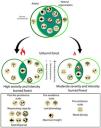
Fires are recurrent in moist tropical savannas, but in recent decades, Neotropical forests have become more affected due to the increased frequency of fires and the extent of burned areas. Currently, there is still limited knowledge on whether and how these disturbance events generate changes in taxonomic and functional diversity that can ultimately lead to the degradation and loss of resilience of tropical forests. To understand the response of Neotropical moist seasonally flooded forests to fire and the impact on taxonomic and functional diversity, we studied forests affected by fires with three degrees of severity and intensity: unburned, severity and intensity burned, and high severity and intensity burned. Regardless of the severity, fire generates a high taxonomic and functional homogenization in the tree and palm community by reducing α and β taxonomic and functional diversity and increasing functional homogenization by filtering species with similar traits. We found that adults with fire avoidance traits, such as deciduousness, and persistence traits, such as resprouting ability, were the ones that better survived the fire. Fire significantly reduced the abundance of evergreen species and those that were dispersed by zoochory. Our findings provide insight into the functional trajectory of Neotropical moist seasonally flooded forests after the fire, indicating that even moderate fire events may lead to a homogenization of these ecosystems and threaten their persistence.
Several types of ecosystems around the world are becoming increasingly affected by forest fires. While the total global area burned has decreased in recent years (Andela et al., 2017), nearly one-third of the global landmass experiences recurring fire activity, with an annual burned area of ∼4.5 M km2 (Robinne et al., 2018). The savannas and forests of the tropics are two of the biomes in which the greatest fire activity is concentrated (Andela et al., 2017). Savanna fires account for 65% of the total greenhouse gas (GHG) emissions from burning events (Van Der Werf, 2018). Fires in tropical forests have increased considerably since the 1980s, and they contribute to a further 15% of total GHG emissions (François-Nicolas et al., 2018).
The effects of fires on vegetation vary according to the characteristics of the disturbance, e.g., the extent, frequency, intensity, or severity (Turner, 2010). The larger the extent, the larger the impact, although increases in fire frequency may also lead to higher vulnerability and impose a greater risk of irreversible forest loss (Armenteras et al., 2021a). In the case of the tropical moist seasonally flooded forest, it has been found that fire generates high mortality of trees, causing a series of cascading effects such as biomass loss (Armenteras et al., 2021b) and the loss of taxonomic diversity which increases with fire frequency (Barlow and Peres, 2008a,b; Da Silva et al., 2018). These changes not only affect carbon stocks (Silva et al., 2018) but also modify the composition and structure of both vegetation (Armenteras et al., 2021b; Barlow and Peres, 2008a,b; Cochrane and Schulze, 1999) and faunal communities, restricting or promoting the appearance and occupation of different species (Balch et al., 2015; González et al., 2021).
Specifically, in Neotropical floodplain forests, most studies have focused on forests associated with blackwater streams in the Amazon. Using spectral vegetation indexes and fieldwork, this studies have reported a stronger and longer-lasting impact on process that determine the temporal dynamics of communities such as plant recruitment and mortality (Maracahipes et al., 2014), leading to changes in forest composition and structure, the tree seed bank, the invasion of herbaceous plants and the soil fertility (Flores et al., 2014, 2016, 2017; Da Silva et al., 2018). Fewer studies have been carried out on clear and mixed water Neotropical flooded forests, such as those in the Orinoco region; where changes in species composition of plant and animal communities occur as well (Armenteras et al., 2021c; González et al., 2021).
Although progress has been made in understanding the effect of fire regimen characteristics, such as fire frequency on vegetation and faunal communities of moist seasonally flooded forests, to the best of our knowledge, the effect of different fire severities on taxonomic and functional diversity, has not been evaluated. Specifically, functional diversity can provide a broader approach to understanding community responses to fire (Nóbrega et al., 2019) since it provides us with information on the degree of difference in functional traits between and within species (Mason and De Bello, 2013). This influences the system’s capacity to respond to disturbances, as well as ecosystem processes and functionality (Suding et al., 2008). Higher functional diversity is expected to support more ecosystem services (Díaz et al., 2007). Furthermore, plant communities with diversified functional characteristics support a greater number of biotic interactions, such as pollination (Martins and Batalha, 2006), seed dispersal (Cadotte, 2006), and herbivory (Ristok et al., 2020). Likewise, it is expected that systems with greater species richness and functional diversity are more resilient to disturbance (Bhaskar et al., 2018), owing to the higher stability provided by redundancy i.e. different species have the same functional strategies within an ecosystem. Redundancy contributes to the system’s resilience; if a species is lost due to a disturbance event, other species will play a similar role in the ecosystem (White and Jentsch, 2001).
