The presence of domestic dogs (Canis lupus familiaris) in Brazilian protected areas is fairly frequent. The interaction of such dogs with native animals leads to population declines for many species, particularly carnivores. In this paper the main threats dogs bring about Brazilian biodiversity are assessed with a focus on protected areas. We collected information from papers on the interaction of dogs and wildlife species as well as from interviews with National Park managers. Studies in protected areas in Brazil listed 37 native species affected by the presence of dogs due to competition, predation, or pathogen transmission. Among the 69 threatened species of the Brazilian fauna, 55% have been cited in studies on dogs. Dog occurrence was assessed for 31 National Parks in Brazil. The presence of human residents and hunters in protected areas were the factors most often quoted as facilitating dog occurrence. These may be feral, street or domestically owned dogs found in protected areas in urban, rural or natural areas. Effective actions to control this invasive alien species in natural areas must consider dog dependence upon humans, pathways of entry, and the surrounding landscape and context.
The introduction of alien species is one of the most significant threats inflicted by humans on biodiversity (Scholes and Biggs, 2005). Invasive alien species may alter environmental conditions and cause severe impacts in natural community composition and structure (Richardson, 2011). The Convention on Biological Diversity defines invasive alien species as a species outside its native range which threatens the integrity of ecosystems, habitats, and the permanence of indigenous species. Interactions such as predation, competition, pathogen transmission and hybridization initiate ecological processes that lead to native species population declines and changes in ecosystem dynamics (Simberloff and Von Holle, 1999). Domestic cats and dogs are considered invasive alien species when using or living in natural areas without human assistance. Cats are listed as one of the 100 worst invasive alien species on the planet (Lowe et al., 2000) and the majority of papers published in the past ten years on the interaction of dogs and native animals stress their negative impacts on biodiversity (Hughes and Macdonald, 2013), even in protected areas.
Domestic dogs (Canis lupus familiaris) may be considered a potential threat to the integrity of protected areas in Brazil, particularly of those in the highest level of protection. The presence of these animals in protected areas or their surroundings may reduce effectiveness in conserving biodiversity (MMA, 2013). The National Biodiversity Policy defines that it is vital to foresee, prevent, and take action against the origin of processes leading to considerable biodiversity decline or loss (Decree no. 4.339, August 22nd, 2002), such as invasive alien species. In this study we assessed information published on the impact of domestic dogs in protected areas, described these impacts particularly for Brazilian protected areas, and provided directions for protected area management in dealing with the problem. A literature review on the topic was carried out and complemented by interviews with National Park managers in Brazil. The information gathered was classified in five topics, the first two on basic information on dog natural history and interactions with native species, the third on papers published covering dog impacts in protected areas around the world, the fourth on problems in Brazilian national parks, and the last one on guidance for invasive dog management in protected areas in Brazil. This study is considered a preliminary approach to the problem and a source of information for future action and research for controlling domestic dogs in Brazilian protected areas.
Canis lupus familiaris (Linnaeus, 1758) natural historyThe global population of domestic dogs has been estimated at 700 million widely distributed around the world (Hughes and Macdonald, 2013). Brazil ranks as third in highest dog numbers after the United States and all European countries considered as a unit, with about 27 million dogs (Hughes and Macdonald, 2013). The highest density registered to this moment is 76 dogs per km2 in a rural area in Brazil in Piracicaba, in São Paulo state (Campos et al., 2007). Dogs are distributed in different landscapes, mostly urban and rural under human intervention, but also in protected areas under the strict protection category in Brazil. Dogs have been associated with human populations for more than 33,000 years (Ovodov et al., 2011). In spite of providing some benefits to society, domestic dogs have generated many negative impacts on biodiversity, particularly due to interactions with native animals.
To better define the relationship of dogs with biodiversity they have been classified according to their dependence upon humans: owned dogs; street or free living dogs; and feral dogs (Srbek-Araujo and Chiarello, 2008; Campos et al., 2007; Lacerda et al., 2009; Hughes and Macdonald, 2013). Owned dogs live in properties with resources such as food, shelter, and interactions provided by humans. Street dogs are not under human care, surviving opportunistically on food resources offered by humans. This class represents 75% of the 700 million dogs in the world (Hughes and Macdonald, 2013). Feral dogs live in natural areas, legally protected or not, yet close to human dwellings. These dogs may occasionally feed on resources offered by humans, but are not dependent upon them. They have a generalist diet (Macdonald and Carr, 1995; Campos et al., 2007), often feeding on food resources made available by humans, but also on animal carcasses and a great variety of animal and vegetal food items (Campos et al., 2007).
Domestication efforts have made dogs react with specific behavior responses when prompted by rewards in the form of food, playing, petting or simply attention (Scott and Fuller, 1974). Dogs in natural areas, however (alone or accompanied by humans), are stimulated by the environment and react similarly to their wild ancestors (Scott and Fuller, 1974; Gompper, 2013). These dogs develop greater hunting abilities and make better use the natural areas, changing their social behavior by forming packs (Rubin and Beck, 1982). The presence of dogs is therefore a threat to biodiversity and needs to be treated with effective management actions targeted at specific dog profiles in each protected area (Beck, 1973; Lavigne, 2015; Gompper, 2013; Young et al., 2011).
Main threats to biodiversity by dogsCompetition for territoryDogs are considered the most abundant carnivores in several natural areas (Hughes and Macdonald, 2013), including the Brazilian Atlantic Forest (Paschoal et al., 2012). They often occur in much higher numbers than native carnivores, usually present in low densities. This indicates the potential high impact of dogs on the community as a whole, and particularly on vertebrates (Vanak and Gompper, 2009; Vanak et al., 2013). High dog densities in natural areas may, at first, affect native carnivores due to competition. Dog density, predatory behavior, and pathogen transmission will determine the spatial range of competition and its resulting impact on native fauna as assessed through modeling based on empirical data (Vanak and Gompper, 2009). The mere presence of dogs in areas with native species intensifies competition for space and resources (Atickem et al., 2010). The presence of dogs in natural areas in India negatively affects the spatial distribution of the Indian fox, Vulpes bengalensis; the probability of site use by the fox is directly proportional to the distance from sites used by dogs, regardless of resource availability for the fox (Vanak and Gompper, 2010). In Brazilian Savannas the maned wolf (Chrysocyon brachyurus) avoids areas where domestic dogs are present, possible evidence of competition for territory between dogs and native carnivores (Lacerda et al., 2009).
