Callithrix is a genus of small sized primates endemic to Brazil. Callithrix jacchus and C. penicillata have become invasive in the Atlantic Forest, outside their native range, being a concern especially for birds. We reviewed the literature to understand the impact of these marmosets on native species. Most studies were about marmoset's diet, as opposed to its impacts on birds, reporting a diet dominated by plant items, complemented by animal items (mostly arthropods) particularly during the dry season. The studies found low rates of marmoset predation of birds. 90% of the 19 species preyed by these marmosets were widespread non-threatened birds, and only five were recorded within marmoset's invaded range. Of the four studies focused on the impacts of invasive marmoset's predation on birds, two found no impact and two found a strong negative impact of marmoset egg predation on two birds in coastal restinga vegetation.
We conclude that there is weak evidence available to date of a negative impact of invasive marmosets on Atlantic Forest birds, but with a clear need for studies that focus on population trends of preyed birds, instead of studies on marmoset's diet, to determine whether invasive marmoset are harmful to Atlantic Forest birds.
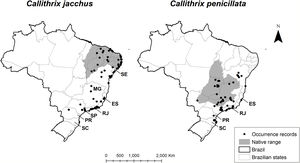
Callithrix Erxleben, 1777 (Primates: Callithrichidae) is a genus of small sized primates, with six species parapatrically distributed within Brazil (Kinzey, 1982; Braz et al., 2019). Two marmoset species were introduced and became invasive in the Atlantic Forest of southeastern Brazil: the common marmoset, Callithrix jacchus, native xeric shrublands (locally known as the Caatinga) and some localized Atlantic Forest enclaves in northeastern Brazil and the black-pencilled marmoset, C. penicillata, native to seasonal savannas in central Brazil (locally known as Cerrado) (Braz et al., 2019) (Fig. 1). Marmosets are rarely hunted for food, but used to be heavily captured for illegal pet trade (Mittermeier et al., 1982). Both species have the largest ranges within the genus (Braz et al., 2019), increasing their availability for pet trading. Young marmosets were captured in the wild and brought to large cities, especially those in wealthier southeastern Brazil, to enter the national and international pet market (Mittermeier et al., 1982; Ruiz-Miranda et al., 2006). Additionally, in the past, individuals rescued from the illegal trade were released in the wild by government agents, irrespective of being within its original range or not (Coimbra-Filho, 1983).
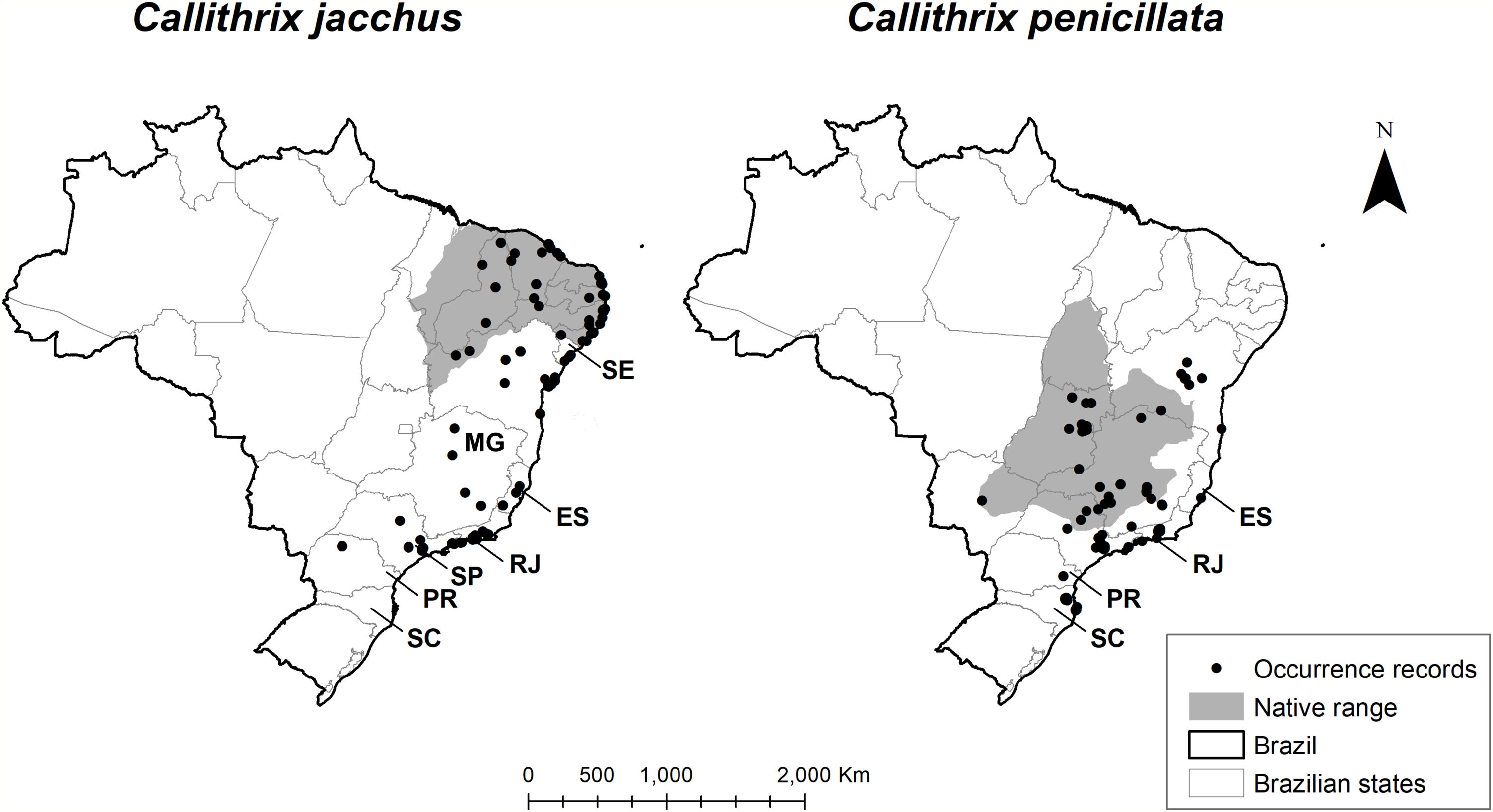
Invasive marmosets distribution. Distribution of C. jacchus (left) and C. penicillata (right) showing occurrence records (from Global Biodiversity Information facility), within and outside species’ native range (according to the International Union for Conservation of Nature). Brazilian states where the species are considered invasive: Sergipe (SE), Bahia (BA), Espírito Santo (ES), Rio de Janeiro (RJ), São Paulo (SP), Paraná (PR), Santa Catarina (SC).
