Habitat loss has major impacts on biodiversity. Yet, such impacts are not always linear, as there can be threshold values of habitat amount below which species become extirpated from human-modified landscapes (extinction thresholds). This may be particularly the case for species with high habitat spatial requirements, especially in regions with a long land-use history, which have a lower extinction debt. To address these issues, we evaluated the linear and non-linear effects of landscape-scale forest (habitat) loss on primate species richness in regions with relatively new (Amazon) and old (Atlantic Forest) histories of land-use change. We also evaluated the role of mean home range size in regulating species responses to forest loss. Extinction thresholds were higher in the Atlantic Forest (78% remaining forest cover) than in the Amazon (45%), but primate-landscape associations were stronger in the Amazon. Thus, despite its recent land-use history, Amazon primates are more sensitive to habitat loss. As predicted, mean home range size decreased with forest loss in both biomes. Our findings highlight the importance of stopping deforestation in both biomes to maintain habitat amount above these thresholds. Yet, as <30% of the Atlantic Forest cover remains today, promoting restoration initiatives across this biome is paramount.
The growing demand for new agricultural lands is causing the annual loss of millions of hectares of old-growth tropical forests (Global Forest Watch, 2021). Such a massive forest loss is a well-known driver of species extinction in human-modified landscapes (Fahrig, 2003; Newbold et al., 2016). Nevertheless, population decline (and extinction) is not always directly proportional to the amount of forest that is lost (i.e., linear relationship). In many cases, there can be threshold values of forest cover (i.e., habitat amount) below which populations decline abruptly, increasing their probability of local extirpation (“extinction threshold”; Lande, 1987; Andrén, 1994; Swift and Hannon, 2010). Although empirical studies suggest that most species require at least 10-30% of habitat amount in a landscape for their survival (Andrén, 1994; Swift and Hannon, 2010), relatively higher thresholds (30–50%) are also commonly documented (Swift and Hannon, 2010; Arroyo-Rodríguez et al., 2021; Brindis-Badillo et al., 2022). Therefore, additional studies on this topic are needed to know how much habitat should be preserved in a landscape to prevent species extirpation in human-modified landscapes (Arroyo-Rodríguez et al., 2020, 2021; Banks-Leite et al., 2021).
Surprisingly, the impact of habitat loss is not always negative, as some species are able to tolerate some degree of habitat loss and crowd in the remaining habitat patches (Ewers and Didham, 2006). Because of this “crowding effect”, the density of some animals can be higher in landscapes with lower habitat amount (Gestich et al., 2021). The sensitivity of species to habitat loss may depend on intrinsic factors such as their life-history traits (Newbold et al., 2013). Some studies highlight the importance of home range size in predicting species’ sensitivity to habitat loss (Mönkkönen and Reunanen, 1999; Newbold et al., 2013), as species with large home ranges tend to have large group sizes and specialized diets (Milton and May, 1976), which can increase their sensitivity to habitat loss and disturbance (Keinath et al., 2017). Species with small home ranges, instead, tend to have more generalist and/or folivorous diets, and smaller group sizes, decreasing their spatial needs (Milton and May, 1976; Johns and Skorupa, 1987). However, the importance of home range size in regulating extinction thresholds is not well understood.
The degree and history of land-use change at the regional scale can also drive the response of species to habitat loss (Andrén, 1994; Metzger et al., 2009; Galán-Acedo et al., 2021). For example, there is evidence that species diversity decreases in smaller habitat patches, but only when located in regions with intermediate percentage of habitat amount (30–50%), where there is a higher variation in patch size (Pardini et al., 2010). Other studies argue that patch size effects are stronger in highly modified regions (<30% of habitat amount; Andrén, 1994), where interpatch isolation distance is typically higher (Fahrig, 2003). Such increase in isolation can decrease landscape connectivity (Goodwin and Fahrig, 2002), potentially increasing the risk of dispersal mortality (Fahrig et al., 1995; Sreekar et al., 2015). Regarding the importance of land-use history, there is evidence that habitat loss effects may go undetected if studied in recently modified regions, especially in long-lived organisms such as primates, which respond slowly to environmental fluctuations, potentially causing time-delayed extinctions (Metzger et al., 2009). Nevertheless, the influence of the regional context in regulating species responses to habitat loss is poorly known.