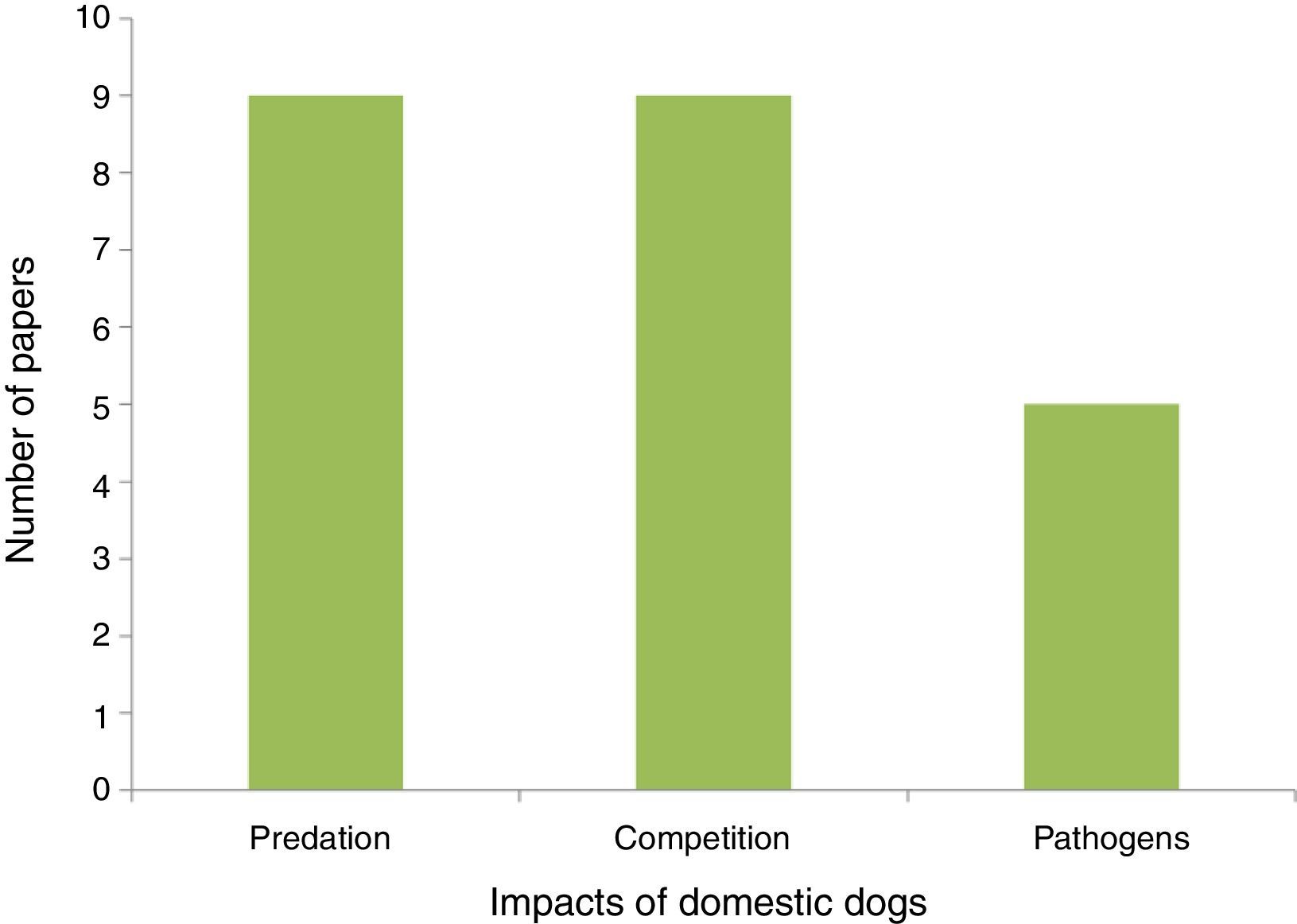
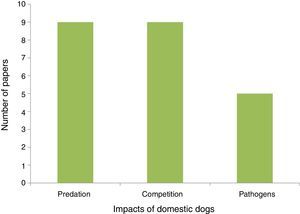
PredationDogs often do not truly prey, as predation is defined as the act of capturing (directly or indirectly) and feeding on the prey (Strauss, 1991). Dogs usually chase and capture other species for fun. In these predator – prey games they may injure animals, leading to death, while not always feeding on them (Gompper, 2013). Because this direct interaction with wild species is the most frequent topic in the studies assessed we maintained the use of the term predation for this type of impact (Fig. 1).
Hughes and Macdonald (2013) indicate that predation is the highest impact caused by dogs on native species, leading to population decline even of rare or threatened species such as the deer Pudu puda in the Andes (Silva-Rodriguez et al., 2010) and marine iguanas in the Galapagos Islands (Kruuk and Snell, 1981). The most emblematic case registered is the annihilation of a kiwi bird (Apteryx mantelli) population by one single dog on an island in New Zealand (Taborsky, 1988). Dogs have been identified as potentially efficient predators in several parts of the world such as Australia, Africa, and New Zealand (Butler et al., 2004). Published scientific information on native species preyed by dogs in Brazil is scarce. These publications most often refer to small and medium mammals killed by dogs, but also to deer, tapir, and primates (Galetti and Sazima, 2006; Campos et al., 2007; Oliveira et al., 2008; Lacerda et al., 2009).