Callithrix jacchus was first cited as an invasive species (although not using the term) in 1971 (Coimbra-Filho et al., 1971), in 2011 both C. jacchus and C. penicillata were included in the Brazilian list of invasive species (Rocha et al., 2011), and since 2012 they figure in the Global Invasive Species Database (2019). Invasive marmosets are a cause for concern for their potential for competition and predation of native species (Bicca-Marques et al., 2006; Ruiz-Miranda et al., 2006; Traad et al., 2012). Marmosets are believed to be particularly harmful to the avifauna in the areas where they are introduced (Traad et al., 2012), due to numerous records of marmosets preying on eggs, nestlings and chicks (e.g. Lyra-Neves et al., 2007; Galetti et al., 2009; Alexandrino et al., 2012). In the early 70s, for example, a study already stated that native bird populations were threatened by invasive marmoset egg predation (Coimbra-Filho et al., 1971), although without any quantification of the impact of marmosets predation on bird populations.
The establishment of self-sustaining populations of C. jacchus and C. penicillata following introduction outside their original range is often attributed to their great ecological plasticity (Coimbra-Filho, 1983; Stevenson and Rylands, 1988). Where they are introduced, the two species seem to have a preference for secondary and edge forests, surviving well in degraded and fragmented landscapes (Rylands, 1996; Cerqueira et al., 1998; Vilela and Del-Claro, 2011; Ferrari et al., 2013; Bruno and Bard, 2016).
The negative impact of invasive marmoset on birds is often stated with certainty. Marmosets, however, are considered as predominantly frugivorous-gummivorous primates (Rylands, 1996; Miranda and Faria, 2001; Schiel et al., 2010). Furthermore, in invaded areas they tend to occur in disturbed and edge forests, where presumably there is a prevalence of more adaptable bird species. Here we carried a systematic review of the literature to investigate the evidence of negative impacts of these marmosets on birds within their native and invaded ranges.
MethodsWe did a bibliographic survey in three databases: Web of Science database for papers from 1945 until June 2019 (isiwebofknowledge.com), and Scopus (www.scopus.com) and Scielo (www.scielo.br) for papers from all years until June 2019. We used the combination of the words: Callithrix AND bird, mammal, amphibian, reptile, fish, frog, lizard, vertebrate, invertebrate, predation, and feeding (Table S1 in the Appendix A). We also selected additional papers from the references cited in the papers retrieved in the systematic survey. In this review we use the Oxford Dictionary definition of “evidence”, i.e. the available body of facts or information indicating whether a belief or proposition is true or valid.
For studies reporting bird predation by marmosets, we compiled conservation and natural history traits of the prey species: conservation status from IUCN (2019), forest dependency and distribution range size (km2) from Birdlife Data Zone (www.birdlife.org/datazone/species), body mass (g) from Birdlife Data Zone and the Handbook of the Birds of the World Alive (www.hbw.com/species), and nest type (open vs. closed) from the Birds of the World (2020). We searched additional literature when information was not available from these sources.
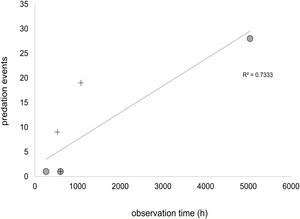
We also made a regression between observation time (h) and the number of bird predation events, to evaluate whether a lack of records might be due to low observation time, plotting the seven studies that specified the number of bird predation events and observation time (Lyra-Neves et al., 2007; Galetti et al., 2009; Schiel et al., 2010; Abreu et al., 2016; Ballarini, 2016; Patiu, 2017; Borges et al., 2018).