Understanding the impact of habitat loss is particularly valuable for primates, as they are one of the world’s most at risk taxa (Estrada et al., 2017). Primates are of critical importance for ecosystem functioning, acting as herbivores, seed dispersers, pollinators, predators and preys, and even as ecosystem engineers (Estrada et al., 2017; Andresen et al., 2018). Therefore, preserving primates implies preserving many important ecological processes. However, ∼66% of primate species are threatened with extinction and ∼75% have declining populations (IUCN, 2023) mostly due to habitat loss, which restricts their distribution and abundance in anthropogenic landscapes (Galán-Acedo et al., 2019c). Unfortunately, the few studies that assess the effect of landscape-scale habitat loss on primates are typically focused on linear effects (Galán-Acedo et al., 2019c), thus overlooking the potential existence of extinction thresholds. Identifying such extinction thresholds is of critical importance to design human-modified landscapes of high conservation value for primates (Arroyo-Rodríguez et al., 2020, 2021).
Here, we evaluated the linear and non-linear effects of landscape-scale forest loss on primate species richness and the associations between forest loss and mean home range size in Brazil – the world’s richest country in primate species (131 out of 515 species, https://icmbio.gov.br/) and one of the countries with highest deforestation rates worldwide (>28 million hectares of old-growth forest in the last two decades; Global Forest Watch, 2021). We compiled information for 85 sites distributed in the Amazon and the Atlantic Forest, two biomes with relatively new and old histories of land-use change, respectively. As all Brazilian primates are arboreal and forest-dependent species (Galán-Acedo et al., 2019b), forest loss can be considered a good proxy of habitat loss. We predict that species richness and mean home range size are negatively related to forest loss in both biomes. These relationships are likely weaker in the Amazon, which maintains forest cover and has a shorter land-use history, so there could be a higher number of time-delayed extinctions (i.e., higher extinction debt).
MethodsStudy designWe used a site-landscape study design (sensuMcGarigal & Cushman, 2002), as the response variables (i.e., primate species richness, and mean home range size) were measured in different sites (i.e., transects) within forest patches, and the predictor variable (i.e., forest cover) was measured within a specified radius from each focal site. This study design is widely considered adequate to make accurate landscape-scale inferences (McGarigal & Cushman, 2002; Galán-Acedo et al., 2019c).
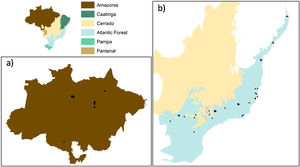
Data compilationWe compiled a database including scientific and grey literature by performing a search of studies reporting primate species richness in Brazilian forest patches (Fig. 1), using SCOPUS (www.scopus.com) and Google Scholar (https://scholar.google.com/). We conducted the search on April 22nd 2020, for literature containing the following terms: [(primate* OR monk*) AND (‘richness’ AND/OR ‘diversity’ AND/OR ‘community’ AND/OR ‘presence’ AND/OR ‘occurrence’ AND/OR ‘fragment’ AND/OR ‘patch’ AND/OR ‘fragmentation’ AND/OR ‘landscape’)]. In addition, we included data on species richness in forest patches from the ATLANTIC-PRIMATES database (Culot et al., 2019).
To adequately assess the effects of forest loss on species richness, we selected studies performed in sites immersed in fragmented landscapes. We only included studies that reported accurate geographic coordinates (i.e., <500m error) and that disclosed the kilometers walked within transects as a measure of sampling effort to be controlled in the models. Finally, we excluded the study sites located in biomes with less than 8 sites (i.e., Pampa and Caatinga biomes) to avoid overfitting. To increase the independence among samples, we also excluded the study patches in which the largest landscape buffer (i.e., 2,500-m radius, see below) overlapped with another study landscape. In total, we selected 85 forests sites from 22 studies reporting primate species richness in three biomes: Amazon forest (26 sites), Atlantic Forest (42) and Cerrado (17). However, as sites from the Cerrado were located very close (25km away) to the Atlantic Forest, in the ecotone between these two biomes (Fig. 1), we considered the Cerrado sites as part of the Atlantic Forest. Yet, we included the analyses of the Atlantic Forest sites (excluding the 17 Cerrado sites) in the Supplementary Material, see Supplementary results Fig. S1 and Table S1).