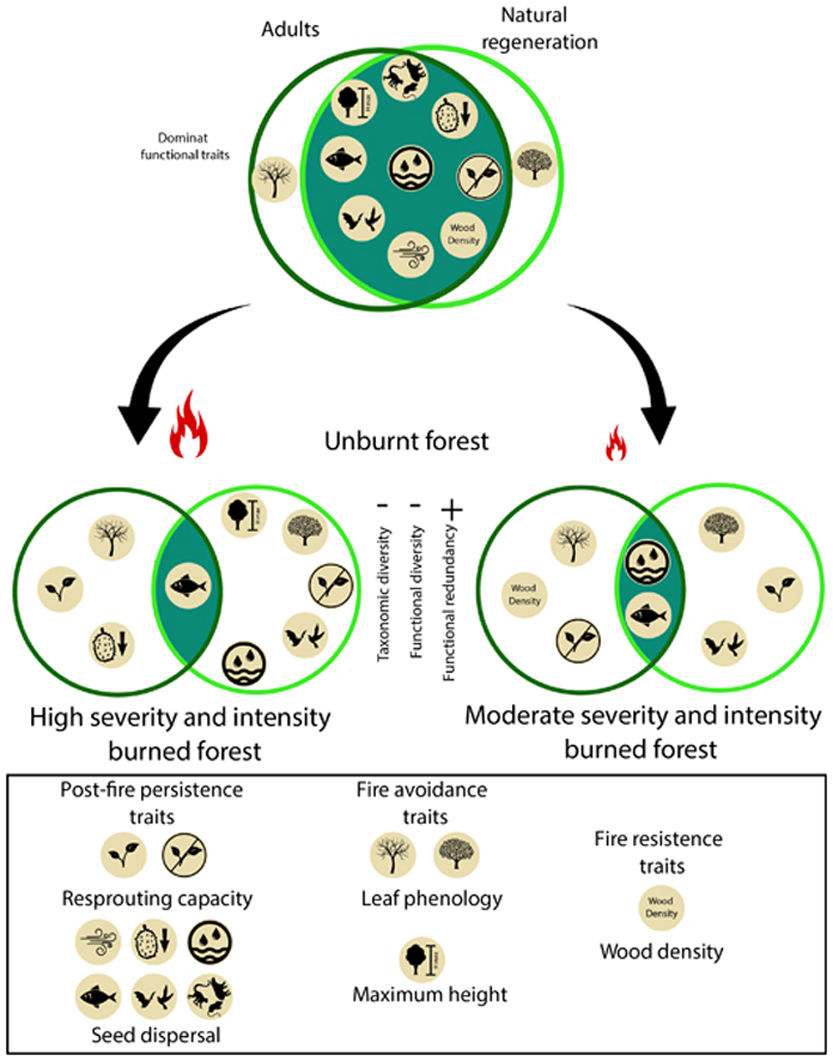
When referring to fire-affected ecosystems, functional diversity can be evaluated through functional traits that can facilitate species persistence during a fire or post-fire (Pausas, 2019). For instance, some traits, such as very thick bark or high wood density, confer resistance (Cornelissen et al., 2003). Other traits, such as leaf deciduousness, allow plants to reduce flammability and avoid burning (Cornelissen et al., 2003), while maximum height can protect key regenerative and reproductive organs by keeping them are out of reach of flames (Cornelissen et al., 2003). Furthermore, the presence of some traits, as well as their values, depends on the plant’s age or size (Hammond et al., 2015; Kuusk et al., 2018; Liu et al., 2019). Often, young trees may lack some of the traits that could allow them to survive a fire. Yet, some species which may not have the capacity to directly survive fire nonetheless manage to maintain themselves after a fire, due to, recolonization mechanisms mediated by seed dispersal (Pérez-Harguindeguy et al., 2013; Pausas, 2019). Those species that manage to establish postfire will exhibit succession patterns that help to identify whether the community recovers its composition, structure, and function before the fire or if, on the contrary, a significant post-fire replacement occurs (Freeman and Kobziar, 2011). Therefore, to better understand forest recovery processes and rates after a disturbance, the analysis should focus not only on the response of adult stems (≥10 cm DBH) but also on saplings (Berenguer et al., 2018).
Despite advances in knowledge of the ecological effects and responses of tropical forests to fires, few studies have focused on how fires influence functional diversity. Furthermore, despite the fact that fire intensity and severity are measurable parameters both on the ground and with remote sensing (Keeley, 2009), few studies to date have evaluated the response of forests to different fire intensities and severities, and large gaps remain to be filled in the Neotropics (Armenteras et al., 2021b; Nóbrega et al., 2019). In this study, we evaluated changes in taxonomic and functional diversity under different fire severities in tropical moist seasonally flooded forests, both at the local (α) and (β) landscape levels. We used forests of the Orinoco, Colombia, as a case study. We measured traits associated with fire resistance and postfire persistence in both adults and natural regeneration. Our central hypothesis was that fire causes a taxonomic and functional simplification of the tree community, by decreasing functional diversity and filtering certain traits, such as the dominance of deciduous species with resprouting capacity and high wood density. Moreover, we consider that this simplification increases with fire severity. This knowledge can help guide the design of forest fire prevention and restoration strategies in fire-sensitive systems with a high conservation value such as Neotropical moist seasonally flooded forests.