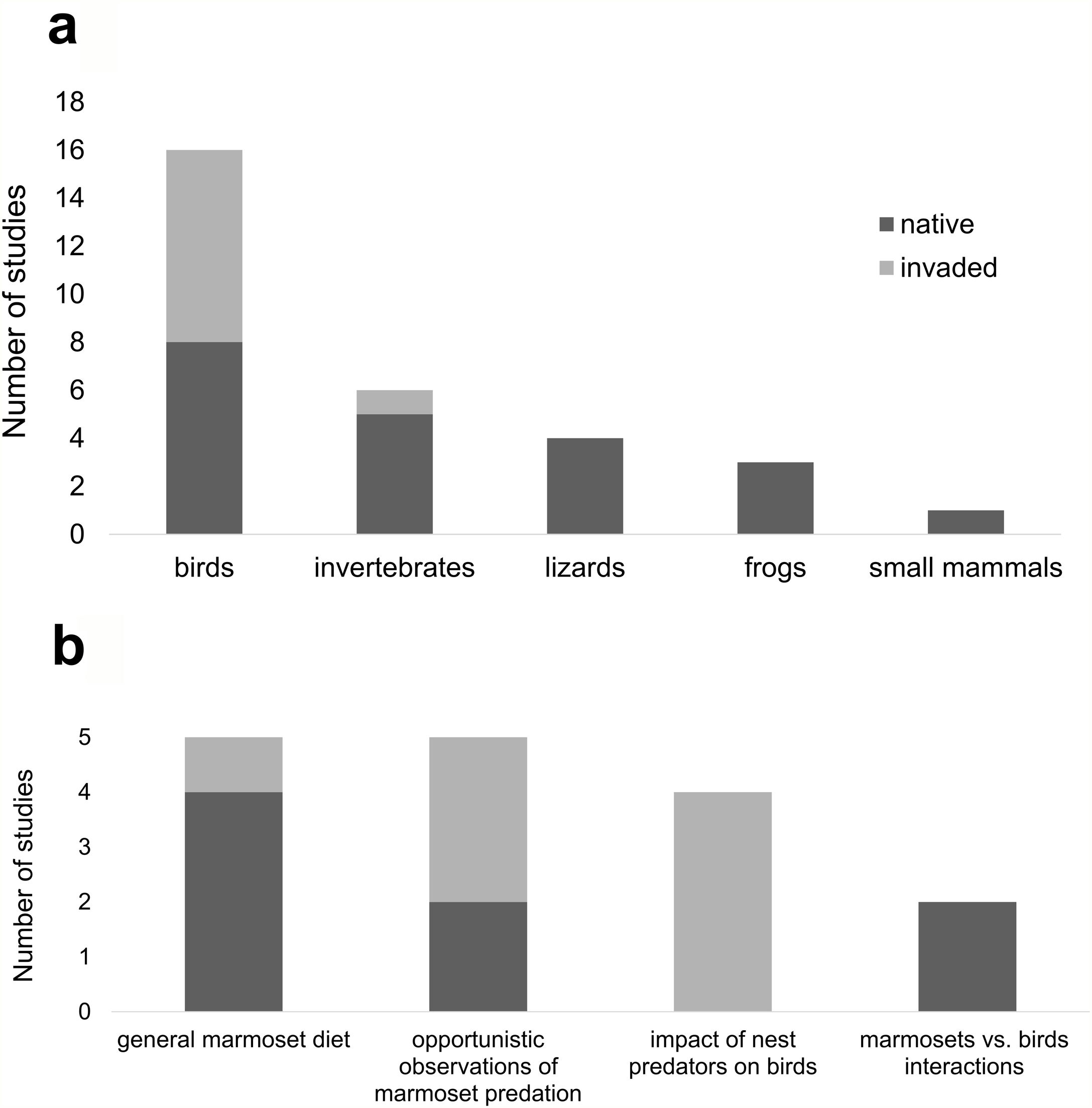
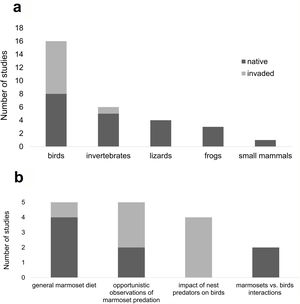
ResultsThe survey returned 1.842 papers, of which 20 were used in the study (Table S1 in the Appendix A), plus nine retrieved from the references cited in those. Of those, only ten (37%) were studies conducted within marmoset's invaded range. There were only three studies reporting interspecific competition of marmosets with birds, all within marmoset's native range. There were 24 studies reporting marmoset predation, 15 within marmoset's native range and nine within the invaded range, with records of both invertebrate (mostly arthropods) and vertebrate predation (mostly birds) (Fig. 2a). Most studies were focused on marmoset diet or were opportunistic observations of marmoset bird predation (Fig. 2b); only four studies were focused on invasive marmoset's impact on birds (Fig. 2b). We found records of 19 bird species preyed by marmoset, only five of which (26%) within marmoset's invaded range (Table 1, Table S2 in the Appendix A). We also found records of other vertebrate prey such as small mammals (Camargo et al., 2016), frogs (Santos, 2009; Schiel et al., 2010; Beltrão-Mendes et al., 2018) and lizards (Mendes Pontes and Soares, 2005; Schiel et al., 2010; Amora et al., 2014; Melo et al., 2018) (Fig. 2a).