Study biomesThe Amazon and Atlantic Forest have different degree and history of deforestation. The Amazon forest is the most extensive tropical rainforest in the world (Silveira et al., 2022). Land use change in the Amazon started in the 1970s, and ∼78% of forest cover remains today (Silveira et al., 2022). The Atlantic Forest has a longer history of deforestation dating back to the Portuguese colonization in the 16th century (Dean, 1997). Currently, only ∼28% of their native forest cover remains (Silveira et al., 2022).
Response variablesWe recorded the total number of primate species and mean home range size (ha) per forest patch, excluding alien species. Nocturnal species were also excluded because most studies did not perform nocturnal surveys. We also excluded hybrids; only parental species were included. We recorded the home range size of all 31 primate species included in the present study from Galán-Acedo et al. (2019b), and then calculated the mean home range size per study site. We selected this ecological trait because it is usually considered a good predictor of species’ sensitivity to forest loss and disturbance (Mönkkönen and Reunanen, 1999; Boyle and Smith, 2010).
Forest coverAs all Brazilian primates are forest-dependent species, we used forest cover as a proxy of habitat amount. We measured forest cover based on a 30-m resolution map of Brazil provided by MapBIOMAS (Collection 5; Souza and Azevedo, 2017). Forest cover included the following MapBIOMAS-Collection 5 categories: forest (1), which includes the class natural forest (1.1) (i.e., forest formation (1.1.1), savanna formation (1.1.2), and mangroves (1.1.3)), and forest plantations (1.2) (Souza and Azevedo, 2017). For each study site, we used the map corresponding to the year when fieldwork was conducted (from 1995 to 2017). We calculated landscape forest cover using ArcGIS 10.5 software. In particular, we calculated forest cover within 11 different spatial scales (i.e., buffers) ranging from 500 to 2500-m radius (at 200-m intervals) from the center of each study site (i.e., transect). Following Jackson and Fahrig (2015), the largest landscape was much larger than the maximum home range size of the studied species (∼846ha, Sapajus xanthosternos; Galán-Acedo et al., 2019b).
Statistical analysesTo evaluate the linear effect of forest cover on primate species richness and mean home range size in each biome, we performed generalized linear models (GLM). In the first case, we fixed a Poisson error distribution, which provides an adequate representation of the variability observed in count data (Crawley, 2012). Yet, as recommended for continuous response variables (Crawley, 2012), mean home range size was assessed with a Gaussian distribution. The non-linear responses were modeled with a four-parameters logistic regression, which follow a sigmoidal or “s” shaped curve that makes them appropriate to find zones of inflection points, which represents extinction thresholds (Ficetola and Denoel, 2009). Such models have been used for assessing ecological thresholds previously (e.g., Morante-Filho et al., 2015). In each region, we used the same dataset (same landscapes and response variables) to perform the linear and the logistic models.
As forest loss effects may go undetected if studied at the wrong scale, we identified the so-called “scale of effect” (Jackson and Fahrig, 2015). For each dataset, we fitted 11 linear models (one per scale) and 11 logistic models and selected the linear and non-linear model with highest goodness-of-fit (i.e., pseudo-R2) among the 11 scales considered. We then calculated the Akaike information criterion corrected for small samples (AICc) to compare the best linear model and the best logistic model with the null model (i.e., including only the intercept), and considered the models with the lowest AICc as the best model (Burnham and Anderson, 2002). In all models, we included the sampling effort (i.e., km walked in each study site) as a covariate to avoid its potential confounding effect. We performed all statistical analyses and plots with R 4.0.4.