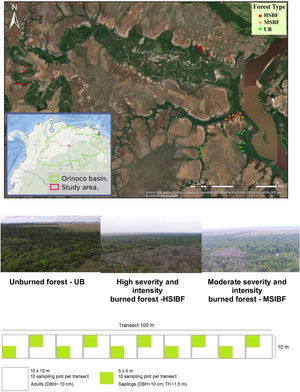
Materials and methodsStudy siteThe study area is located in the savannas of northern South America, which constitute the second-largest savanna complex in the neotropics after the Brazilian Cerrado (Romero-Ruiz et al., 2010). Specifically, we investigated seasonally flooded forests present within a matrix of savannas in Colombian Guiana (Fig. 1). The area is of special interest for conservation since the Orinoco basin is in one of the most important river ecosystem complexes in the world which also happens to be one of the most important reservoirs of biodiversity in the Neotropics (Gassón, 2002). The sample plots were established in burned and unburned forests in the Biosphere Reserve “El Tuparro.” This area has a tropical climate with average temperatures that range between 27° and 36 °C and a monomodal rainfall regime of 2000–3500 mm per year. The forests experience a period of flooding from April to November and a dry season between December and March.
Study designThe anthropogenic fires at the study sites occurred during January and March in 2015 and 2019, which are the hottest and driest months of the year for the region. To classify fire intensity and fire severity, we adopted the parameters associated with changes in the vegetation proposed by Keeley (2009). Thus, a visual inspection was carried out to define fire intensity by measuring the height of the fire scar in the adult trees (Keeley, 2009; Pausas, 2017) and estimating fire severity based on the canopy opening. We classified three forest conditions as: 1) High severity and intensity burned forest (HSIBF), forests with fire scars reaching the crowns, and charred trunks with a diameter greater than 10 cm and superficial burned debris of all sizes; 2) Moderate severity and intensity burned forest (MSIBF), tree trunk scars at variable heights, trees with some dead canopy cover but the foliage not entirely consumed, thin branches on the soil surface; and 3) Unburned forest (UB): forests that in the last 30 years have not been subjected to burning, logging, or grazing.
We used a design of contiguous plots nested within transects following a sampling strategy successfully tested in previous similar studies (Maracahipes et al., 2014; Da Silva et al., 2018; Molina et al., 2018; Armenteras et al., 2021c). This was done to minimize vegetation disturbance by trampling during sampling and optimize the time spent in the Natural Reserve. We established thirty-three transects with a length of 100 m and a width of 10 m, separated by at least 400 m. Each transect was divided into ten sub-plots of 100 m2. Specifically, for the different fire severities we established 9 UB transects (90 sub-plots), 6 MSFB transects (60 sub-plots), and 18 HSIBF transects (180 sub-plots).
Measurements were conducted in the selected locations two years after each fire event (2017 and 2021). In each 100 m2 plot, we recorded and identified all trees and palms with a DBH ≥ 10 cm (survivors), hereafter considered adults. Each adult was measured for diameter at breast height (DBH) and total height. To evaluate tree regeneration after the fire, each of the 10 m2 plots was subdivided into sampling units of 25 m2 for the recording of natural regeneration (saplings). Saplings were considered all individual trees and palms with a DBH < 10 cm and heights greater than 1.5 m.
Plant traits that influence the fire responseWe assessed the following traits for all species: a) fire avoidance traits: the maximum height (Hmax, m), and the leaf phenology (Cornelissen et al., 2003); b) fire resistance traits: wood density (WD, g/cm3) (Cornelissen et al., 2003); c) post-fire persistence traits: resprouting capacity and dispersion type (Cornelissen et al., 2003; Clarke et al., 2012; Pérez-Harguindeguy et al., 2013).
Hmax values were measured during the field campaign with a TruPulse 200 L laser rangefinder. As trait value we used the 95% of the values registered for each species in the adult stratum. In the case of species for which this information was not available eg. because were not present in the adult stratum, we complemented the data obtained from scientific papers and herbarium records. Like Casanoves et al. (2011), the assignment of leaf phenology traits was made from observations in the field and specialized bibliography. We classified the species into three categories: a) evergreen, those that keep their leaves coverage throughout the year; continuously change leaves, b) deciduous, those that lose their leaves during the same period year, and c) semideciduous, those that lose part of their foliage in the year either due to climatic seasons or reproductive events (flowering or fruiting).
Wood density (WD) values were obtained from the Global wood density database (Zanne et al., 2009) and complemented with reports in the peer reviewed literature. When no information was available at the species level, we used the wood density average value for the genus.
We determined resprout capacity monitoring all individuals with a DBH ≥ 10 cm in the dry season during the period 2017–2021 We classified species into two categories: a) species with resprouting capacity, those species that can resprout roots either underground or in the trunk and branches (Salazar et al., 2020), and b) species without resprouting capacity, species without regrowth observed. Finally, the type of dispersion of each species was assigned based on the information obtained from published peer reviewed papers. In the case of the species for which no specific information was found, we used the dispersal agent or dispersal syndrome reported for the genus or family according to the characteristics of the fruit. Based on the information collected, we employed the dispersal categories established by Salgado-Negret (2007): i.e. anemochory, hydrochory, autochory (explosive and gravity) and zoochory, subdivided into three subcategories corresponding to terrestrial (small and large mammals), aerial (bats and birds), and icthyochory (fish). This decision was taken given that the study system is subject to flooding and fish are an important dispersal agent. The information about the species and their traits can be seen in Table S1.
