Riparian deforestation may strongly affect stream functioning, with consequences for biodiversity and ecosystem services. These effects can be assessed using bioindicators relating to biotic community structure and ecosystem functioning. We evaluated the effects of riparian deforestation on 1. measures of community structure using aquatic benthic invertebrates, and 2. an aspect of ecosystem functioning, aquatic leaf processing. We selected sites along gradients of riparian land use in four Atlantic rainforest streams and measured physical and chemical properties for their association with riparian deforestation. We sampled benthic invertebrates and calculated metrics of community structure at each site. We measured rates of leaf processing using leaves of a common riparian tree, Guarea guidonia. Riparian deforestation was accompanied by increasing concentration of ammonia, water current and temperature and decreasing nightly oxygen saturation. Invertebrate diversity decreased and community metrics changed with deforestation as expected of negative impacts. Leaf processing decreased with deforestation. Although there were significant differences in physical and chemical measurements among streams, the gradients in community and ecosystem responses were similar, thus suggesting that both types of bioindicators were useful for monitoring changes and relating them to loss of biodiversity and ecosystem function.
Lotic ecosystems provide a diverse suite of ecosystem services but are highly vulnerable due to their landscape position which integrates ecological stressors across their catchments (Vörösmarty et al., 2010). Streams and rivers are energetically coupled with their surrounding vegetation, which controls light availability for instream photosynthesis and the supply of organic matter in the form of terrestrial plant production (Webster and Benfield, 1986). Linkages between riparian and stream conditions are often most intense in small headwater streams and deforestation in these reaches can cause severe changes in ecosystem structure and function (Richardson and Danehy, 2007).
In forested headwater streams, allochthonous organic matter in the form of leaf inputs constitutes an important food resource and substrate for benthic invertebrates (Wallace and Eggert, 2009). Microorganisms (principally hyphomycete fungi) colonize leaves and use leaf carbon to support growth and respiration, which enhances leaf palatability for detritivores (France, 2011). Stream invertebrates also colonize leaves promoting their breakdown through fragmentation and ingestion, especially after initial microbial colonization (Gessner et al., 1999).
In the tropics, our understanding of the importance of riparian vegetation for streams lags behind what we know about their temperate counterparts, particularly in the context of how their alteration affects ecosystem processes. In the Atlantic Forest, headwater streams represent a large proportion of river length and have largely avoided the impact of human activity, allowing them to serve as biodiversity reservoirs capable of supplying colonists to downstream reaches (Oliveira, 2011). These streams also influence the downstream delivery of nutrients and organic matter with important implications for the maintenance of ecological integrity of reaches lower in the network (Benstead and Leigh, 2012).
A full inventory of ecosystem responses to environmental change is a huge task and is seldom undertaken. Instead, the intactness and functioning of the impacted ecosystem is usually assessed by measurement of indicator organisms and processes. Within these we can broadly separate two classes of measurement: those associated with structural aspects of the biological community (biodiversity, bio-indicator organisms) and those relating directly to ecosystem processes (metabolism, nutrient cycling, decomposition). Indicator organisms are potentially sensitive to environmental changes and may differentially indicate the stressor (Rosenberg and Resh, 1993; Baptista et al., 2006; Oliveira et al., 2011). On the other hand, changes in ecosystem processes may indicate profound impact on the functioning of the ecosystem and the provision of ecosystem services (Gessner and Chauvet, 2002; Fellows et al., 2006; Bunn et al., 2010; Silva-Junior et al., 2014).