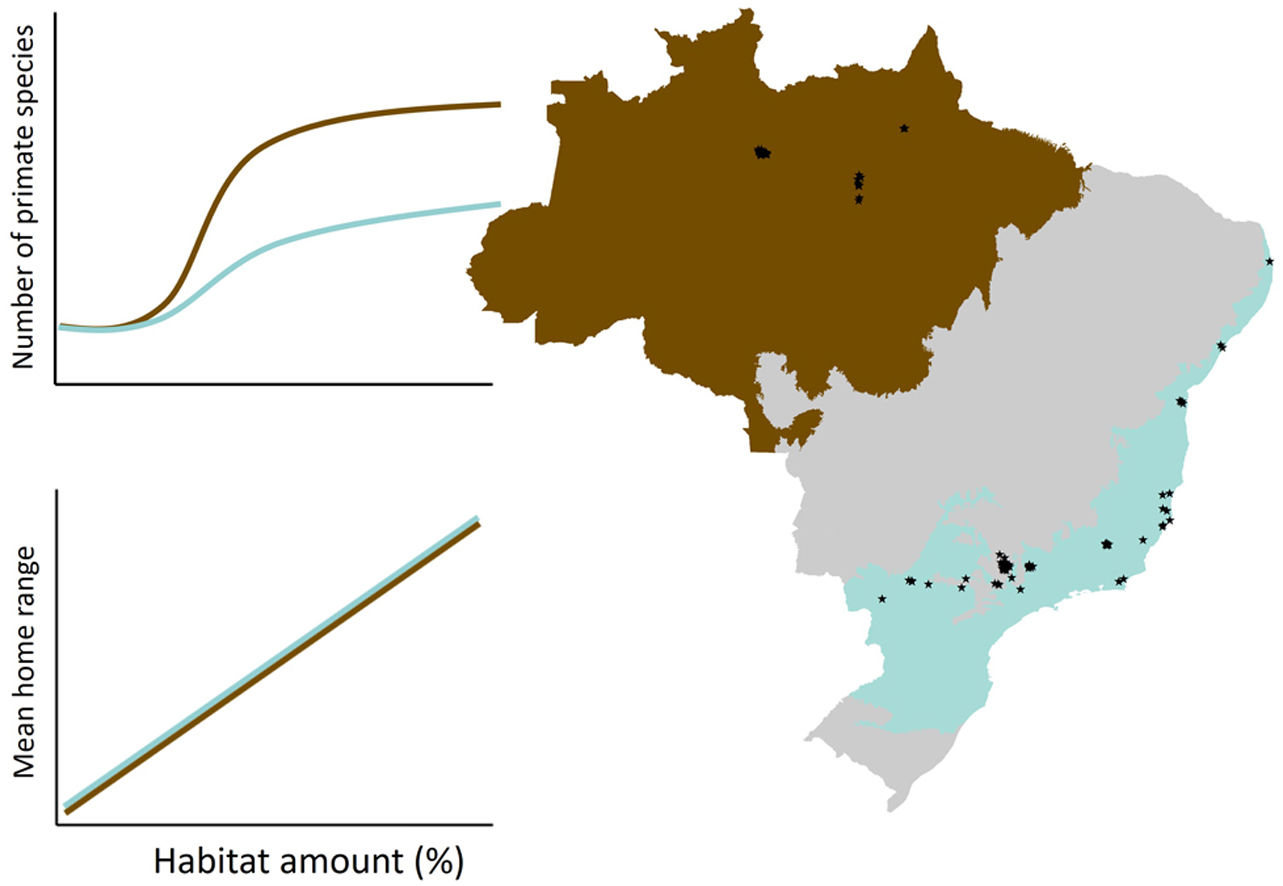
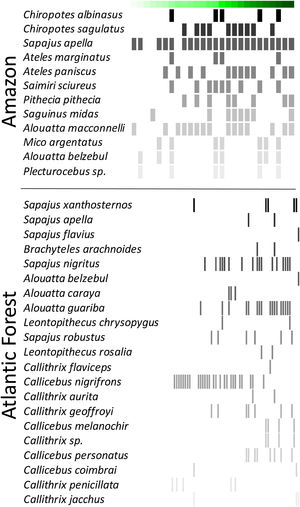
ResultsPrimate communityWe recorded 31 primate species in 80 of the 85 forest sites (94%), 12 species in the Amazon and 21 species in the Atlantic Forest (Fig. 2). Amazon primates occupied 25 of 26 (96%) study sites, whereas in the Atlantic Forest primates occurred in 55 of 59 sites (93%). Species richness ranged from 0 to 7 species (mean=4.4 species) in the Amazon, and from 0 to 5 species (mean=2 species) in the Atlantic Forest. Home range size ranged from 8 to 605ha in Amazon primates and from 3.6 to 846ha in the Atlantic Forest (Fig. 2; Table S2 in Supplementary Material). Four species (i.e., Sapajus apella, Alouatta macconnelli, Alouatta belzebul and Plecturocebus sp.) in the Amazon, and one species (i.e., Callicebus nigrifrons) in the Atlantic Forest were ubiquitous, occurring in landscapes with a wide range of forest cover (Fig. 2). In contrast, some Atlantic Forest species (e.g., Sapajus flavius, Alouatta belzebul and Callithrix flaviceps) were only recorded in more forested landscapes, whereas Callithrix penicillata was recorded in intermediate to highly deforested landscapes in the Atlantic Forest and no Amazon species was exclusively recorded in highly deforested landscapes (Fig. 2).