Statistical analysisTaxonomic diversityDue to the size difference between sample units (sub-plots) for measuring the adults and saplings, we used rarefaction curves to determine if differences in sample sizes influenced the results. The rarefaction curves were constructed using the abundance of the species sampled per sub-plots (Fig. S1 in the material Supplementary). For this analysis, we used the iNEXT package (Hsieh et al., 2016) in R software (version 1.3.959).We calculated plant community richness (species number), specific diversity (Shannon–Wiener index, hereafter Shaw) and Pielou index at the level of each stratum (adults and natural regeneration) for each transect. We also Fisher’s alpha index that quantifies diversity and intrinsically involves the relationship between the number of individuals and the number of species present. In these analyses we considered all adult species recorded in the sampling plots. For natural regeneration (saplings), we excluded from the analysis rare species with an abundance of fewer than ten individuals. To analyze the influence on the taxonomic diversity of stratum and fire severity amongst sites, we constructed several models depending on the distribution of the errors of each variable. For Richness, Pielou index, and Alpha Fisher we used a generalized linear mixed-effects model (GLMM) with a Poisson distribution. While for the ShaW index we used a general lineal model (GLM). In this analysis, fire severity and intensity (unburned, moderate, high) was included as a fixed factor, and we included the transects as random factors. We used Moran's test to analyze the spatial autocorrelation of the models. Moran I static values were calculated in R using the code from Bivand and Wong (2018), and are presented in Table S4.
To analyse the effects of fire severity on taxonomic diversity at the landscape level (beta - β diversity), the changes in species composition between the different communities were analysed by means of the Sørensen index (βsor), which measures species dissimilarity. Following the approach proposed by Baselga (2010), we partitioned the potential changes in βsor into two components: species replacement (βsim) and nesting (βnes). A arger nesting value (βnes) indicates that differences in species composition among sites mostly arise from the presence of sub-groups of species contained (nested) in the site with the higher species richness, while larger number of replacement (βsim) indicates that differences in species composition among sites mostly stem from the turnover of different species (see the seminal paper by Baselga (2010) and Lourenço‐de‐Moraes et al. (2019)). For the calculation of the three indices, we used the betapart package (Baselga et al., 2021) in R (version 1.3.959).
Considering that dissimilarity metrics cannot discern whether differences in dissimilarity are due to changes in the underlying structuring of community composition across sites or changes in α-diversity alone (Chase et al., 2011), we used the Raup-Crick β-diversity metric (βRC), as configured by Chase et al. (2011), in a complementary way βRC s explicitly conditioned upon variation in α-diversity and therefore provides a more appropriate metric than other measures of dissimilarity for comparing the dissimilarity among communities that vary in α-diversity (Chase et al., 2011).
Plant traits that influence the fire responseTo analyze the differences in functional traits among forest conditions, we calculated community weighted means (CWM). To determine the CWM, we first calculated the weights (wts) based on the abundance of each species in the transect. Then, we created a matrix that included the values of the traits for each species. Qualitative traits such as leaf phenology, resprouting capacity, and dispersion type were represented as binary values (0 for absence and 1 for presence). For quantitative traits, we used the reported values of wood density by species and 95% of the maximum height values recorded for each species in the adult stratum. We used the FD package (Laliberté et al., 2022) in RStudio to calculate the CWM for each trait. The CWM was defined as the mean of values present in the community (i.e., transect) weighed by the relative abundance of taxa bearing each value (Lavorel et al., 2008). The values of CWM for each functional trait in different forest types and strata are presented in Table S5.
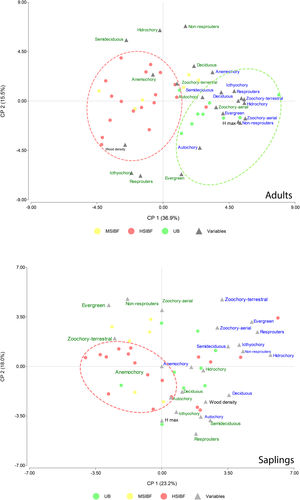
For each stratum (adults and saplings), we did a principal component analysis (PCA) to represent the variability of the data of richness for each functional trait and CWM between transects and forest conditions (Fig. 4). For this analysis, we used the software INFOSTAT 2017.
Finally, to determine whether there were significant differences in trait abundance (CWM) and trait richness, we used generalized linear mixed-effects models (GLMM) with a Poisson distribution and general linear models (GLM), according to the error distributions of each variable. In all the models, we include as fixed effects the forest conditions, the stratum, and the interaction of these two factors. As random effects, we included the transects. To analyse the spatial autocorrelation of the models we used Moran’s test. Moran I static values were calculated in R using the code from Bivand and Wong (2018), and are shown in Tables S7 and S9.
Functional diversityFunctional diversity was determined from multidimensional multitrait indices that account for the abundance of individuals. We used the functional dispersion index (FDis), functional richness (FRic), functional evenness (FEve) and functional divergence (FDiv) proposed by Laliberte and Legendre (2010), as it allows for the calculation of functional diversity for communities including a minimum of two species (see Espelta et al. (2020)). The calculation was performed in RStudio (version 1.4.1717) using the functional diversity (FD) library (Laliberté et al., 2014). Additionally, redundancy of functional traits was estimated by means of the unidimensional multitrait index based on Rao abundance and species richness. To analyse the effects of fire severity on functional diversity, a general lineal model (GLM) was chosen. The fixed effects included in the model were forest conditions (UB, MSIBF, HSIBF), stratum, and their respective interactions, while the plot nested within the transect was included as the random factor.