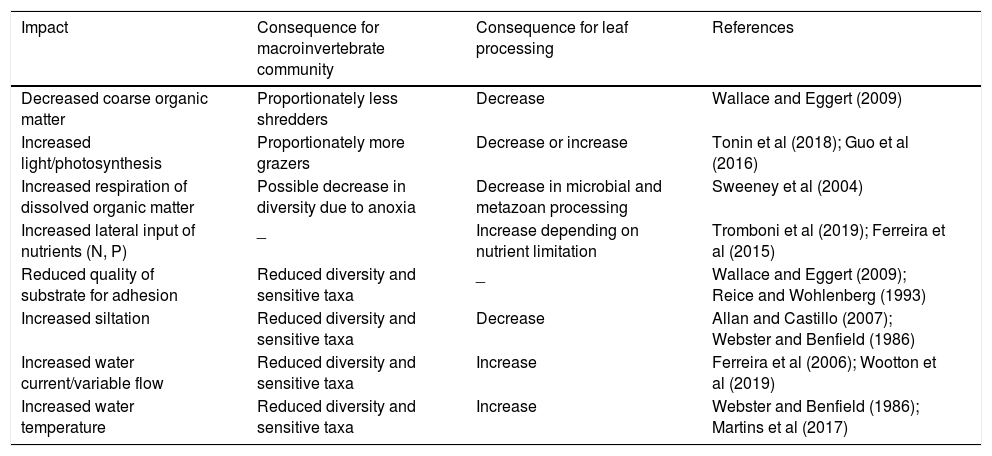
Land is often transformed from forests into pasture and agriculture causing large changes in the land-to-stream interface and the aquatic ecosystem. Because allochthonous organic input to the river is relatively large in forested streams, we expect that its removal will cause changes in the processes of decomposition of coarse organic matter (CPOM) and the organisms associated with CPOM (Casotti et al., 2015; Tanaka et al., 2015). Other changes are expected to be brought about by exposing the stream to higher levels of light, which can have negative effects on decomposition due to inhibition of fungi and associated fauna (Tonin et al., 2018) or positive effects if macroinvertebrates are stimulated by high-quality associated algae (Guo et al., 2016). Other possible effects of riparian vegetation loss can be caused by larger lateral inputs of nutrients, fine and dissolved organic and inorganic matter (Table 1).
Expected impacts caused by riparian deforestation on aquatic benthic macroinvertebrate community and leaf processing.
| Impact | Consequence for macroinvertebrate community | Consequence for leaf processing | References |
|---|---|---|---|
| Decreased coarse organic matter | Proportionately less shredders | Decrease | Wallace and Eggert (2009) |
| Increased light/photosynthesis | Proportionately more grazers | Decrease or increase | Tonin et al (2018); Guo et al (2016) |
| Increased respiration of dissolved organic matter | Possible decrease in diversity due to anoxia | Decrease in microbial and metazoan processing | Sweeney et al (2004) |
| Increased lateral input of nutrients (N, P) | _ | Increase depending on nutrient limitation | Tromboni et al (2019); Ferreira et al (2015) |
| Reduced quality of substrate for adhesion | Reduced diversity and sensitive taxa | _ | Wallace and Eggert (2009); Reice and Wohlenberg (1993) |
| Increased siltation | Reduced diversity and sensitive taxa | Decrease | Allan and Castillo (2007); Webster and Benfield (1986) |
| Increased water current/variable flow | Reduced diversity and sensitive taxa | Increase | Ferreira et al (2006); Wootton et al (2019) |
| Increased water temperature | Reduced diversity and sensitive taxa | Increase | Webster and Benfield (1986); Martins et al (2017) |
Recent work has demonstrated that leaf decomposition in tropical streams may respond significantly to changes in vegetation structure (Casotti et al., 2015; Figueiredo et al., 2018) even when there may be no evident response of more traditional variables like those describing the invertebrate community structure (Silva-Junior and Moulton, 2011; Silva-Junior et al., 2014). The more traditional view was that sensitive taxa of macroinvertebrates would indicate environmental impairment at much lower levels of impact than ecosystem-level properties (Reice and Wohlenberg, 1993; Rosenberg and Resh, 1993).