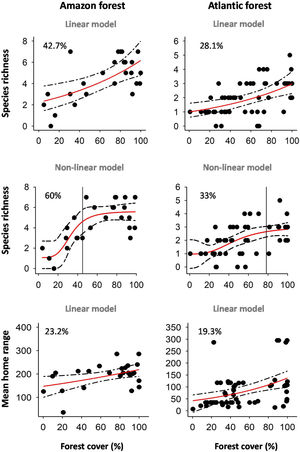
Primate responses to forest coverThe linear and/or non-linear models showed higher empirical support than the null models (Table 1) and indicated that primate species richness and mean home range size increase with forest cover in both biomes (Fig. 3). In all cases, the non-linear models showed higher goodness-of-fit (pseudo-R2) than the linear model, and suggest that extinction thresholds are higher in the Atlantic Forest (78% remaining forest cover; Fig. 3b) than in the Amazon (45%; Fig. 3e). However, all primate-landscape associations were stronger (higher goodness-of-fit) in the Amazon than in the Atlantic Forest (Table 1).
Linear vs. non-linear effects of forest cover on primate species richness and mean home range in the Amazon and Atlantic Forest, Brazil. The linear effect was assessed with generalized linear models (GLM), and the non-linear effect with logistic models. We ranked the models from the lowest to the highest Akaike information criterion corrected for small samples (AICc), and indicated the Akaike weight (wi) and goodness-of-fit (pseudo-R2) in percentage.
| Biome | Taxon | Modela | AICc | ΔAICc | wi | pseudo-R2 |
|---|---|---|---|---|---|---|
| Amazon | Community | GLM700 | 105.7 | 0.0 | 0.62 | 42.7 |
| Logistic1100 | 106.8 | 1.1 | 0.36 | 60.0 | ||
| Null | 112.5 | 6.8 | 0.02 | 0 | ||
| Mean home range | GLM500 | 272.8 | 2.3 | 0.21 | 23.2 | |
| Null | 273.9 | 3.4 | 0.12 | 0 | ||
| Atlantic Forest | Community | Logistic1100 | 177.6 | 0.0 | 0.80 | 33.0 |
| GLM900 | 180.4 | 2.8 | 0.19 | 28.1 | ||
| Null | 188.4 | 10.8 | <0.01 | 0 | ||
| Mean home range | GLM1300 | 628.6 | 2.6 | 0.10 | 19.3 | |
| Null | 635.8 | 9.8 | <0.01 | 0 |
Linear and non-linear effects of landscape-scale forest cover (i.e., habitat amount) on primate species richness and mean home range size in the Amazon and Atlantic Forest, Brazil. We show both the linear models and the non-linear (logistic) models. The pseudo-R2 (in percentage) is indicated in each panel. Lines indicate the extinction thresholds based on the inflection points. Note that forest cover is different in linear and non-linear models because they showed different scales of effect (see details in Table 2).
This study evaluates the linear and non-linear effects of landscape-scale forest loss (i.e., habitat loss) on primate species richness in two biomes (the Amazon and Atlantic Forest) with different land-use history, and the role of home range size in regulating such effects. Our findings support the hypothesis that habitat loss has negative impacts on primates, as in both biomes, primate species richness decreased in more deforested landscapes. The non-linear models showed higher goodness-of-fit (pseudo-R2) than the linear models, suggesting that there could be extinction thresholds in the Amazon and Atlantic Forest. In particular, extinction thresholds tended to be higher in the Atlantic Forest (78% remaining forest cover) than in the Amazon (45%). Nevertheless, contrary to our expectations, primate-landscape relationships were stronger in the Amazon, thus suggesting that despite its recent history of land-use change, Amazon primates are likely more sensitive to habitat loss. Importantly, home range size seems to play a major role in regulating primate responses to habitat loss, as species with mean home range decreased with forest loss in both biomes.