There are few studies on dogs as prey, but one particular example from Africa reports leopard populations depending upon dog feeding for survival in a farming area where natural resources and animal populations are depleted and few leopards remain due to hunting and disease (Bodendorfer et al., 2006). The jaguar is a potential predator of domestic animals in Brazil, including dogs (Leite et al., 2002; Whiteman et al., 2007), but would hardly be dependent upon dogs as a food source, given the abundance of other species. The interaction of domestic dogs with jaguars could still be negative for the native species, as packs of dogs may ambush and transmit pathogens even to large cats (Furtado et al., 2008).
Pathogen transmissionDogs function as parasite and pathogen reservoirs for native animals and human populations. They are potential vectors of distemper, parvovirus, rabies, leishmaniosis and heartworm, which threaten native vertebrate populations. Canine distemper is a viral disease which has been a significant cause of the decline of wild carnivore populations (Appel and Summers, 1995; Cleaveland et al., 2000). The weasel Mustela nigripes was included in the list of threatened species in the United States because the population greatly declined mainly due to distemper (Thorne and Williams, 1988). The best studied carnivore population decline cases are in Africa, where an epidemic of the distemper virus transmitted by dogs killed 30% of a lion (Panthera leo) population in the Serengeti National Park in Tanzania (Roelke-Parker et al., 1996). Contamination by this same virus increased mortality of the African wolf (Lycaon pictus), which was already threatened with extinction (Alexander and Appel, 1994). The frequent contact of wild dogs (Cerdocyon thous) with domestic dogs in an area in the Brazilian Amazon explained the high likelihood of distemper and heartworm transmission (Courtenay et al., 2001). Distemper has been detected in nine jaguars (Panthera onca) in the Ivinhema State Park (Sao Paulo) in the Brazilian Atlantic Forest, representing 60% of its population. Pathogens were possibly transmitted by dogs in the park surroundings, as 100% of the dogs tested positive for canine distemper (Nava et al., 2009). A recent study showed that dogs are more exposed to canine distemper virus and parvovirus in small protected areas than in larger ones, and that exposure was associated with the sex, age, and lack of health care of dogs (Curi et al., 2016). Such studies provide strong evidence to support management actions for the prevention of virus transmission. Studies providing information on factors which explain how domestic dogs become a threat to other species are important to support preventative management actions.
Rabies is another zoonosis caused by a virus and transmitted by domestic dogs to native animals, but corroborating studies are scant. The presence of this virus has been diminishing in domestic dogs while increasing in wild animals, especially in carnivores and bats (Rupprecht et al., 1995; Iamamoto, 2005).
Dogs are the most frequent reservoir (91%) of leishmaniosis protozoans transmitted by phlebotomic mosquitoes, but only 9% of native canids function as reservoirs (Courtenay et al., 2002). The factors facilitating transmission require further studies. Approximately two million people in the world are contaminated by leishmaniosis mosquitoes every year (WHO, 2013). Besides leishmaniosis, heartworm can be conveyed to dogs, native mammals, and humans by a nematode transmitted by mosquitoes (genera Culex, Aedes and Anopheles). Half of the street dogs in the USA (50%) are infected with this parasite (Nayar and Knight, 1999), while in Spain 433 red foxes (Vulpes vulpes) were infected (Gortázar et al., 1994). The prevalence of this pathogen was verified in Brazil in 40% of street dogs in cities in the northeastern region, and 30% in the southeast (Ahid and Lourenço-de-Oliveira, 1999; Labarthe et al., 1998). Advances in research on potential disease transmission by domestic dogs as well as the severity of disease impacts on native vertebrates is essential for the conservation of the latter.
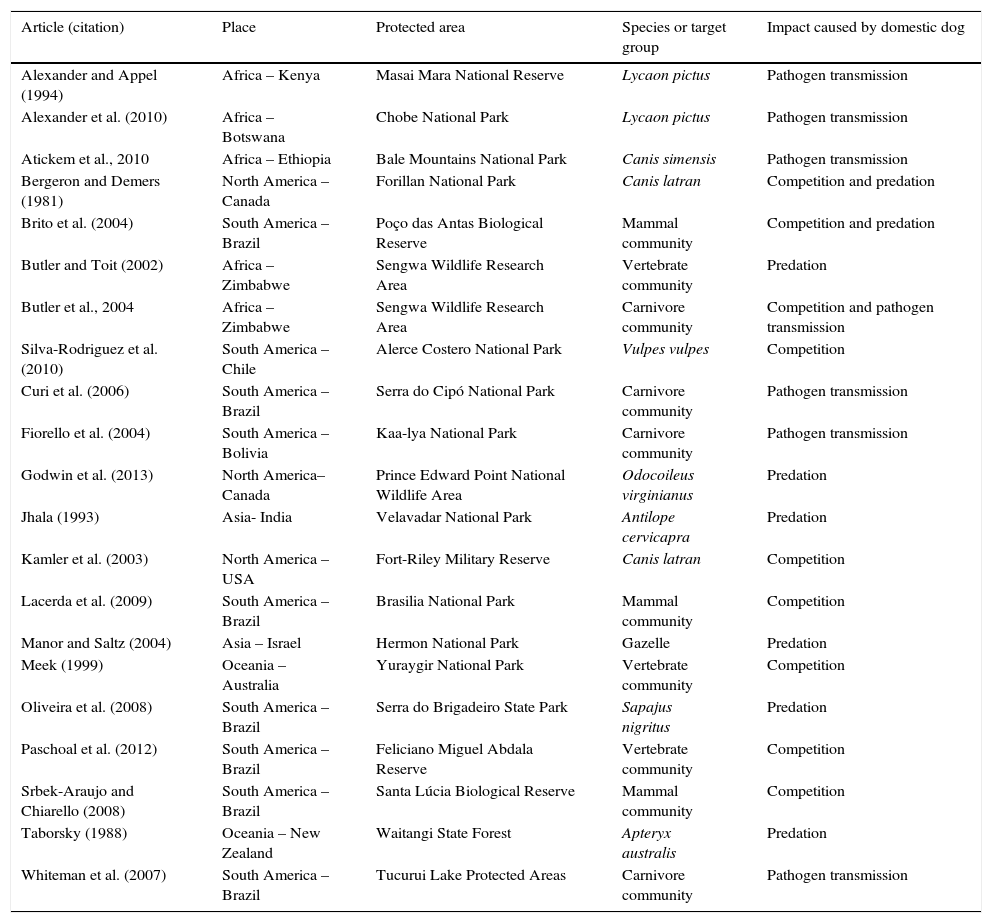