The objective of this study was to evaluate how riparian deforestation affects 1. measures of community structure using aquatic benthic invertebrates, and 2. an aspect of ecosystem functioning, aquatic leaf processing. We expected that riparian deforestation would affect measures of community structure to indicate adverse impacts (decrease in biodiversity, sensitive species, etc., as indicated in Table 1). We expected that aquatic leaf processing would decrease, although some factors associated with riparian vegetation loss might increase processing (Table 1). We sought to explain the observed changes by correlating them with measurements of environmental factors (such as nutrients) but our design did not allow us to isolate the effects of the different, simultaneous, changes (Table 1).
Material and methodsStudy area, land cover characterization and environmental variablesThe study was conducted in 28 sites distributed among four streams, Anil River (ANL), Santa Maria River (STM), Mariquita River (MRQ) and Itaperiti River (ITA), located in the Guapiaçu River basin, Rio de Janeiro, Brazil (Figure S1). We studied 7 sites in each stream along a deforestation gradient, ranging from intact forest to pasture and croplands; a comprehensive description of the riparian vegetation is found in (Azevedo, 2012; Azevedo et al., 2018). Riparian land cover was characterized using satellite images and field assessment. We used ArcGIS software (ESRI, Redlands, CA, EUA) and a supervised classification scheme to estimate the proportion of deforested area in a 30-m wide riparian corridor in the 300 m immediately upstream of each sampling site. We measured a set of physical and chemical variables at each site including: water temperature, depth, discharge, width, water current velocity, pH, minimum daily O2 saturation, canopy cover, and ammonium (NH4+) and soluble reactive phosphate (SRP) concentration. Details of methodology used are presented in supplementary material. The leaf-pack deployment (below) and measurement of stream characteristics were carried out in August-September 2013. The sampling of stream invertebrates was carried out in September-October 2015. We assumed that the characteristics of the sampled sites stayed constant over this period; we observed no changes.
Benthic invertebrate faunaWe sampled stream benthic invertebrates at each sampling site using a D-frame net with a 250-μm mesh and collecting 20 samples by disturbing 1-m long segments in front of the net. Samples were divided into 8 parts and at least 300 cumulative invertebrates were collected from a randomly-selected sub-sample.
Invertebrates were identified to the level of family except leaf-mining chironomids (genus), Collembola (order) and Acari (order). We classified the invertebrate community into functional feeding groups (e.g. shredders, collectors) using published information (Table S2), and calculated community metrics: invertebrate family richness, Shannon diversity of families, percentage of shredders among all invertebrates by abundance, percentage of Ephemeroptera, Plecoptera and Trichoptera (%EPT) among all invertebrates by abundance, and percentage of Chironomidae among Diptera.
Leaf decomposition ratesWe quantified leaf decomposition rates using Guarea guidonia, (L.) Sleuner, Meliaceae, which is the most common riparian tree in the study area. At each sampling site, we deployed five pre-weighed bagless leaf packs each containing five fresh leaves (∼ 8−11 g) kept together with a binder clip. We retrieved a randomly-selected leaf pack from each site after 2, 6, 15 and 28 days of immersion. The leaf material was oven-dried at 60 °C for 48 h and weighed (with an analytical balance, ±0.0001 g) to determine the remaining dry mass.
Decomposition rates (k, day−1) for each site were determined by plotting the natural log of the proportion of remaining leaf mass against time and using linear regression to estimate the slope of that relationship (k = - slope) (Webster and Benfield, 1986; Silva-Junior and Moulton, 2011). The proportion of leaf mass at time zero (100%) was excluded from the regression. We corrected the mass of the fresh leaves to the mass expected after drying, using the relationship between wet and dry mass that we established using 7 leaf packs which were not deployed.
Data analysisWe explored the relationships between deforestation and environmental variables, invertebrate metrics and decomposition using Pearson’s correlation analysis and analysis of covariance (ANCOVA) with streams as a fixed factor and deforestation as the covariate. Data normality and the homogeneity of variances were evaluated with boxplots, normal Q-Q plots, and residuals. The data were Log10 or Arcsin√(x) transformed when necessary. The proportion of deforested area was Arcsin√(x) transformed in all analyses and graphs and is referred to as “deforestation”. The statistical analyses were performed using R software (R Core Team, 2015) and SYSTAT (ver. 12, SYSTAT Software Inc., Chicago, IL).
