBio, 2020). This species was never kept under human care in history, and its ex situ program started with the collection of a few eggs in the field by conservation managers for founding a captive population. This is a piscivorous duck, and husbandry techniques had to be developed to supply these animals with alive fish, which constitutes an important part of its diet also in captivity. In only seven years of intensive management, the total population under human care (genetically managed) reached about 60 individuals, with the majority of the birds born in captivity. It is worthy to mention that the global wild population of this duck, formerly distributed in Brazil, Paraguay (extinct), and Argentina (extinct) is under 250 individuals (BirdLife International, 2021). To our knowledge, the Brazilian Merganser, the Red-billed Curassow, and the Alagoas Curassow were the only species in Brazil for which founder individuals were collected in the wild specifically for conservation purposes. Three recently globally extinct taxa were insectivorous birds, endemic to the Atlantic Forest of northeastern Brazil (Lees et al., 2014; Pereira et al., 2014). This region concentrates most of the Brazilian taxa that will likely become extinct in the next years, and maybe now it is too late to implement ex situ conservation plans due to the small numbers of extant individuals (see Pereira et al., 2014 and Francisco et al., 2021), in such a way that the risk of taking individuals from the wild could be a serious threat for the taxa itself. Insectivorous birds represent the guild that concentrates the most probable candidates for the next global extinctions. Due to cultural or logistic reasons, they have been totally excluded from Brazilian aviaries, but insectivorous birds have been successfully raised worldwide in zoos and also in laboratories for experimental research, and many were proven to adapt to artificial diets (Dilks, 1993; Verbeek et al., 1994; Owen, 2008; Aplin et al., 2015). Further, in terms of space and amount of food, they may be less demanding than, for instance, most psittacines or cracids. The list of Brazilian endangered birds is certainly too extensive for all of the taxa to be covered by ex situ plans and many of them would not qualify for this type of conservation. However, we suggest that investing in the development of husbandry techniques, especially for insectivorous passerines, and incorporating the foundation of captive populations of taxa chosen by scientific criteria in conservation managers’ agenda, could be important actions to avoid some of the most imminent Brazilian bird extinctions. Certainly, it is too late for the implementation of ex situ programs for many Brazilian taxa, but managers, governmental agencies, and field biologists should start to develop the required husbandry techniques using non-threatened species as models, and focusing the breeding programs especially in taxa currently considered as vulnerable or near-threatened.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank to our dearest colleagues working hard every day in breeding centers and zoos for generously sharing their knowledge, experiences, and concerns. We thank specially to Roberto Azeredo, James Simpson, Nelson M. Kawall (in memoriam), Moacyr de C. Dias (in memoriam), Alcides Vertematti, Rolf Grantsau (in memoriam), Werner Bokermann (in memoriam), Herculano Alvarenga, Paulo Flecha, Ana C. P. de Azevedo Lopes, Epitacio C. Farias Junior, Bruno Ehlers, Cynthia Cipreste, Mara Marques, Fernanda Guida, Carlos Keller, Mauro G. Diniz, Robert Kooij, Maria F. Gondim, Camila Piovani, José Selmi, Geraldo M. Belo (in memoriam), Valter Silveira, Carlos A. Polezel, Rosemary Low, William Wittkoff, Andrey Naves, Fernando Pinto, Priscila P. Amaral, Marina Somenzari, Érika Machado, Ricardo J.G. Pereira, Alberto Fonseca, José S. Nogueira, and Antônio Guimarães (Zu, in memoriam). This work was due to ARCA project supported by São Paulo Research Foundation - FAPESP (Thematic project 2017/23548-2). We also acknowledge FAPESP (#2017/23458-2 and 2018/20249-7) and Brazilian Research Council - CNPq for further grants (#308337/2019-0, 158845/2018-8, and 308337/2019-0). ABL received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (#88887519041/2020-00). We especially thank two anonymous referees for providing valuable comments in the early versions of this manuscript.
The following are Supplementary data to this article:
List of threatened Brazilian avian taxa and raw data used in the MCMCglmm analyses, including taxa scientific names as in the Brazilian Red List; scientific names as in birdtree.org; confirmed presence in non-conservation facilities (yes/not); confirmed simple presence in ex situ conservation facilities (yes/not); and confirmed presence in organized ex situ conservation plans (yes/not), together with the respective sources of information (literature = LT; personal observation = PO; Action Plans = AP; Stoodbooks = ST; Web Sites = WS). The taxa characteristics were diet, body mass, and levels of threat in the Brazilian Red List. The complete reference list is presented at the end of the table.
A — Numbers of threatened Brazilian avian taxa per family compared to (A) numbers of threatened taxa recorded in captivity for non-conservation purposes, (B) numbers of threatened taxa recorded in ex situ conservation facilities, and (C) numbers of threatened taxa with organized ex situ conservation plans.
Proportions of threatened Brazilian avian taxa according to their diets, including (A) all of the taxa from the Brazilian Red List, (B) the taxa recorded in captivity for non-conservation purposes, (C) the taxa recorded in ex situ conservation facilities, and (D) those with organized ex situ conservation plans.