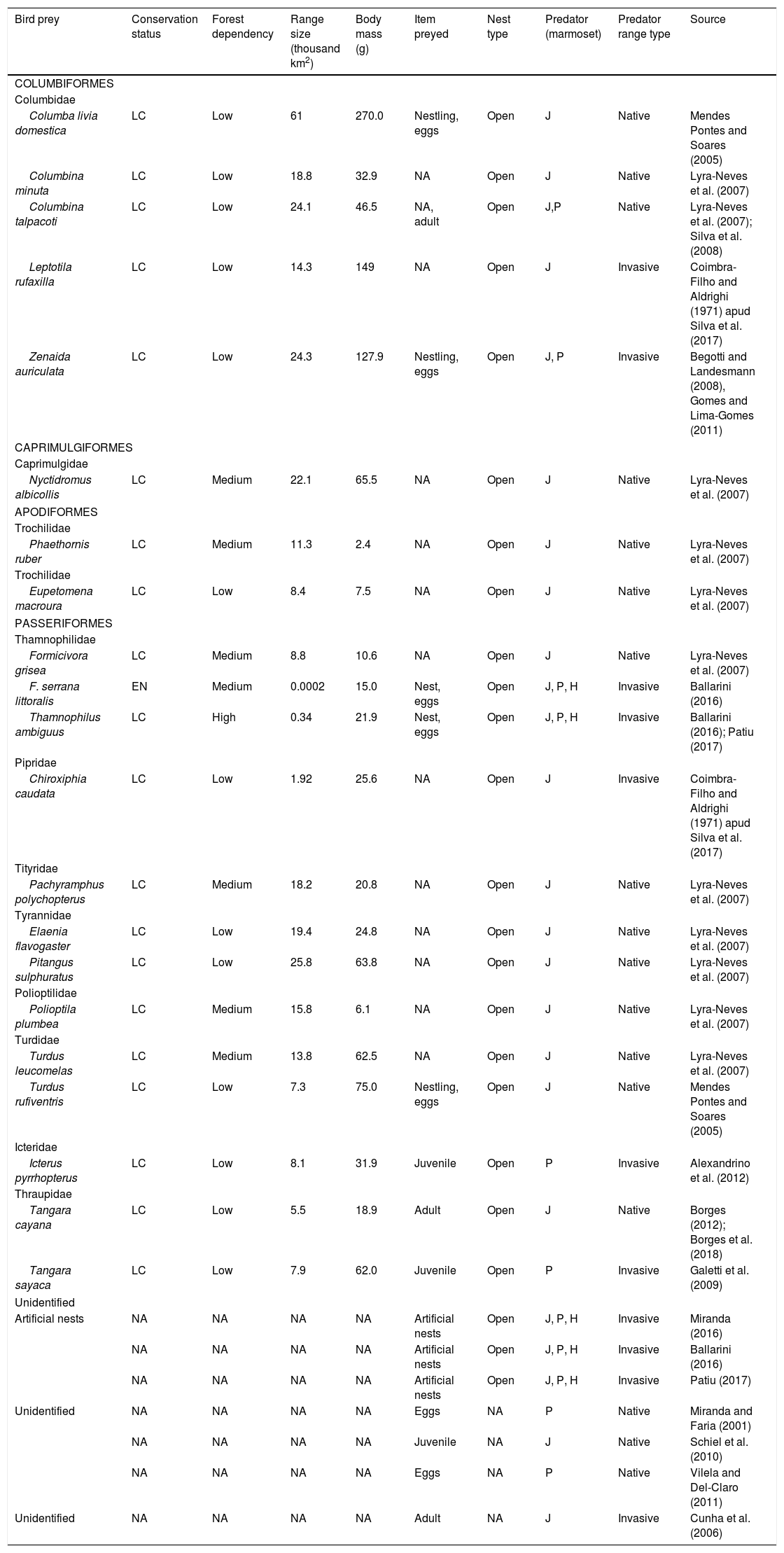
Natural history and conservation traits of bird prey and marmoset predators reported in the literature. Bird taxonomy according to the Brazilian Committee on Ornithological Records (Piacentini et al., 2015); Conservation status: Least concern (LC), Endangered (EN); Item preyed according to the most detailed information from the studies, where it says “nest” there was no information whether it was nestling or egg; Nest type according (Birds of the World, 2020), when the information was unavailable, it was according Wikiaves (https://www.wikiaves.com.br/); Predator (marmoset): Callithrix jacchus (J), C. penicillata (P), and hybrid between the two species (H).
| Bird prey | Conservation status | Forest dependency | Range size (thousand km2) | Body mass (g) | Item preyed | Nest type | Predator (marmoset) | Predator range type | Source |
|---|---|---|---|---|---|---|---|---|---|
| COLUMBIFORMES | |||||||||
| Columbidae | |||||||||
| Columba livia domestica | LC | Low | 61 | 270.0 | Nestling, eggs | Open | J | Native | Mendes Pontes and Soares (2005) |
| Columbina minuta | LC | Low | 18.8 | 32.9 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Columbina talpacoti | LC | Low | 24.1 | 46.5 | NA, adult | Open | J,P | Native | Lyra-Neves et al. (2007); Silva et al. (2008) |
| Leptotila rufaxilla | LC | Low | 14.3 | 149 | NA | Open | J | Invasive | Coimbra-Filho and Aldrighi (1971) apud Silva et al. (2017) |
| Zenaida auriculata | LC | Low | 24.3 | 127.9 | Nestling, eggs | Open | J, P | Invasive | Begotti and Landesmann (2008), Gomes and Lima-Gomes (2011) |
| CAPRIMULGIFORMES | |||||||||
| Caprimulgidae | |||||||||
| Nyctidromus albicollis | LC | Medium | 22.1 | 65.5 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| APODIFORMES | |||||||||
| Trochilidae | |||||||||
| Phaethornis ruber | LC | Medium | 11.3 | 2.4 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Trochilidae | |||||||||
| Eupetomena macroura | LC | Low | 8.4 | 7.5 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| PASSERIFORMES | |||||||||
| Thamnophilidae | |||||||||
| Formicivora grisea | LC | Medium | 8.8 | 10.6 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| F. serrana littoralis | EN | Medium | 0.0002 | 15.0 | Nest, eggs | Open | J, P, H | Invasive | Ballarini (2016) |
| Thamnophilus ambiguus | LC | High | 0.34 | 21.9 | Nest, eggs | Open | J, P, H | Invasive | Ballarini (2016); Patiu (2017) |
| Pipridae | |||||||||
| Chiroxiphia caudata | LC | Low | 1.92 | 25.6 | NA | Open | J | Invasive | Coimbra-Filho and Aldrighi (1971) apud Silva et al. (2017) |
| Tityridae | |||||||||
| Pachyramphus polychopterus | LC | Medium | 18.2 | 20.8 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Tyrannidae | |||||||||
| Elaenia flavogaster | LC | Low | 19.4 | 24.8 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Pitangus sulphuratus | LC | Low | 25.8 | 63.8 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Polioptilidae | |||||||||
| Polioptila plumbea | LC | Medium | 15.8 | 6.1 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Turdidae | |||||||||
| Turdus leucomelas | LC | Medium | 13.8 | 62.5 | NA | Open | J | Native | Lyra-Neves et al. (2007) |
| Turdus rufiventris | LC | Low | 7.3 | 75.0 | Nestling, eggs | Open | J | Native | Mendes Pontes and Soares (2005) |
| Icteridae | |||||||||
| Icterus pyrrhopterus | LC | Low | 8.1 | 31.9 | Juvenile | Open | P | Invasive | Alexandrino et al. (2012) |
| Thraupidae | |||||||||
| Tangara cayana | LC | Low | 5.5 | 18.9 | Adult | Open | J | Native | Borges (2012); Borges et al. (2018) |
| Tangara sayaca | LC | Low | 7.9 | 62.0 | Juvenile | Open | P | Invasive | Galetti et al. (2009) |
| Unidentified | |||||||||
| Artificial nests | NA | NA | NA | NA | Artificial nests | Open | J, P, H | Invasive | Miranda (2016) |
| NA | NA | NA | NA | Artificial nests | Open | J, P, H | Invasive | Ballarini (2016) | |