ResultsCanopy cover (Pearson´s correlation, p < 0.001, r = −0.77) and 24 -h O2 saturation minimum (p = 0.004; r = −0.52) decreased with deforestation, while temperature (p = 0.002, r = 0.55), NH4+ (p = 0.001, r = 0.62), and current velocity (p = 0.004, r = 0.52) were positively correlated with deforestation (Table S1, supplementary material).
ANL stream differed from the others and had higher NH4+ concentrations (ANCOVA: F3,22 = 22.78; p < 0.001) and lower daily O2 minimum saturation (F3,22 = 8.87; p < 0.001), while STM stream had higher temperature compared to other streams (F3.22 = 4.08; p = 0.019).
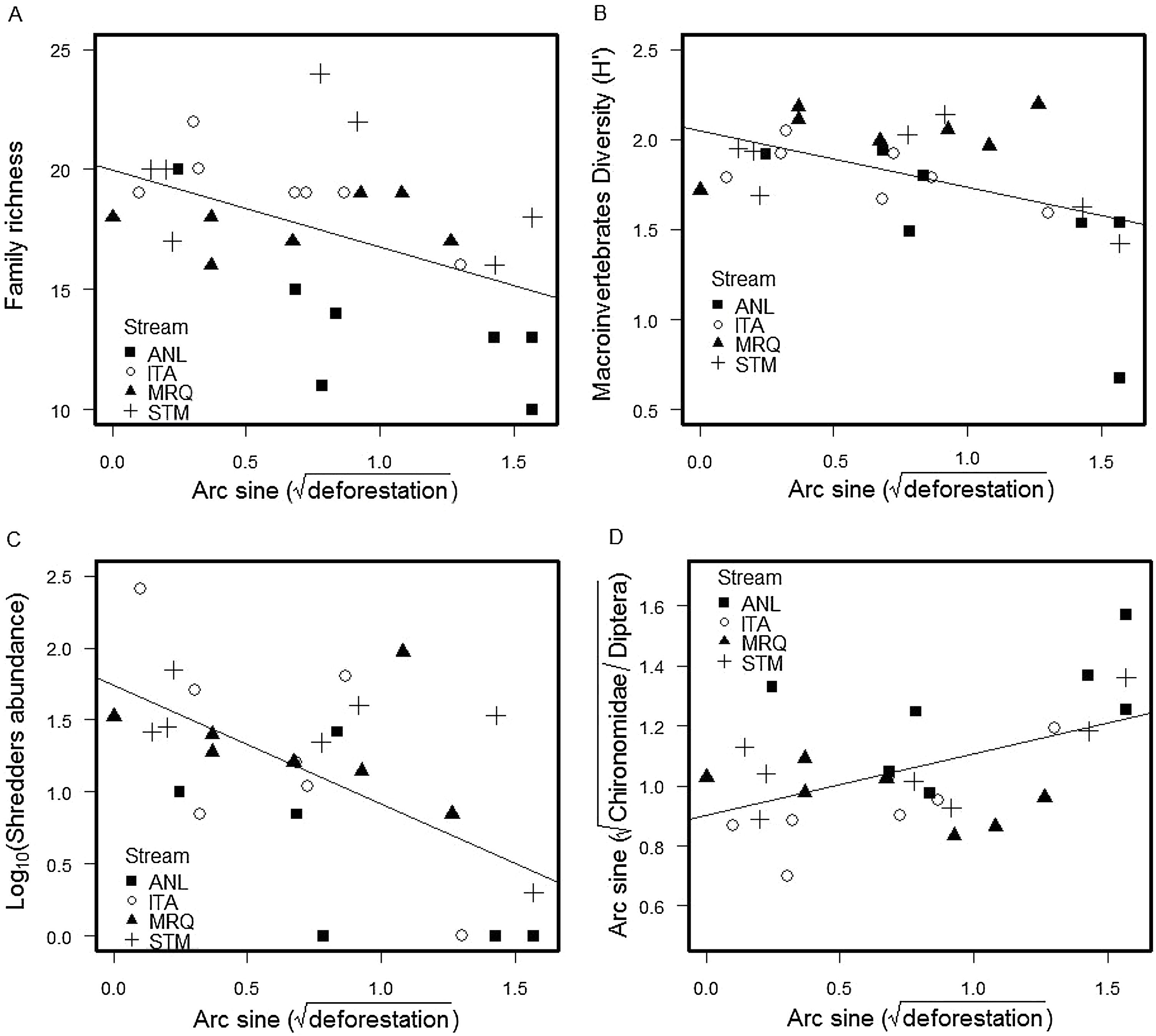
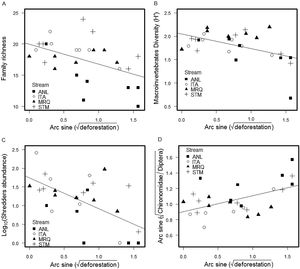
We found a total of 53,697 benthic invertebrates comprising 49 taxa in the stream sampling (Tables S2, S4). Collector-gatherer and scraper taxa were most abundant. Invertebrate abundance per sample ranged from 138 (ANL5) to 3280 specimens (STM5) (1918 ± 874, mean and SD) (Table S4). Family richness varied from 10 (ANL8) to 24 taxa (STM5) (17.5 ± 3.26). Percentage of shredders varied from 0.0% (ANL8) to 8.9% (ITA1) (2.24% ± 2.11%) and percentage of EPT (Ephemeroptera, Plecoptera and Trichoptera) ranged from 8.9% (ANL8) to 66.7% (ITA7) (37% ± 16%). Deforestation