Dogs in protected areasWe assessed 23 papers on dog impacts in protected areas around the world from which three types of interaction stood out (predation, competition, and pathogen transmission; Fig. 1, Annex I). Predation, meaning attacking prey, was registered in nine of the studies assessed. Research work carried out in Brazil shows that predation probably takes place both during the day and at night (Srbek-Araujo and Chiarello, 2008; Galetti and Sazima, 2006). Competition was inferred wherever dogs co-occurred with native species. Moreover, spatial overlap was also considered as an indicator of possible pathogen transmission and predation. The pathogen transmission studies assessed indicate high potential for negative impacts on native animals. Distemper tested positive in 27% of 101 domestic dogs sampled in the Tucurui Environmental Protection Area in the Amazon region (Whiteman et al., 2007). At Serra do Cipó in Minas Gerais 19% of the native canids sampled showed prevalence for the leishmaniosis protozoan, which is very likely transmitted by domestic dogs in rural areas as well as feral dogs (Curi et al., 2006, 2012). Despite the relevance of this issue none of the studies, particularly in Brazil, has assessed control and management alternatives for invasive dogs in protected areas.
The domestic dog survey in protected areas in Brazilian National Parks was implemented using questionnaires directed at park managers by telephone or In this initial phase information was sought based on the following questions: (i) Are there records of domestic dogs (feral or not) inside the park?; (ii) Are there records of dog interaction with native wildlife? (Please give details such as which native species have been registered, type and frequency of interaction, range within the park.); (iii) Which factors facilitate domestic dog entry in the park: residents, visitors, hunters, others? (please specify); (iv) Are there any dog management or control actions in place (which)?; (v) Is there any research concluded or in process about dogs in the park? This approach allowed us to collect basic data to direct future research and management strategies for protected areas.
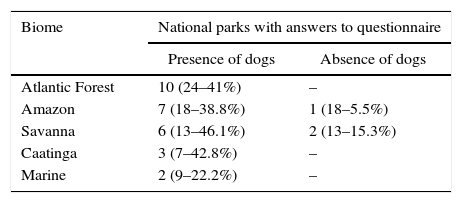
All managers in the 71 national parks in all Brazilian biomes were contacted (Annex III), including marine national parks which include terrestrial areas. Thirty-one park managers answered the questionnaire, 28 (90%) of which corroborated dog presence, and 26 (84%) confirmed existing interactions between dogs and wildlife. No differences in dog presence were observed in national parks between Brazilian biomes (χ2=3.829, p=0.43, gl=4; see Table 1 and Annex 1 for replies from all biomes). Furthermore there was no difference neither in the proportion of national parks with dogs in forest areas, open vegetation, or marine parks (χ2=1.474, p=0.48, gl=2) nor between densely populated biomes and those of lower human population density (χ2=0.207, p=0.88, gl=2). Hunting was reported as a dog pathway of entry for one in every three national parks, and is one of the major factors leading to biodiversity decline, particularly threatening mammals and compromising the effectiveness of protected areas (Chiarello, 2000). As many hunters use dogs to catch the desired prey this becomes an impact intensification factor. In other 11 national parks (40%) residents within and around the parks were responsible for the presence of dogs. The lack of land compensation to private owners upon the establishment of protected areas is a relevant factor in facilitating dog presence, as people remain on their land awaiting payment. There are about six thousand residents inside the Lençóis Maranhenses National Park, a common situation in many others. Although the number of dogs present in protected areas in Brazil has not yet been estimated, their high frequency in the parks assessed in this study indicate that this is a relevant impact factor on biodiversity.
Occurrence of dogs as declared by National Park managers in each biome; “Marine” refers to the terrestrial areas which are included in marine national parks. In total 31 managers answered the questionnaire. The relative percentage of parks with answers on the presence or absence of dogs is given within the total number of parks in each biome.
| Biome | National parks with answers to questionnaire | |
|---|---|---|
| Presence of dogs | Absence of dogs | |
| Atlantic Forest | 10 (24–41%) | – |
| Amazon | 7 (18–38.8%) | 1 (18–5.5%) |
| Savanna | 6 (13–46.1%) | 2 (13–15.3%) |
| Caatinga | 3 (7–42.8%) | – |
| Marine | 2 (9–22.2%) | – |
Among the protected areas with dog records whose managers answered our questionnaire are the following national parks: Cavernas do Peruaçu, Amazonia, Chapada das Mesas, Chapada Diamantina, Chapada dos Guimarães, Serra da Capivara, Serra do Divisor, Serra do Itajaí, Serra dos Órgaos, Emas, Sempre Vivas, Boa Nova, Brasília, Ilha Grande, Pacaás Novos, Saint-Hillaire/Lange, São Joaquim, Catimbau, Jaú, Juruena, Monte Pascoal, Pico da Neblina, Superagui, Lençóis Maranhenses, Itatiaia, Fernando de Noronha, Montanhas do Tumucumaque, and Pau Brasil. National parks where dogs have not been recorded are Chapada dos Veadeiros, Serra da Canastra, and Serra da Cutia.