| NA | NA | NA | NA | Artificial nests | Open | J, P, H | Invasive | Patiu (2017) | |
| Unidentified | NA | NA | NA | NA | Eggs | NA | P | Native | Miranda and Faria (2001) |
| NA | NA | NA | NA | Juvenile | NA | J | Native | Schiel et al. (2010) | |
| NA | NA | NA | NA | Eggs | NA | P | Native | Vilela and Del-Claro (2011) | |
| Unidentified | NA | NA | NA | NA | Adult | NA | J | Invasive | Cunha et al. (2006) |
We found many more studies on marmoset predation than on competition. The three studies reporting interspecific competition of marmosets with birds were all in Pernambuco State, within marmoset's native range (Fig. 1). The studies found that during the dry season, when food availability decreases for both marmosets and birds, there is an increase in conflictive interactions between C. jacchus and birds (Borges, 2012; Borges et al., 2018), in the overlap of their foraging grounds, and in the competition for resources, especially arthropods (Lyra-Neves et al., 2007). Because none of these studies were within marmoset's invaded range or estimated the impact of this competition on native birds, there is no evidence of a negative impact of competition of invasive marmosets with native birds.
Just as there seems to be an increase in competition between marmosets and native species in the dry season, so does in predation. There seems to be a pattern of a higher consumption of vegetable material (fruits and exudates) in general, and a seasonal fluctuation in the consumption of animal items. Fruits and insects are more abundant in the rainy season (Magalhães et al., 2007; Vilela, 2007), so in the dry season there is an increase in the consumption of other items, such as exudates and small vertebrates.
There are many studies of marmoset diet within their native range, especially on C. jacchus in Pernambuco State (Table S2 in the Appendix A). The longest one lasted 30 months and in 5.040h of observation 28 nest predation events were recorded, involving 11 bird species (Lyra-Neves et al., 2007). Most species had one to three recorded attacks, except for the tropical gnatcatcher (Polioptila plumbea), a widespread and common native species, with eight records (Table 1). The study found a greater number of bird predation events in the dry season, when the supply of insects and fruits was lower. In 600h of observation, another study made many records of insect predation, but very few of vertebrates (one frog, one lizard, and one nestling, all unidentified) (Schiel et al., 2010). In 488h of observation, a study recorded the predation of nestlings and eggs in two bird species: the rock dove (Columba livia domestica), which is itself an invasive species, and the rufous-bellied thrush (Turdus rufiventris), a widespread and common native species (Mendes Pontes and Soares, 2005) (Table 1). Another study recorded, in 262h of observation, only one bird predation, an adult burnished-buff tanager (Tangara cayana), again a widespread and common native species (Borges, 2012; Borges et al., 2018) (Table 1). The study also found more interactions between birds and marmosets in the dry season (N=54) in relation to the rainy season (N=14). Among the interactions in the dry season, 81.5% were conflictive and 18.5% were non-contact interactions.
Another study on C. jacchus diet in Pernambuco, however, found the opposite pattern, with feeding and foraging behavior being more frequent in the rainy season than in the dry season (Pinheiro and Mendes Pontes, 2015). The study also found a diet predominantly composed of plant items (83.4%), and animal items including only insects. Interestingly, a study with 263h of observation in Paraíba, another state within C. jacchus’ native range (see Fig. 1 for state location), found a higher consumption of animal items (90.4%) than vegetable items (Abreu et al., 2016), but still mostly composed of arthropods (94%), recording only one unidentified bird egg predation.