was negatively correlated with invertebrate family richness (ANCOVA, F1,20 = 5.7, p = 0.027), Shannon diversity (F1,20 = 5.3, p = 0.033), percentage of shredders (F1,20 = 22, p = 0.0002) and %EPT (F1,20 = 5.4, p = 0.030), while the percent Chironomidae among dipterans increased with deforestation (F1,20 = 5, p = 0.037) (Fig. 1) (Table S3). The relationships with deforestation were not significantly different among streams (i.e. not significant interactions in the ANCOVAs), and there were no significant differences among streams except that percent Chironomidae among dipterans was greater in ANL than in the other streams (Table S3).
The mining chironomid, Stenochironomus, was the most abundant shredder taxon, followed by elmid adults; only 5 taxa were shredders (Table S2). The most common ephemeropterans, Leptohyphidae and Baetidae, were classified as scrapers.
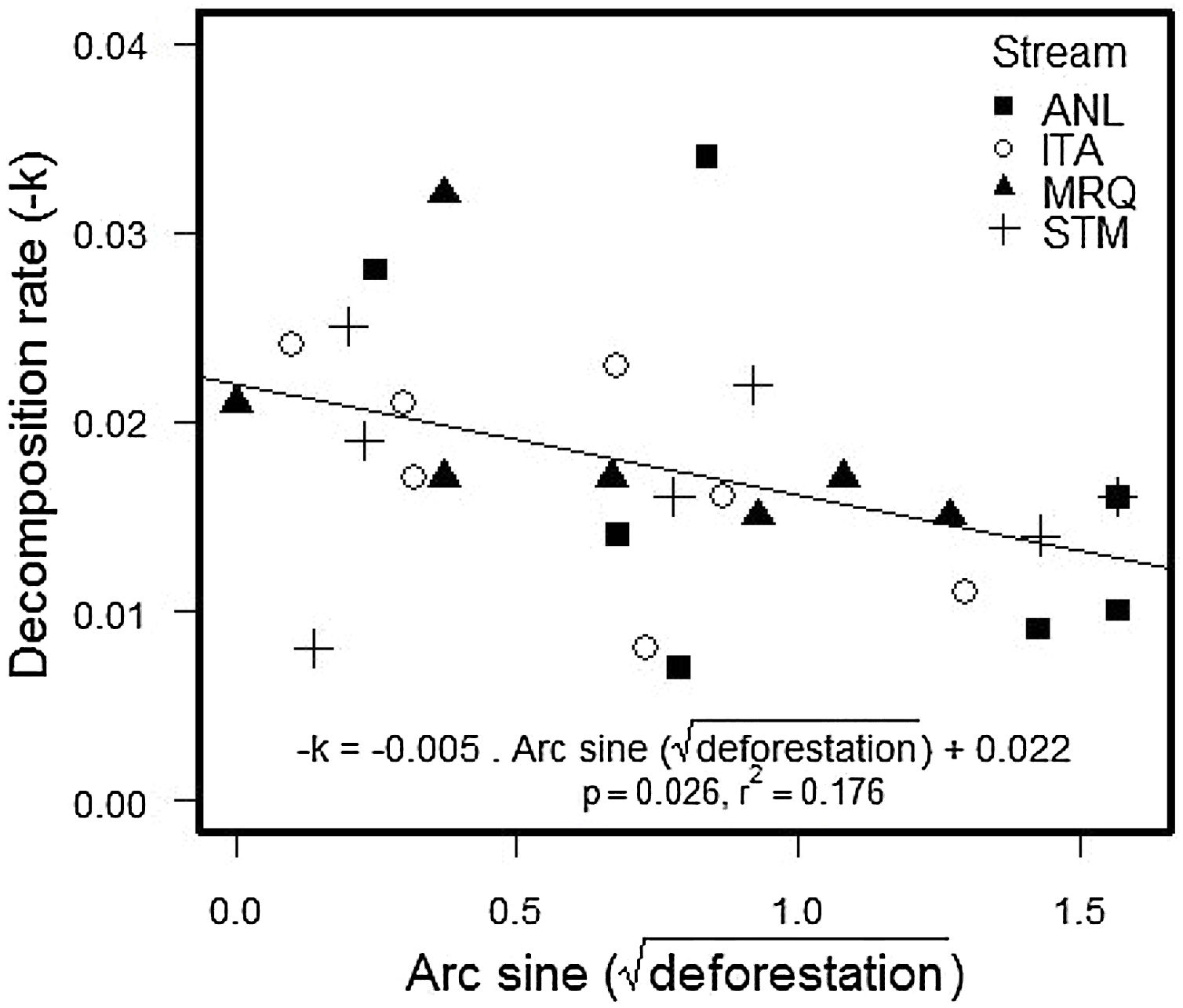
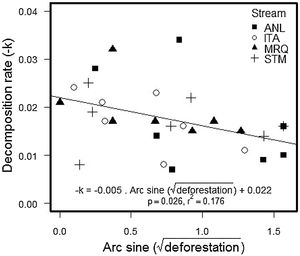
The percentage of remaining leaf mass after 28 days in the stream ranged from 34.7% in ANL1 to 81.9% in MRQ7 (58.1% ±10.97%). The leaf decomposition rates (k) varied from 0.007 d−1 in ANL3 to 0.034 d−1 in ANL2 (0.018 ± 0.007) (Table S5). Leaf decay functions of each site are presented in supplementary material (Figure S2). Decomposition rates decreased with deforestation in all studied streams (ANCOVA: F1,20 = 5.1; p = 0.035), with no significant difference between streams (F3,20 = 0.2; p = 0.9) or a deforestation by stream interaction (F3,20 = 0.68; p = 0.6) (Fig. 2). The overall relationship with (arcsine transformed) deforestation implies a reduction of k from 0.022 to 0.014 d-1 (36% reduction) over the gradient from 0 to 100% deforestation.
Leaf decomposition and macroinvertebrate abundance and indices were correlated with physical and chemical factors (Table S6). Since many of the factors were correlated with deforestation, their relationships with macroinvertebrates and decomposition were expected. Canopy cover was positively correlated with diversity of macroinvertebrates and shredders and with decomposition. Water current velocity was negatively correlated with macroinvertebrate abundance and diversity and negatively with decomposition. However minimum daily percent oxygen saturation was positively correlated with macroinvertebrate abundance and diversity and EPT taxa and negatively correlated with proportion of Chironomidae, but without a significant relationship with leaf decomposition (Table S6).