Potential impact of dogs on biodiversityIn a review by Hughes and Macdonald (2013) 64 wild animal species interacting with dogs were listed, showing expressive impacts on native bird and mammal populations. Sixty-three of these species are part of the IUCN (International Union for Conservation of Nature) Red List of Threatened Species (IUCN, 2012), 33% of which are threatened at the global level. We listed the species with records of interactions with dogs in Brazil as well as interaction types according to the present assessment. Native species status was assessed based on the IUCN Red List (IUCN, 2012) and on the National Official list of Brazilian fauna threatened of extinction (Portaria MMA n° 444 published on December 17, 2014).
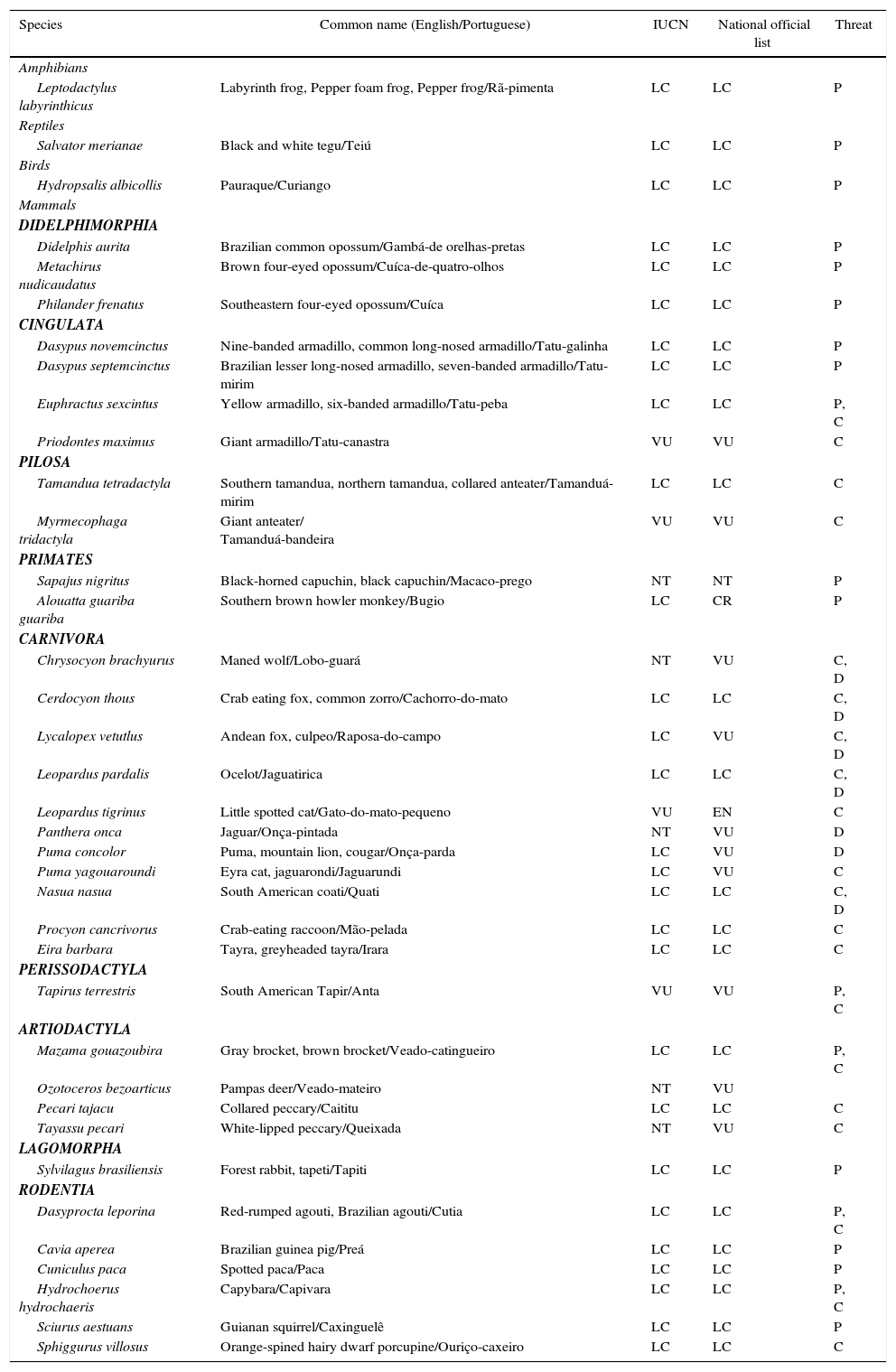
Thirty-seven native vertebrate species were listed from the 23 studies assessed (Table 2). Only three of these species are not mammals, while 85% (27) are medium or large-size mammals (heavier than 1kg). Eight (18%) species are in the IUCN Red List and 19 (55%) are listed as threatened in Brazil. Considering that these species are already severely threatened of extinction by several other factors such as habitat loss and hunting the results of the current assessment bring up strong reasons for concern. Protected areas established in the strict protection category are the last refuge for medium and large-size mammals, particularly in the Atlantic Forest and Savanna biomes in Brazil. As the results of the research papers assessed show by consistent records of predation, competition and pathogen transmission, the presence, invasion, and impacts of dogs in these areas affect the structure of vertebrate communities and contribute to local extinctions.