There were fewer studies on the diet of C. penicillata in its native range, as compared to C. jacchus. There was an opportunistic observation of the predation of one ruddy ground-dove (Columbina talpacoti) in Goiás State (see Fig. 1), again a common and widespread species (Silva et al., 2008). A 10-month long study in Brazil's capital city, Brasília, during one year, recorded bird egg predation events of unidentified birds (Miranda and Faria, 2001). Another study, also in Brasília, found that insect foraging, gummivory and feeding behavior were higher in the dry season than in the rainy season (Vilela and Faria, 2004). The feeding behavior of C. penicillata continued to be studied in the same area, revealing that insect larvae were consumed only in the dry season, while adult insects were consumed year round, although more frequently during the rainy season, probably because they were more abundant during that period (Vilela, 2007). A one-year long study in Uberlândia, Minas Gerais State, totaling 67h of observation, also recorded bird egg predation (Vilela and Del-Claro, 2011). Again, egg predation occurred during the dry season, when there was lower food resource availability. In the dry season these marmosets fed on the sap of many tree species, while in the rainy season they fed on fruits, flowers, and the sap of only one tree species (Vilela and Del-Claro, 2011).
There were fewer records of bird predation within the invaded range of C. jacchus and C. penicillata. There are a number of opportunistic records in São Paulo State (see Fig. 1), including predation of two nestlings of the eared dove (Zenaida auriculata) (Gomes and Lima-Gomes, 2011) and another two of the variable oriole (Icterus pyrrhopterus) (Alexandrino et al., 2012), both widespread and common native bird species (Table 1, Table S2 in the Appendix A). Predation of eared dove's eggs by a mixed group of C. jacchus and C. penicillata was also opportunistically recorded in another study (Begotti and Landesmann, 2008). The same group of marmosets also attempted to attack a nest of chalk-browed mockingbird (Mimus saturninus), but the defensive behavior of the bird incubating the nest drove the group away.
Studies on marmoset diet were also carried out within the invaded range of C. jacchus and C. penicillata, generally showing a low threat to the bird fauna in urban and peri-urban forests, but a high threat in coastal restinga vegetation. A number of studies were carried in Tijuca Forest, a 95km2 forest fragment within Rio de Janeiro city (Abreu, 2014), with a superabundance of invasive marmosets, mostly composed by hybrids between C. jacchus and C. penicillata (Cunha, 2005; Cunha et al., 2006). In Tijuca National Park, Coimbra-Filho and Aldrighi (1971) apud Silva et al. (2017) made the opportunistic observation of C. jacchus predation of eggs from the grey-fronted dove (Leptotila rufaxilla), a common and widespread non threatened native bird, and of the blue manakin (Chiroxiphia caudata), a common non threatened bird endemic to the Atlantic forest (Vale et al., 2018 (Table 1). in. Many years later, a study of marmosets’ feeding behavior in the area showed a prevalence of plant items, but with 20% of the observations composed of marmoset chasing birds, with no recorded bird consumption, although one third party opportunistic observation of the consumption of an unidentified bird was mentioned (Cunha et al., 2006). Although the authors considered the results to represent an “intense faunivory” never reported before, the five studies on marmoset diet (Fig. 2) reveal that their findings were pretty typical, with a confirmed low consumption of vertebrate items. The prevalence of plant items in the diet of marmosets in the Tijuca Forest was confirmed by another study, which raises the hypothesis that marmosets may be important seed dispersers in this highly disturbed forest and, therefore, any population control should be carried only after other (native) seed dispersers are reintroduced in the area (Silva et al., 2017). There were only four studies that actually focused on the potential impacts of invasive marmoset predation on birds. All these studies used camera trapping, which is among the best techniques to monitor bird nest predation in tropical forests (Ribeiro-Silva et al., 2018). Among these studies, two found no significant impact of marmoset predation in disturbed forests in Rio de Janeiro (Miranda, 2016) and São Paulo (Galetti et al., 2009), and the other two found significant impacts on two bird populations in sandy coastal plain vegetation in Rio de Janeiro, locally known as restinga (Ballarini, 2016; Patiu, 2017). The Rio de Janeiro study was in the Rio de Janeiro Botanical Garden, also inside the Tijuca Forest, using artificial nests with common quail (Corturnix cortunix) eggs, recording in 3200 days of sampling effort only five egg predation by invasive marmosets, but 35 by the native black capuchin monkey (Sapajus nigritus) (Miranda, 2016). Still, birdwatchers in the garden attribute a perceived bird decline in the area to the presence of invasive marmosets (Cunha et al., 2006). The São Paulo study was in the Anchieta Island, where there is a well-known decline in bird populations, also using artificial nests with quail eggs (Galetti et al., 2009). They found that mesopredators, such as domestic cats and dogs, were the main culprit, although other species were also implicated, including native ones such as the red-rumped agouti (Dasyprocta leporina), big-eared opossum (Didelphis aurita), tegus lizard (Tupinambus merianae), and the coati (Nasua nasua). In 599h of sampling effort, there was no record of marmoset egg predation. During the fieldwork, however, the authors made an opportunistic sighting of C. penicillata predation of a juvenile sayaca tanager (Tangara sayaca). Despite the very low marmoset predation, the authors say that, as “first step, we suggest the complete eradication of marmoset because it is the only “truly” exotic species, since C. penicillata does not occur in the coastal Atlantic Forest” (Galetti et al., 2009). It is important to note, however, that these two studies that found no significant impact of invasive marmosets egg predation on native birds used artificial nests, which are criticized for possibly attracting different predators than real nests (Moore and Robinson, 2004). It is also important to note that invasive marmosets seem to avoid eating common quail eggs (Maria Alice S. Alves, pers. comm., see Alvarez and Galetti 2007, Oliveira et al. 2013 and Roper 1992 for use of quail eggs in nest predation studies involving predators in general). Therefore, these results may underestimate marmoset egg predation.