As expected, all associations with forest cover were positive in both biomes. Positive responses to forest cover are hardly surprising, as both resource availability and connectivity are expected to increase in more forested landscapes (Fahrig, 2003). In fact, there is ample evidence on the pervasive impact of habitat loss of different organisms (Fahrig, 2003; Newbold et al., 2016), and primates are no exception (reviewed by Galán-Acedo et al., 2019c). Yet, species responses to habitat loss are not always linear (i.e., proportional). Our findings suggest the existence of extinction thresholds in the Amazon and Atlantic Forest. Although the number of species decreased linearly with forest loss in both biomes, non-linear models showed higher goodness-of-fit. These non-linear trends were stronger in the Amazon than in the Atlantic Forest, where we found a decrease in the number of species in landscapes with <45% of forest cover. This is somewhat surprising, as given the relatively high remaining forest cover in this biome, its relatively short history of land-use change, and primates’ long lives (Jones, 2011), we expected weaker responses to forest loss in the Amazon. However, there are also many studies indicating that this biome is extremely sensitive to contemporary human disturbances (Ochoa-Quintero et al., 2015), so our findings add novel evidence for primates.
Importantly, the extinction thresholds found for the Amazon are consistent with those reported for other organisms in the Amazon (30–40%: Ochoa-Quintero et al., 2015) and other ecosystems (40%: Brindis-Badillo et al., 2022; 46%: Morante-Filho et al., 2015). But why forest loss showed a relatively weaker effect on Atlantic Forest primates? We think that the extremely high deforestation degree and widespread defaunation level across this biome have probably caused the impoverishment of primate assemblages in most sites, potentially weakening primate-landscape associations. Alternatively, primate species may be using other elements available in the matrix to move across the landscape and persist in highly modified regions (Anderson et al., 2007; Galán-Acedo et al., 2019a), decreasing their dependency on the remaining habitat. This is an interesting avenue for future research.
Consistent with our hypothesis, mean home range size was negatively related to forest loss. This indicates that this ecological trait is an important predictor of primate responses to habitat loss, as suggested in previous works (Mönkkönen and Reunanen, 1999; Boyle and Smith, 2010). This is likely caused by the fact that species with smaller home ranges usually live in smaller groups and have less specialized diets (Milton and May, 1976), which may decrease the amount of habitat needed for their survival. This is, for example, the case of Plecturocebus sp., Alouatta belzebul and Callithrix penicillata, species that occurred in landscapes with a wide range of forest cover, including highly deforested landscapes. In contrast, the high spatial requirements of species with large home ranges can force them to use frequently the anthropogenic matrix in more deforested landscapes. Although this behavior may be valuable for obtaining some supplementary resources (reviewed by Galán-Acedo et al., 2019a), it can also expose primates to numerous threats, such as hunting (Sreekar et al., 2015) and road-kill (Fahrig et al., 1995), making them more prone to extinction. Therefore, our results indicate that the conservation of species with small home ranges is likely less challenging than conservation of species with large home ranges.
Implications for conservationOur findings indicate that to prevent primate extinction in the Amazon and Atlantic Forest, stopping forest loss is paramount. In particular, we should maintain the percentage of forest cover above the extinction thresholds, which is a great challenge in some regions of the Atlantic Forest, where most of the original forest cover has disappeared (Banks-Leite et al., 2021). Yet, it is important to note that, although higher, the extinction thresholds in the Atlantic Forest were not as clear as in the Amazon. Therefore, rather than focusing conservation efforts on extremely difficult (if not impossible) goals (Banks-Leite et al., 2021), preserving all remaining forests in this biome and focusing on effective restoration strategies is paramount, especially in top priority areas (Crouzeilles et al., 2019; Strassburg et al., 2020). This is particularly urgent for species with large home range requirements in both biomes, such as Sapajus xanthosternos, S. flavius and Ateles marginatus, which are all threatened with extinction (IUCN, 2022). The implementation of these measures will not be only critical to prevent primate extinction, but to maintain their ecological roles in the ecosystem, thus contributing to maintaining ecosystem functioning.
Conflict of interest statementThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to affect the work reported in this paper.
CG-A and MM-R received a postdoctoral scholarship from DGAPA-UNAM and support from Idea Wild. Part of the writing was done while CG-A was on a postdoctoral stay at Carleton University, funded by the Natural Sciences and Engineering Research Council of Canada.