To analyse the effects of fire severity on the changes or replacement in the functional diversity between the different communities (beta - β functional diversity), we analysed the multiple-site functional dissimilarities, separating the turnover and nestedness-resultant components of functional diversity, as in the above mentioned procedure conducted for taxonomic diversity following Baselga (2010). In this analysis, the number of dimensions should not exceed four; therefore, we performed two analyses: the first for the presence/absence traits (leaf phenology, resprouting capacity, animal dispersal, or physical dispersal) and the second for the quantitative traits (maximum height and wood density). We used the functional betapart package (Baselga et al., 2021) in R (version 1.3.959) for the calculations of the Sørensen index (βsor), the Simpson Index (βsim), the Nesting Index (βnes), and the calculation of the multiple-site functional beta diversity measures and pairwise functional dissimilarity matrices.
ResultsTaxonomic diversityIn total, we registered 182 species of trees and palms belonging to thirty-eight families (Table S1). The most representative families were Fabaceae, with thirty-eight species, including Chrysobalanaceae (16), Myrtaceae (13), Euphorbiaceae (10), Lecythidaceae (8), and Sapotaceae (7).
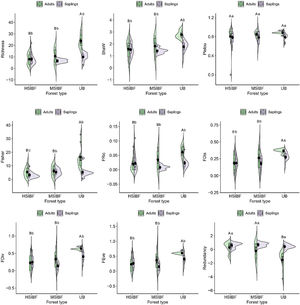
Our results showed a negative effect of fire on the taxonomic and functional diversity of the canopy (adults) and the natural regeneration (saplings) stratum, with some differences between moderate (MSIBF) and highly severe burned forest (HSIBF) depending on the vegetation strata (interaction forest conditions × stratum in Table S3). Regardless of the fire severity degree, both high- and moderate-severity burned forests exhibited lower tree richness in their canopy stratum in comparison to unburned forests (respectively, HSIBFadults = 2.2 ± 1.4, MSIBFadults = 2.8 ± 1.6 and UBadults = 4.3 ± 1.9). Similarly, burned forests showed a lower tree taxonomic diversity (Shaw HSIBFadults = 0.59 ± 0.58, Shaw MSIBFadults = 0.78 ± 0.65) in comparison to unburned areas (Shaw = 1.27 ± 0.50). Alpha Fisher index showed significant differences between the burned forests andunburned forest that loses the greatest diversity (HSIBFadults = 5.42 ± 3.88, MSIBFadults = 6.19 ± 4.26 and UBadults = 16.44 ± 8.44; HSIBFsaplings = 3.27 ± 2.24, MSIBFsaplings = 5.33 ± 2.49 and UBsaplings = 5.42 ± 4.16). We did not find significant differences in species evenness (Pielou index) among burned (HSIBFadults = 0.54 ± 0.47, MSIBFadults = 0.65 ± 0.4, HSIBFsaplings = 0.48 ± 0.45, MSIBFsaplings = 0.54 ± 0.46) and unburned forests (UBadults = 0.90 ± 0.21 and UBsaplings = 0.71 ± 0.38).
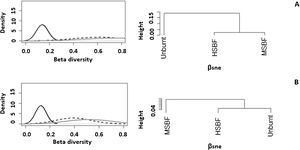
In addition to exhibiting lower taxonomic diversity at the plot level (α-diversity), the analysis of β diversity (Fig. 2), Sørensen index and βRC (Table S11) revealed that the adult stratum composition of moderate and high severity burned forests was more similar among plots at the landscape level and had higher dissimilarity with unburnt forests (βsorHSIBF = 0.65, βsorMBSF = 0.67 and βsorUB = 0.81). In natural regeneration, βRC shows that the unburned forest has high dissimilarity in species richness with HSBF but maintains a medium similarity with MSIBF (Table S11). However, we did not observe differences in the values of the Sorensen index in natural regeneration among forest conditions (βsorHSIBF = 0.47, βsorMBSF = 0.47 and βsorUB = 0.44). For both the adult stratum and the regeneration stratum, differences in species composition among plots were more related to species substitution (βsimadults = 0.62 and βsimsaplings = 0.39) than to nestedness (βsneadults = 0.14 and βsnesaplings = 0.13).
The partition of βsor (gray solid line) into βsim (black dashed line) and βnes (black solid line) and clustering using average linkage of the βsim and βnes components of species dissimilarity between forest conditions. Where A: adults and B: natural regeneration (Saplings) and HSIBF: high severity burned forest, MSIBF: moderate severity burned forest and UB: unburned forest.
The models showed that there are no significant differences in the communities of burned and unburned forests in relation to the CWM values of maximum height (Table S8), but models showed a decrease in the proportion of individuals with denser woods in the natural regeneration of high severity and intensity burned forests (HSIBFsaplings = 0.58 g/cm3).