The other two studies focused on potential impacts of invasive marmosets on birds, however, showed truly alarming negative impacts. During one year, Ballarini (2016) monitored nests with camera traps in coastal restinga vegetation in Rio de Janeiro State: active nests of restinga antwrens (Formicivora serrana littoralis) and sooretama antshrikes (Thamnophilus ambiguus), abandoned nests restinga antwrens with Atlantic canary (Serinus canarius) eggs, and artificial nests with common quail eggs. Although none of the 30 artificial nests were preyed by the marmosets, they preyed 20% of the 40 abandoned antwren nests, 75% of the 12 antwren active natural nests and 23.5% of the 17 antshrike active natural nests monitored. The author suggests that the presence of marmosets may cause a decrease in reproductive success of these birds in the region, and highlights the urgent need for efficient measures for marmoset control in the area (Ballarini, 2016). Indeed, a follow-up study with the sooretama antshrike using natural and inactive nests with Atlantic canary eggs revealed that the greatest cause of reproductive failure was nest predation, 80% of which was attributed to Callithix spp. Marmosets preyed on 40% of the 10 sooretama antshrike nests monitored and 33.3% of the inactive nests with canary eggs. The study was conducted both in restinga and in Atlantic Forest with invasive marmosets, but nest predation occurred only in restinga. Patiu (2017) attributed this result to the difference in vegetation cover and food resources in the two areas. They hypothesize that forests’ denser and closed vegetation would hinder movement and reduce nest visualization by predators, while restingas’ open vegetation would facilitate nest finding (Patiu, 2017). In addition, the restinga is drier and has less plant resources, increasing the search for alternative food items (Patiu, 2017). The data from the natural nests of the two antbirds studied in restinga by Ballarini (2016) and Patiu (2017) were recently published by Ballarini et al. (2021), who indicated that the invasive marmosets depredated 81% of the 16 nests whose predators were identified.
The high impact of marmosets on restinga birds, as compared to Atlantic Forests, may also be explained by the fact that the restinga antwren and sooretama antshrike build their nest at low height (<1m, Ballarini, 2016), and marmosets preferentially use the low stratification (Zaluar et al., 2014). Additionally, they build open nests, which can be more susceptible to predation. Indeed, all records of egg predation by marmosets were from open nests, both in native and invaded ranges (Table 1). Also, there is no native Callithrix in Rio de Janeiro restingas (Cerqueira et al., 1998), and the Golden Lion Tamarin (Leontopithecus rosalia), another primate in the Callithrichidae family, have long been extinct in Rio de Janeiro restingas (Cerqueira et al., 1998). Therefore, restinga birds in Rio de Janeiro are presumably not well adapted to the presence of marmosets. Finally, C. jacchus and C. penicillata are originally predominantly from Caatinga xeric vegetation and Cerrado savanna vegetation (Braz et al., 2019), both physiognomies that are more similar to restingas than Atlantic Forest. This, presumably, implies that invasive marmosets are better adapted to restingas than Atlantic Forest were they are generally found, which may enhance their success as predators in restinga.
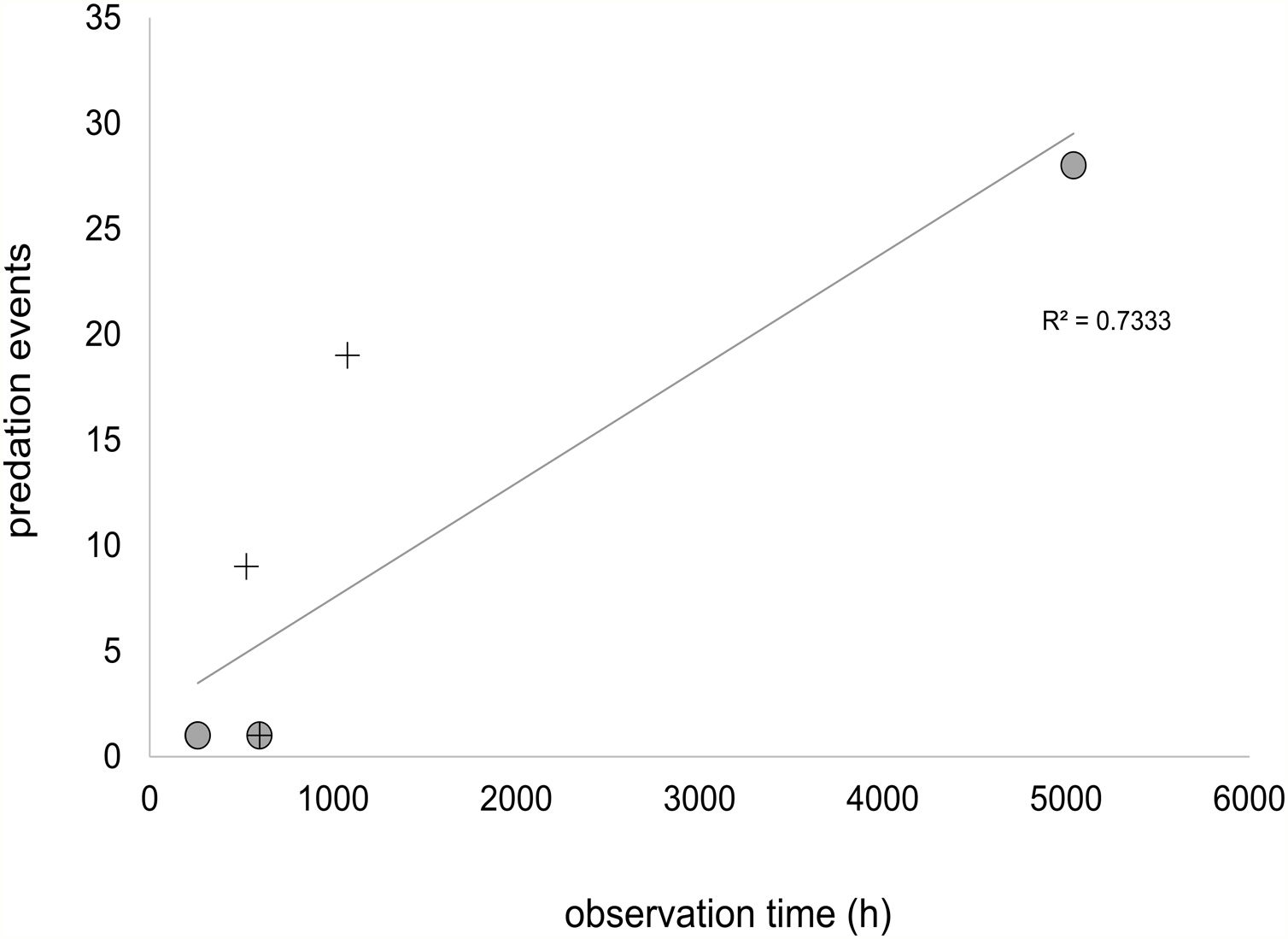
Except for the high impact of invasive marmosets in birds in Rio de Janeiro restingas, we found little evidence of a generalized negative impact of invasive marmosets in the avifauna. In studies that recorded sampling effort, at least 200h of observation was needed to record a marmoset bird predation event (Fig. 3). It is important to note that this estimate includes studies based on scan sampling and artificial nest methods that probably underestimates the number of bird predation occurrences. Therefore, although the rate of bird predation by marmosets should not be alarming in invaded areas, it is likely greater than one event per 200h of observation. Still, most preyed birds were small (X¯=50.8 g, SE=14.1g), non-threatened and widespread (X¯=15.8 million km2, SE=3.1millionkm2), with low forest dependency (58% of the records) (Table 1). The exception was again the two restinga birds, which have small ranges. Furthermore, although both species are not threatened at the global level, the restinga antwren subspecies (Fomicivora serrana littoralis) is regionally endangered (Mattos et al., 2009; Table 1).