DiscussionDecrease in riparian vegetation (deforestation) was associated with changes in physical, chemical and metabolic factors in the four streams. These in turn were associated with changes in community structure of the benthic macroinvertebrates and in leaf processing, although not all components behaved as expected in Table 1.
Deforestation led to a decrease in invertebrate family richness, diversity, percent shredder abundance, percent EPT taxa, and an increase in percentage of Chironomidae among Diptera. Shredders are expected to be highly responsive to deforestation, since they depend upon allochthonous material (Moretti et al., 2007; Wallace and Eggert, 2009). Indeed, decreases in shredder abundance in impacted riparian conditions have been detected elsewhere in impacts of agricultural use in Atlantic forest (Casotti et al., 2015) and natural canopy differences in high-altitude Atlantic forest streams (Tonin et al., 2018). The other community metrics (invertebrate family richness, Shannon diversity, %EPT and percentage of Chironomidae in Diptera) are often correlated with impacts (Rosenberg and Resh, 1993; Baptista et al., 2006; Oliveira et al., 2011), presumably through reduction and loss of sensitive taxa (e.g. EPT) and relative increase of resistant taxa (Chironomidae).
Decomposition rates reported here are slightly higher than those found for other leaf species in Atlantic rainforest (Moulton et al., 2010; Casotti et al., 2015; Andrade et al., 2017) and similar to those found for the same leaf species in the same catchment (Silva-Junior and Moulton, 2011). Decomposition rates were in the upper range of values reported by Bruder et al. (2014), who studied decomposition of three tropical species and Alnus glutinosa in Amazon forest streams. We used bag-less, tethered, leaf packs, which are expected to decompose faster than leaf packs in bags, which are used in many other studies (Moulton et al., 2019). We used bagless, tethered, leaf packs because experiments in nearby streams showed that bags attracted fauna and particularly shrimps that apparently produced artificially high leaf processing (Moulton et al., 2019). Deforestation significantly reduced decomposition rates in our study (Fig. 2) which is consistent with the results reported for other Atlantic rainforest streams (Silva-Junior and Moulton, 2011; Casotti et al., 2015; Tanaka et al., 2015). This relationship was consistent across our streams, despite their differences in NH4+ concentrations, daily minimum O2 saturation and temperature.
A metanalysis of the effects of increased nutrient concentrations reported increased leaf decomposition (Ferreira et al., 2015), although the effects varied greatly among studies and depended on concomitant other impacts. Increasing nutrient concentrations are expected to stimulate leaf decomposition if the decomposer microorganisms are nutrient limited. Periphyton algae of streams of this study were found to be variously limited by nitrogen and phosphorus, especially in stretches with open canopy (Tromboni et al., 2019). Thus we had reason to expect that decomposition would increase with increasing nutrients, in particular with NH4+ which varied both among streams and with deforestation. The fact that decomposition did not vary with NH4+ apparently indicates that other factors were more important. The level of nutrients is important in this context; the relationship of decomposition and nutrients was found to be hump-shaped and high concentrations were found to decrease leaf decomposition (Woodward et al., 2012). The levels of phosphorus (SRP) and nitrogen (NH4+) of this study lie well below those expected for inhibition of decomposition.