List of native species reported to interact with domestic dogs cited in studies carried out in Brazilian protected areas. The categories used in the IUCN Red List of Threatened Species and in the National Official Brazil Red List (Portaria MMA n° 444, December 17, 2014) are indicated in the table as well as the type of damage caused by dogs (P, predation; C, competition; D, disease transmission) according to each reference.
| Species | Common name (English/Portuguese) | IUCN | National official list | Threat |
|---|---|---|---|---|
| Amphibians | ||||
| Leptodactylus labyrinthicus | Labyrinth frog, Pepper foam frog, Pepper frog/Rã-pimenta | LC | LC | P |
| Reptiles | ||||
| Salvator merianae | Black and white tegu/Teiú | LC | LC | P |
| Birds | ||||
| Hydropsalis albicollis | Pauraque/Curiango | LC | LC | P |
| Mammals | ||||
| DIDELPHIMORPHIA | ||||
| Didelphis aurita | Brazilian common opossum/Gambá-de orelhas-pretas | LC | LC | P |
| Metachirus nudicaudatus | Brown four-eyed opossum/Cuíca-de-quatro-olhos | LC | LC | P |
| Philander frenatus | Southeastern four-eyed opossum/Cuíca | LC | LC | P |
| CINGULATA | ||||
| Dasypus novemcinctus | Nine-banded armadillo, common long-nosed armadillo/Tatu-galinha | LC | LC | P |
| Dasypus septemcinctus | Brazilian lesser long-nosed armadillo, seven-banded armadillo/Tatu-mirim | LC | LC | P |
| Euphractus sexcintus | Yellow armadillo, six-banded armadillo/Tatu-peba | LC | LC | P, C |
| Priodontes maximus | Giant armadillo/Tatu-canastra | VU | VU | C |
| PILOSA | ||||
| Tamandua tetradactyla | Southern tamandua, northern tamandua, collared anteater/Tamanduá-mirim | LC | LC | C |
| Myrmecophaga tridactyla | Giant anteater/ Tamanduá-bandeira | VU | VU | C |
| PRIMATES | ||||
| Sapajus nigritus | Black-horned capuchin, black capuchin/Macaco-prego | NT | NT | P |
| Alouatta guariba guariba | Southern brown howler monkey/Bugio | LC | CR | P |
| CARNIVORA | ||||
| Chrysocyon brachyurus | Maned wolf/Lobo-guará | NT | VU | C, D |
| Cerdocyon thous | Crab eating fox, common zorro/Cachorro-do-mato | LC | LC | C, D |
| Lycalopex vetutlus | Andean fox, culpeo/Raposa-do-campo | LC | VU | C, D |
| Leopardus pardalis | Ocelot/Jaguatirica | LC | LC | C, D |
| Leopardus tigrinus | Little spotted cat/Gato-do-mato-pequeno | VU | EN | C |
| Panthera onca | Jaguar/Onça-pintada | NT | VU | D |
| Puma concolor | Puma, mountain lion, cougar/Onça-parda | LC | VU | D |
| Puma yagouaroundi | Eyra cat, jaguarondi/Jaguarundi | LC | VU | C |
| Nasua nasua | South American coati/Quati | LC | LC | C, D |
| Procyon cancrivorus | Crab-eating raccoon/Mão-pelada | LC | LC | C |
| Eira barbara | Tayra, greyheaded tayra/Irara | LC | LC | C |
| PERISSODACTYLA | ||||
| Tapirus terrestris | South American Tapir/Anta | VU | VU | P, C |
| ARTIODACTYLA | ||||
| Mazama gouazoubira | Gray brocket, brown brocket/Veado-catingueiro | LC | LC | P, C |
| Ozotoceros bezoarticus | Pampas deer/Veado-mateiro | NT | VU | |
| Pecari tajacu | Collared peccary/Caititu | LC | LC | C |
| Tayassu pecari | White-lipped peccary/Queixada | NT | VU | C |
| LAGOMORPHA | ||||
| Sylvilagus brasiliensis | Forest rabbit, tapeti/Tapiti | LC | LC | P |
| RODENTIA | ||||
| Dasyprocta leporina | Red-rumped agouti, Brazilian agouti/Cutia | LC | LC | P, C |
| Cavia aperea | Brazilian guinea pig/Preá | LC | LC | P |
| Cuniculus paca | Spotted paca/Paca | LC | LC | P |
| Hydrochoerus hydrochaeris | Capybara/Capivara | LC | LC | P, C |
| Sciurus aestuans | Guianan squirrel/Caxinguelê | LC | LC | P |
| Sphiggurus villosus | Orange-spined hairy dwarf porcupine/Ouriço-caxeiro | LC | LC | C |
NT, nearly threatened; LC, least concern; CR, critically endangered; EN, endangered; VU, vulnerable.
Among the papers reviewed only Paschoal et al. (2012) estimated dog abundance in a protected area fragment in Atlantic Forest. Camera traps generated 173 records of 32 domestic dogs in contrast with 13 records of the only native wild canid in the area (Cerdocyon thous) and two ocelot (L. pardalis) records. The highest frequency of records among carnivores belongs to domestic dogs. They were not restricted to the borders of this protected area but were found almost two kilometers inward. Besides representing the most abundant carnivore, the domestic dog was also the fourth most frequent species registered in a Biological Reserve in Atlantic Forest (Srbek-Araujo and Chiarello, 2008).
Records of dog interactions in Brazil were found with a critically endangered species (CR), an endangered one (EN), and ten vulnerable (VU) species according to the national official list (Table 2). Among these species is the maned wolf, the jaguar, and the pampas deer, classified as nearly threatened (NT) at the global level (Table 2). In the revision produced by Hughes and Macdonald (2013) two critically threatened species are wild canids whose populations were reduced by hybridization with domestic dogs. No studies regarding hybridization have been identified so far for Brazil.
Dogs preying on small to large-size animals such as the giant anteater are the major cause of mortality of wild animals in the Brasilia National Park (Lacerda et al., 2009). Dogs are suspected of having contributed to the decline of bush dog (Speothos venaticus, cachorro-do-mato-vinagre) populations in the park. The presence of dogs indicated negative associations with species such as the maned wolf, which was found to be 1.53 times more frequent in areas without dogs, showing an inverse and significant relation (p<0.05) (Lacerda et al., 2009). In the same study the authors proved dogs to be infected with rabies and leishmaniosis. In a visit to the Brasilia National Park in April, 2013 we noted dogs retaining their wild behavior, including formation of packs. One visitor gave up hiking for fear of attack by a pack of five dogs found on the way. A few days later park rangers reported saving a tapir (Tapirus terrestris) ambushed by dogs. The Brasilia National Park has several entry points for dogs due to its urban surroundings with human communities, private condominiums, and a garbage dump. Placed in an urban setting, this park has become an enclave so dog invasion may be considered a border effect (Lacerda et al., 2009) as is the case of other protected areas in a similar context.