Adult survivors of the HSIBF mostly corresponded to deciduous individuals with resprouting capacity (HSIBFadults = 53.21%), whereas the unburned forest and moderate severity burned forest did not differ significantly from each other, hosting mainly deciduous species without resprouting capacity (MSIBFadults = 56.91% and UBadults = 50.10%). The models did not show significative differences in CWM of evergreen, deciduous, and semi-deciduous individuals between forest conditions. But a lower richness of evergreen species was recorded in the burned forests in the adult stratum and in natural regeneration
Out results found that fire do not influence dispersal mechanisms such as anemochory but can positively and negatively influence other mechanisms of dispersion. We identified that high-severity fire promoted a significant reduction in water-dispersed species richness, but the values of CWM are greater in the communities of burned forest of high intensity and severity.
It was found that dispersal involving animal interactions is affected by fire, CWM and the richness of species that are dispersed by terrestrial animals were significantly reduced in burned forests, regardless of the severity and intensity of the fire. In the case of dispersal by aerial zoochory, highly severe and intensely burned forests displayed a lower richness of species dispersed by birds and bats, although there are no differences in CWM values between burned and unburned communities. Fire also reduces the richness of species that are dispersed by icthyochory, but in terms of the relative abundance of individuals with this means of dispersal, there is only a significant reduction in CWM in MSIBF, and there are no differences in CWM between UB and HSIBF.
Regarding regrowth capacity, the loss of species due to fire also led a reduction in the richness of those with regrowth capacity in burned forests. However, there were no differences in CWM values between burned communities and unburnt. Differences were reflected between the adult stratum and the natural regeneration of all forest conditions. In adults, there is a greater proportion of individuals with regrowth capacity while there is a greater abundance of saplings without regrowth capacity. The results of the models that evaluated the differences between the richness and CWM of each functional trait according to stratum, forest conditions, and their interactions are shown in Table S6 and Table S8, respectively.
Functional diversityRegarding functional diversity, as shown by the linear mixed effect model in Table S3, we found that fire regardless of severity reduces functional diversity in the adult stratum and in natural regeneration (FDis HSIBFadults = 0.22 ± 0.19, MSIBFadults = 0.28 ± 0.21, UBadults = 0.36 ± 0.12; and HSIBFsaplings = 0.19 ± 0.19, MSIBFsaplings = 0.18 ± 0.17, UBsaplings = 0.28 ± 0.16). Loss of functional diversity resulted in an increase in the redundancy of some functional traits in burned forests (redundancy HSIBFadults = 0.73 ± 0.32, MSIBFadults = 0.55 ± 0.47, UBadults = 0.34 ± 0.46; and HSIBFsaplings = 0.73 ± 0.38, MSIBFsaplings = 0.85 ± 0.17, UBsaplings = 0.68 ± 0.28). The FEve, FRic and FDiv indexes had the same behavior as FDis as evidenced in Fig. 3 and Table S3.
Taxonomic and functional diversity index. The contours of the violin plots provide information about the probability density of the data, and boxplots within violin plots show the median, quartiles, and range. HSIBF: high severity burned forest, MSIBF: moderate severity burned forest and UB: unburned forest. Means with a common letter are not significantly different (p > 0.05). Capital letters represent the values of the adult community and lowercase letters the natural regeneration community.
Regarding functional β diversity, we observed a turnover that generated functional dissimilarity among forest conditions in the adult and natural regeneration strata of the functional traits associated with phenology, regrowth capacity, and type of dispersal (βSIMadults = 0.92, BsaNEadults = 0.0093, βSORadults = 0.93 and βSIMsaplings = 0.81, βSNEsaplings = 0.046, βSORsaplings = 0.86). There were no differences in the beta diversity associated with the traits of maximum height and wood density (βSIMadults = 0.07, βSNEadults = 0.12, βSORadults = 0.19 and βSIMsaplings = 0.12, βSNEsaplings = 0.10, βSORsaplings = 0.23, respectively). We did not find an intersection among the vectors (FRic) of functional richness at each site, that is, the values of functional richness in the three forest conditions are significantly different from each other. All the matrices containing the functional richness shared and not shared between pairs of sites are shown in Table S12.
DiscussionOur results show that a single entry of fire decrease species richness and diversity in Neotropical moist seasonally flooded forest. Interestingly, we found that with the Shaw index, the loss is significant in all cases regardless of fire severity, but the reduction is higher for the natural regeneration strata; whereas Fisher’s alpha analysis showed that yet, the degree of fire severity and intensity was relevant for this simplification process as high fire treatment caused a greater loss of diversity in the regeneration compartment. Forests with higher burn severity have a higher mortality of adult trees and, as a result, presented a more open forest cover, which may increase the amount of light reaching the understory limiting the establishment of shade-tolerant species (Bernhardt-Römermann et al., 2015). Conversely, moderate burning allows for greater forest cover and promotes a larger presence of shade-tolerant species (Vetaas et al., 2021). Our results showed that our hypothesis was fulfilled since the severity and intensity of fire caused a taxonomic simplification of the plant community (Fig. 4).
Principal component analysis that considered the values of species richness (letters color blue) and CWM (letters color green) associated with each trait in all the transects in the three forest conditions. The upper figure corresponds to the tree stratum and the lower figure to the natural regeneration stratum (saplings). HSIBF: high severity and intensity burned forest, MSIBF: moderate severity and intensity burned forest and UB: unburned forest.