Other factors showed unexpected trends with respect to leaf decomposition: Temperature should increase heterotrophic metabolism and leaf processing and interact with other environmental components (Martins et al., 2017). Temperature increased with deforestation (R² = 0.30, p = 0.002), contrary to the trend in leaf decomposition. Apparently the expected positive effects of temperature were not as strong as the negative effects of other factors associated with deforestation. Leaf processing also tends to increase with water current due to abrasion (Moulton et al., 2019; Wooton et al. 2019). In our study increasing water current was negatively related to decomposition rates and showed a similar tendency in all streams. This is not likely to be a causal relationship; we cannot imagine a mechanism by which faster current directly reduced leaf decomposition. We assume that the correlation was produced by the correlation of current and deforestation and that other factors associated with deforestation had caused the reduction of leaf decomposition. Since deforestation also led to reduction of stream invertebrate diversity and shredder abundance, we believe that invertebrates possibly had a stronger effect on leaf decomposition, which masked the possible effects of nutrients, temperature and abrasion. Alternatively or additionally other physical and chemical factors associated with deforestation could have contributed more strongly to decreasing leaf processing (Table S6 cf Table 1).
The physical and chemical factors show many strong relationships with the macroinvertebrate abundances and indices (Table S6). However, it is difficult to ascertain which relationships are causal and which are indirectly mediated by correlation with a principal cause. Minimum daily oxygen concentration diminished with deforestation (R² = 0.29, p = 0.003) and was associated with lower overall abundance of macroinvertebrates, lower diversity and reduced proportion of EPT taxa and increased proportion of Chironomidae, as expected of a stress (Table S6). But it apparently did not reduce the abundance and relative abundance of shredders and was not correlated with reduced decomposition (Table S6). On the other hand the range of NH4+ in this study (1.1–14.7 μg L−1) would not be expected to negatively affect abundance and diversity of macroinvertebrates and the negative correlations were probably caused indirectly (Table S6).We found significant relationships with deforestation of both community structure metrics and ecosystem functioning (leaf processing). This is not always the case in studies of this sort; in some cases, community structure has been shown to be more sensitive than ecosystem functioning and vice versa, and proponents of different measures have sought to explain such results (Reice and Wohlenberg, 1993; Bunn et al., 2010). In principle, since there is always a preponderance of rare taxa in any community (cf Table S2, where 65% of the taxa occurred in less than half of the sampled sites and 24% occurred in only one site), there should be a likelihood of sensitive taxa that should respond to environmental changes (impacts). This principle and its empirical observation have led to the general acceptance of bioindicator organisms, and in particular benthic invertebrates, for the detection and monitoring of environmental change (Reice and Wohlenberg, 1993; Rosenberg and Resh, 1993). Indeed, a multimetric index based on benthic macroinvertebrates was developed for the region under study (Oliveira et al., 2011). On the other hand, ecosystem functioning may be more resistant to change because it is mediated by redundant organisms – alternative taxa of shredders, for example, in our case where we have 5 – and thus not respond sensitively. This appears not to be the case here, since rate of leaf processing significantly decreased with deforestation. This was consonant with a previous study in the region (Silva-Junior and Moulton, 2011) and with the general proposal of using leaf processing to detect environmental impacts (Gessner and Chauvet, 2002).
This study showed how community structure and leaf processing are altered when riparian vegetation is removed within the area of 30 m immediately alongside the stream. The effect of deforestation in reducing leaf processing was similar among streams regardless of their differences in the physical template and thus highlighting its potential as indicator of impacts in the functioning of Atlantic rainforest streams. This knowledge about the impact of land cover on stream physical and biological structure, as well as ecosystem processes, contributes substantially to the planning of best stream management practices and highlights the importance of conserving riparian vegetation for maintaining biodiversity and ecosystem function.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the Brazilian “Science Without Borders” program for funding this research (project number 166/2012, pesquisador visitante especial, SAT). We thank the Guapiaçu Ecological Reserve (REGUA) for hospitality and assistance. The research formed part of the Masters dissertation of MS-A), who thanks the Programa de Pós-graduação em Ecologia e Evolução, UERJ, for its support. The authors were supported by scholarships provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 (VN-L; FT; EFS-J; CL-A; RF-L), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (VN-L; FT, EZ - JCNE), CNPq bolsa PQ (TPM, EZ), and UERJ Prociência (EZ, TPM). We also received funding from FAPERJ (E-26/112.066/2013-INST) and CNPq Universal (477503/2013-6) to EZ.