The presence of dogs on islands is also a matter of concern. On Ilha Grande, in Rio de Janeiro state, where 80% of the land area is protected by the Ilha Grande State Park, there is evidence of impacts by dogs as well as by domestic cats (Lessa and Bergallo, 2012) on native animals (Fig. 2A). The island's medium-size mammal populations are lower in density on the northern side of the island where human population density is higher (Lessa, 2012). Dogs in wild conditions with lactating bitches have been registered on this side of the island by camera traps more than 3km away from urban areas, which indicates they have become feral (Lessa, 2012; Fig. 2B). Dogs were also the most frequent carnivores registered by camera traps in the entire region. Residents of the island confirm predation by dogs in forest areas as well as frequent contact between dogs and native animals (Lessa, 2012).
Hikers who visit the Ilha Grande State Park and other protected areas may facilitate the entry of dogs (S. Muniz, Park manager, personal communication). Many stray dogs assume the function of guides for visitors using trails on the island. Many of the visitors are fond of this behavior as they feel welcomed by the dogs and enjoy their company. Dogs also have fun in finding native animals and playing hunting games (Fig. 2A). For this reason, interacting with visitors is a great opportunity for dogs whose behavior contributes to biodiversity decline in these last refuges for native animals.
Guidelines for domestic dog management in protected areasDog management plans for Brazilian protected areas should follow procedures generally adopted to reduce invasive alien species impacts. The invasion stage should be identified as a base to decide whether eradication is feasible or population control and impact mitigation actions should be implemented (Richardson, 2011). An action plan was developed in Australia for dog control in several places, not only targeting dingos, which are a specific Australian issue, but also domestic dogs (Allen and Fleming, 2011; Letnic et al., 2012). Based on guidelines defined in this plan and on information obtained in the present study for Brazilian national parks we offer general guidelines to be implemented according to the scope of dog invasion problems in each protected area.
Although management actions may be classified as control, containment, and eradication (Richardson, 2011), an ideal sequence for protected areas would be to (a) assess pathways of species entry; (b) establish an early detection and rapid response system to maximize potential eradication opportunities; (c) apply containment measures when eradication is no longer feasible but invasion is restricted; (d) carry out permanent control work if prevention and early detection are no longer viable either because the invasive species is already widely distributed or because new specimens keep entering the area and cannot be deterred (ex. dogs living in homes around parks). In the case of dogs in protected areas, control measures using integrated management techniques tend to be urgent in order to avoid damaging native species populations. Containment refers to limiting invasive species spread, requiring population monitoring and blocking protected area borders to avoid new entries. Eradication refers to removing or eliminating invasive species from a certain area and is rarely achieved in continental areas, being more feasible on oceanic islands (Database of Island Invasive Species Eradications, 2015). All these measures must consider secondary effects on biological diversity due to invasive species control (Richardson, 2011).
Protected areas are inserted in particular landscape contexts which require particular management strategies. From the information gathered in this study, some trends in dog invasions in protected areas became clear. Protected areas in urban surroundings such as the Brasilia National Park are more exposed to dog entry as well as to more advanced stages of degradation caused by dogs, so their managers should be more concerned with controlling dog density and isolating protected areas from adjacent urbanization. Protected areas in rural areas such as Chapada dos Veadeiros National Park must direct control actions to rural dwellings, focus on raising awareness and use environmental education strategies to prevent dog invasions and disease transmission to native animals. Protected areas managers in remote regions, where human density is low in the surroundings and dog occurrence is scarce, must be attentive to dogs entering with hunters. In any case it is essential to avert dog ownership by residents around protected areas and prevent dogs from accompanying visitors along trails inside protected areas. If no efficient barrier is built to isolate protected areas from surrounding houses and their animals the likelihood of invasion is very high, particularly when densely populated villages or cities are close by. For management purposes it is ideal to register all dogs and houses in the surroundings (datasheet and photographs) so that, if a dog is found in a protected area, the owner can be accountable. Continuous neutering and sterilization campaigns must be promoted to reduce dog populations and avoid increased numbers of stray and feral dogs.
Containment and eradication actions must be defined and carried out in protected areas. The removal of dogs has proved efficient on an oceanic island, where preventing new dog arrivals is more feasible than in continental areas (Morley, 2006). Capture and removal methods using traps and tranquilizers need to be tested and applied while new dog arrivals must be prevented to ensure that control and containment actions are efficient. If new arrivals are not prevented the removal actions will not generate good results. Eradication projects in continental areas do not work well unless previous removal and control actions are undertaken. The elimination of about 700 dogs in the Brasilia National Park in 1995 (ICMBio, 2013) was not efficient because new dogs kept entering the park afterwards, allowing the population to grow again (Horowitz, 2003). Even the removal of 900 dogs from the Park by the Sanitary and Environmental Agency in July, 2001, did not yield the expected results. Dog presence is persistent, and their abundance, high. Dogs living in the surroundings, and especially the approximately 3000 dogs living freely in a garbage dump adjacent to the park, enter the park along at least 40% of its perimeter (ICMBio, 2013), creating constant invasion events. Even owned dogs enter the park from residential condominiums in the surroundings (ICMBio, 2013).
The lack of public awareness in controlling and containing pet reproduction aggravates the problem of dog impacts on native animals (Lessa and Bergallo, 2012). Protected area effectiveness also depends heavily on management and on the attention given to socio-economic issues affecting each area (Drummond et al., 2009). It is not normally feasible that all the people involved in activities with protected areas are aware of its problems impacting biodiversity and management efficiency. Still, dog management is also needed to ensure safety for human health, as it helps control leishmaniosis, rabies, and distemper (Courtenay et al., 2002; Curi et al., 2006). For this reason, as well as all the consequences of disease transmission and severe negative impacts on biodiversity, it is crucial that environmental education programs and pet sterilization and vaccination campaigns are conceived, planned, and implemented in human settlements in the surroundings of protected areas (Jorge et al., 2010; Curi et al., 2014).