While the impact of forest fires on the taxonomic diversity of rainforests has been documented by several studies, changes in functional diversity have seldom been assessed (Carreño-Rocabado et al., 2012, Armenteras et al., 2021b). Our results show that fire also reduces functional diversity regardless of severity, and this loss is also more noticeable in the regeneration strata. Again, this latter effect may be due to post-fire abiotic habitat filtering (e.g. canopy opening in burned areas) creating divergent plant communities with restricted variation in functional traits (De Pauw et al., 2021). Matching the reduction in functional diversity and the filtering effects of fire, our results showed that there is a higher homogenization of functional traits in high severity burned forests due to the taxonomical and functional simplification (FDis, FEve, FRic and FDiv) and the reduction in species richness (Zhang and Zang, 2021). Indeed, in burned areas, there is the loss of fire-sensitive species (Altomare et al., 2021) and the filtering of species with some particular life history traits (deciduousness, resprouting ability and higher wood density, see also Nóbrega et al. (2019)).
The impact of fire on taxonomic and functional diversity and the increase in homogenization at the local level were also confirmed at the landscape level, as β diversity was lower in burned areas. At the functional level, the beta diversity patterns revealed a substitution of the functional traits of phenology, regrowth capacity and the type of dispersion, thus increasing functional dissimilarity between burned and unburned communities. Studies in other ecosystems, such as savannas of North America, have shown that fire generates rotation-dominated patterns of diversity for different functional groups of plants, even at sites belonging to the same climate subregion (Freeman et al., 2019). This has implications for management, since in these types of landscapes with a high species turnover, conservation is possible only if networks of smaller or species-poor sites are established in a way that they encompass areas which capture the full range of variation within the metacommunity (Socolar et al., 2016).
Our study revealed that species with regrowth capacity and a deciduous leaf habit had an advantage that allowed them to survive fire. On the one hand, the capacity of plants to regrow after their aerial biomass has been destroyed is a key strategy for their persistence in the ecosystem (Clarke et al., 2012; Lawes et al., 2012). On the other hand, as fire events took place during the dry season, which is the time of the year when deciduous species lose their leaves, this could provide an advantage i.e. less flammable structures (Cornelissen et al., 2003; Pausas, 2019). Leaf deciduousness can also be an advantage in postfire conditions, allowing plants to tolerate or avoid hydrological stress (Borchert et al., 2002).
Burned forests presented a predominance of individuals with high WD (>0.5 g/cm3). It has been reported that species that tolerate and survive fire have thicker bark and higher WD (Armenteras et al., 2021b; Balch et al., 2015). Indeed, in Neotropical rainforests affected by medium severity fires, tree mortality decreases significantly as WD, diameter and height increase (Brando et al., 2012). However, our results did not show that Hmax favoured species survival; although it has been associated with plant avoidance strategies (Pausas, 2019; Cornelissen et al., 2003) age or growth stage of trees can limit their ability to reach the maximum possible tree height (Hmax, Hammond et al., 2015; Kuusk et al., 2018; Liu et al., 2019). While species may have traits that allow them to respond to fire regimes, this does not always mean that they can survive events of all fire intensities or a specific severe event (Johnstone et al., 2015).
In relation to natural regeneration, high severity burned forests presented lower WD but did not present significant differences in the values of higher Hmax. These results could be associated with postfire changes in environmental conditions such as higher temperatures and light entry and reduced humidity (Cochrane et al., 1999). These are all microclimatic conditions that favour the establishment of generalist and pioneer species with rapid growth and no defence structures (low WD) but a tendency towards greater heights (Meza-Elizalde and Armenteras-Pascual, 2021).
In reference to phenology and regrowth capacity, we found a mismatch between the adult strata and regeneration. In the three forest conditions evaluated, the abundance of semideciduous and deciduous trees is high in the adult strata, whereas natural regeneration is dominated by evergreen species. In the high severity burned forest, there was a higher proportion of adults with regrowth capacity, whereas in the small trees (DBH < 10 cm, tree height > 1.5 m) category, the proportion of individuals without regrowth capacity increased. This mismatch could indicate that successful establishment of natural regeneration is not part of the legacy of surviving trees but other legacies, such as seed bank or seeds of species from other forests that were able to colonize burned areas (Pausas, 2019). The high abundance of individuals without regrowth capacity in the natural regeneration could compromise plant persistence after disturbance events since regrowth capacity implies the potential for repetitive vegetative regeneration with protected meristems (Clarke et al., 2012), this is an important physiological response to disturbances due to its role in plant persistence after destruction of most aerial biomass (Pérez-Harguindeguy et al., 2013).
On the other hand, we found that fire affected the dispersal mechanisms that involve animals. The loss in the richness of species with specialized reproductive traits and a dependency on animal interactions by the fire is similar to what has been observed in tropical savannas elsewhere (Altomare et al., 2021). Specifically, the significant reduction in CWM and the richness of species that are dispersed by terrestrial animals may not only affect the persistence of plant species with this dispersal mechanism but also generate cascading effects. Changes in the community of small mammals due to changes in vegetation have been reported in burned gallery forests of the Orinoco (González et al., 2021). Our findings could also be an indication of cascade effects since the loss of plants dispersed by terrestrial zoochory can lead to a reduction in food availability for fauna that depends on these fruits.