Relationship between observation time (h) and bird predation events extracted from the literature. Predation within marmoset's native range are represented by circles and within marmoset's invaded area by crosses. Only studies specifying the number of bird predation events and observation time were included.
The invasion of the forests of southern and southeastern Brazil by exotic marmosets is considered a very serious problem that deserves greater societal attention (Modesto and Bergallo, 2008). The main concern is marmosets’ impact on biodiversity through competition and predation of the native fauna in invaded areas (Bicca-Marques et al., 2006; Ruiz-Miranda et al., 2006), especially birds (Traad et al., 2012). We found very few studies on competition between marmosets and birds, all showing increased competition during the dry season when food is scarcer, and no measure of its potential impact on preys’ population. Despite the considerable number opportunistic reports of bird predation by marmosets, our review showed a generally low rate of marmoset predation on birds in more systematic feeding studies, both within native and invaded ranges. This is in agreement with a known diet that is predominantly composed of plant items, complemented by animal items dominated by arthropods, particularly during the dry season (Cunha et al., 2006; Lyra-Neves et al., 2007). The fact that marmosets tend to eat more animal items during the dry season may reduce their impact on birds, as in the Atlantic Forest bird breeding peaks in the rainy season (Davis, 1945). Therefore, marmosets increase consumption of animal items in the period with least availability of bird eggs. The hypothesis of a negative impact of marmosets on the bird fauna is further weakened by the fact that invasive marmosets occur preferentially in disturbed and edge forests (Rylands, 1996; Cerqueira et al., 1998; Vilela and Del-Claro, 2011; Ferrari et al., 2013), where there is a prevalence of more adaptable and generalist bird species (Stotz et al., 1996; Banks-Leite et al., 2010). Indeed, our list of bird species preyed by marmosets is essentially composed of widespread, common and non-threatened birds, with the exception of the two restinga birds.
There is, therefore, weak evidence available to date of a widespread negative impact of invasive marmosets on Atlantic Forest birds, especially in the fragmented and degraded forest edges where they are generally found. It is important to note, however, that the vast majority of the studies retrieved were either studies on feeding behavior of marmosets or opportunistic observation of marmoset bird predation (Fig. 2b), without any assessment of the actual impact of predation on the bird populations. Therefore, in order to fully understand whether invasive marmoset are harmful to Atlantic Forest birds, it would be interesting to foster studies that focus on population trends of bird prey, instead of studies on marmoset's diet. Those studies should also include pristine forests, as most studies conducted to date were in highly disturbed forests. It is important to note, as well, that our study focused only on the likely impact of invasive marmosets on birds, ignoring the likely impact of hybridization with native congeners and competition with native primates, which is also an important issue (Ruiz-Miranda et al., 2006; Oliveira and Grelle, 2012; Aximoff et al., 2019).
Authors’ contributionConceptualization: MMV and MTZ conceived the study, MTZ did the literature search and drafted the work, MMV and MTZ did data analysis and MMV critically revised the work.
We thank Dr. Leonardo Oliveira and Dr. Henrique Rajão for valuable suggestions to the manuscript. The Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) provided a Ph.D. scholarship to MTZ (Grant No. 001), and the Brazilian Council for Scientific and Technological Development (CNPq) provided a fellowship to MMV (Grant No. 304309/2018-4). This paper is developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation, supported by MCTIC/CNpq (No. 465610/2014-5) and FAPEG (No. 201810267000023).