The success of dog control plans in protected areas partly depends on raising public awareness. Because dogs (Canis lupus familiaris) are a pet species highly valued by humans there is much conflict of opinion which affects the success of control and eradication programs. Promoting dialog and partnerships between different stakeholders such as residents from the surroundings, park managers, politicians, and representatives of animal protection organizations is essential for society to understand the relevance of dog management plans and gain more support for protected area managers. Population control by dog sterilization and removal is also beneficial for dog well-being as it reduces their chances of getting hurt in fights with other animals and transmitting diseases.
ConclusionDogs constitute a clear anthropogenically derived threat to indigenous species. The presence of dogs in areas with wildlife increases the risk of disease for dogs, humans and wildlife; moreover, the presence of domestic dogs also interferes with the spatial distribution of populations of wildlife species. Studies undertaken in several parts of the world identify predation as the most frequent result of interaction between dogs and wildlife, followed by pathogen transmission. The species identified as enduring the worst impacts from dog interactions in Brazil are those already severely impacted by hunting and habitat loss or fragmentation, especially medium and large mammals. Within this group, carnivores are more threatened by dogs due to competition and disease transmission, which increase the risk of local extinctions. The presence of dogs is a common problem in nearly all national parks (>90%) and very likely in many other protected areas in all Brazilian biomes, regardless of the density of human populations. Similar records were obtained for remote areas in the Amazon region and for densely populated areas in Atlantic Forest. Dogs inflict negative impacts to at least 63 native animal species, including 12 species threatened of extinction.
The presence of dogs in protected areas is associated to other important impact factors such as hunting and failure at land compensation when protected areas are established. Solving such problems will contribute to reducing impacts by dogs in these areas. Evaluating dog abundance and movement patterns in protected areas is important to assess dog impacts on biodiversity at the local scale. Studies to estimate dog abundance and occurrence in protected areas are urgently needed and should be requested by protected area managers to help define immediate prevention, eradication, and control strategies.
Although scientific information on the impacts by dogs in protected areas is scarce and precise diagnostics are difficult to develop, it is important to define and implement general control actions. These will be more effective if established considering the types of dogs present and the level and intensity of their interactions with native animals. Containment, and particularly eradication actions in continental areas, must be carried out only after dog removal. Once strategies are defined according to the peculiarities of each area, implementation should not be delayed to maximize the chances of reducing biodiversity loss.
Conflicts of interestThe authors declare no conflicts of interest.
We thank all the national park managers who contributed to this assessment. ICML thanks CAPES (for the doctoral scholarship financing this study as well as the Biodiversity and Protected Area Laboratory of the Ecology Department at the Federal University of Brasilia and the Mammal Ecology Laboratory at the Rio de Janeiro State University (UERJ). We also thank IUCN and ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) for the online availability of data on threatened species. This study has been rendered legal status by SISBIO permit n° 39768-1.
| Article (citation) | Place | Protected area | Species or target group | Impact caused by domestic dog |
|---|---|---|---|---|
| Alexander and Appel (1994) | Africa – Kenya | Masai Mara National Reserve | Lycaon pictus | Pathogen transmission |
| Alexander et al. (2010) | Africa – Botswana | Chobe National Park | Lycaon pictus | Pathogen transmission |
| Atickem et al., 2010 | Africa – Ethiopia | Bale Mountains National Park | Canis simensis | Pathogen transmission |
| Bergeron and Demers (1981) | North America – Canada | Forillan National Park | Canis latran | Competition and predation |
| Brito et al. (2004) | South America – Brazil | Poço das Antas Biological Reserve | Mammal community | Competition and predation |
| Butler and Toit (2002) | Africa – Zimbabwe | Sengwa Wildlife Research Area | Vertebrate community | Predation |
| Butler et al., 2004 | Africa – Zimbabwe | Sengwa Wildlife Research Area | Carnivore community | Competition and pathogen transmission |
| Silva-Rodriguez et al. (2010) | South America – Chile | Alerce Costero National Park | Vulpes vulpes | Competition |
| Curi et al. (2006) | South America – Brazil | Serra do Cipó National Park | Carnivore community | Pathogen transmission |
| Fiorello et al. (2004) | South America – Bolivia | Kaa-lya National Park | Carnivore community | Pathogen transmission |
| Godwin et al. (2013) | North America–Canada | Prince Edward Point National Wildlife Area | Odocoileus virginianus | Predation |
| Jhala (1993) | Asia- India | Velavadar National Park | Antilope cervicapra | Predation |
| Kamler et al. (2003) | North America – USA | Fort-Riley Military Reserve | Canis latran | Competition |
| Lacerda et al. (2009) | South America – Brazil | Brasilia National Park | Mammal community | Competition |
| Manor and Saltz (2004) | Asia – Israel | Hermon National Park | Gazelle | Predation |
| Meek (1999) | Oceania – Australia | Yuraygir National Park | Vertebrate community | Competition |
| Oliveira et al. (2008) | South America – Brazil | Serra do Brigadeiro State Park | Sapajus nigritus | Predation |
| Paschoal et al. (2012) | South America – Brazil | Feliciano Miguel Abdala Reserve | Vertebrate community | Competition |
| Srbek-Araujo and Chiarello (2008) | South America – Brazil | Santa Lúcia Biological Reserve | Mammal community | Competition |
| Taborsky (1988) | Oceania – New Zealand | Waitangi State Forest | Apteryx australis | Predation |
| Whiteman et al. (2007) | South America – Brazil | Tucurui Lake Protected Areas | Carnivore community | Pathogen transmission |