Finally, although our study was focusing on species response traits to fire severity and intensity, these characteristics do not necessarily translate to effect traits that matter in ecosystem functioning. Nevertheless, changes in functional traits could potentially be related to different processes and ecosystem services (i.e. Díaz et al., 2004, 2007; Lavorel and Garnier, 2002; Suding et al., 2008). For example, we found that WD decreases in the regeneration of burned forests, which is directly related to a reduction in primary production, measured as standing aerial biomass, which can itself affect aboveground carbon storage (Cornelissen et al., 2003) and negatively influence climatic regulation services (Casanoves et al., 2011). The regeneration of species with low WD in burned forests can also increase forest susceptibility to future disturbance and decrease their resistance. Although functional relations among WD, protection and hydraulic efficiency of the xylem are ambiguous, especially in tropical rainforests (Janssen et al., 2020), the general pattern is that low WD provides less mechanical and hydraulic protection (Hacke et al., 2001; Van Gelder et al., 2006; Fichtler et al., 2010) and thus a lower resistance to physical damage, pathogens, and droughts (Cornelissen et al., 2003). Phenology is also related to ecosystem processes, for example, the higher proportion of semi-deciduous and deciduous individuals in the adult stratum may influence nutrient cycling as these species invest large amounts of nitrogen in the leaves to support high assimilation rates when water is available in the soil; therefore, their short-lived leaves can fix large amounts of carbon in a short period of time (Eamus, 1999).
ConclusionsThis study explores the effects of fire severity on the functional diversity of Neotropical moist seasonally flooded forests. Our study suggests that fire reduces taxonomic and functional diversity at spatial scales α and β regardless of fire intensity and it can modulate plant communities by filtering species with similar functional traits, resulting in functional homogenization. Furthermore, the functional changes observed can endure through time and generate cascade effects that would affect forest recovery trajectories, ecosystem functions and finally forest resilience. Our research focused on trees and did not consider other vegetation types; therefore, the contribution of shrubs and vines to the resilience of burned forests remains to be studied. Finally, our results and interpretations may have been influenced by three situations: trait selection focusing on a few dimensions of fire response, some trait values taken from a global database, and trait imputation for species with missing data. In the future, with more fieldwork, it is hoped that other functional traits of plant species will be evaluated to gain a more detailed understanding of fire response syndromes.
FundingThe research was done under the scientific research permit Resolution 0255 Date March 14, 2014 of the Colombian Autoridad Nacional de Licencias Ambientales (ANLA). Funding was provided by USAID — Partnership for Enhanced Engagement in Research (PEER) and the US National Academies of Science (Subaward 2000007526PEER Cycle 5) with the project “Degradation of Tropical Forest in Colombia: Impacts of Fire and by the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS)”, project No 110180863738 (CT-247-2019) both awarded to DAP. María Meza Elizalde was supported by the Cohort I Bicentennial Doctoral Excellence Scholarship from the Science, Technology and Innovation Fund of the General Royalties System (Vichada Departament). Part of the data analysis was carried out within the framework of a research internship that hosted MME at the Center for Ecological Research and Forestry Applications (CREAF).
CRediT author statementMaríaC.Meza: Conceptualization, Methodology, Investigation, Data Collection, Data Curation, Formal Analysis, Writing - Original Draft, Writing - Review & Editing, Visualization, Funding Adquisition. Josep María Espelta: Formal Analysis, Writing-Review & Editing. Tania Marisol González: Data Collection, Writing Review & Editing, Funding Acquisition. Dolors Armenteras: Conceptualization, Investigation, Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition.
We thank all the people that in one way or another supported the intense field work for this research: we are grateful to the researchers Francisco Castro and Alejandra Reyes Palacios. We also would like to thank the following local researchers: Brayan Marín, Luis A. Piñeros, Luis A. Gutierrez, Beyker Castañeda, Henry Esteves, and as part of the community Nelcy Vega and Jacinto Terán. We would like to thank Fundación Omacha and particularly its scientific director Fernando Trujillo for their continuous support and permission to work in the Bojonawi Civil Society Reserve. Likewise, we express our greatest gratitude to the Civil Society Reserves “Doñana” and “Los Robles” who allowed us to work on their land and who shared with us their experiences, especially to Alejandro Herrera Villegas, Nathaly Herrera Correal, Eusebia Correal Betancour, Jose Sady Bernal, Fanny Tibisay Díaz Franco and Yesid Triana. We appreciate the invaluable expertise and information available at the Colombian National Herbarium from the Natural Science Institute of the National University of Colombia, and we wish to thank all the people who made possible the access to these valuable records and especially to professor: Carlos Parra, Gerardo Antonio Aymard and Jaime Uribe. A special dedication to Professor Gilberto Emilio Mahecha (R.I.P) for their collaboration in the determination of plant material. The support of the following people was important: Joël Morell Simón, Walter Andres García and Juan David González-Trujillo. A final thanks to Charles Tebbutt for his useful comments on the grammar revision of our final version of the manuscript, and to Arturo Cortés for his support in editing the graphs.