The Brazilian Atlantic Forest (BAF) is a global biodiversity hotspot, but its carbon sink capacity, especially in the subtropical portion, is poorly understood. We aimed to evaluate the relationship between biodiversity measures (i.e., taxonomic, functional, and phylogenetic diversity) and net carbon change across subtropical BAF, testing whether there is a win–win situation in the conservation of biodiversity and carbon sink capacity across forests of distinct ages. We obtained the net carbon change from 55 permanent plots, from early successional to old-growth forests, by combining the carbon gains and losses across two censuses. We found that subtropical BAF are on average acting as a carbon sink, but carbon gains and losses varied a lot across plots, especially within late successional/old-growth forests. The carbon sink was consistent across different forest ages, and we did not find a relationship between biodiversity and net carbon change in subtropical BAF. Therefore, conservation programs should aim at both targets in order to maximize the protection of biodiversity and carbon capture across the secondary and old-growth subtropical BAF, especially in a scenario of global changes.
The Brazilian Atlantic Forest (BAF) is widely recognised as a biodiversity hotspot (Myers et al., 2000), making it a global conservation priority. Under the current context of global changes, preserving forests with high capacity to absorb and store carbon becomes a fundamental conservation goal. However, we have only punctual assessments of the capacity of BAF to absorb carbon (Bordin and Müller, 2019; Capellesso et al., 2020; Maia et al., 2020; Rolim et al., 2005). The BAF suffers with historical deforestation, degradation, recent land-use changes (Ribeiro et al., 2009), and land abandonment across the region, meaning that these forests are a mosaic of successional stages (Rosa et al., 2021). The profound human impact across BAF has led to the loss of biodiversity and carbon stocks (Bergamin et al., 2017; Lima et al., 2020), with potential impacts to the carbon sink capacity of these forests. Most conservation programs in the BAF have to date focused on preserving biodiversity (Grelle et al., 2021). However, whether conservation efforts to preserve biodiversity will also lead to the conservation of the carbon sink capacity resulting in a win–win conservation scenario across these forests is still unknown. A win–win scenario where strategies to preserve biodiversity maintain forests with large carbon capture capacity would be especially welcomed facing current biodiversity and climate emergency (Mori et al., 2021; Reside et al., 2017), but the presence of such relationship is likely to be dependent on scale, forest type and biodiversity metric evaluated (Capellesso et al., 2021; Ferreira et al., 2018; Mori et al., 2021).
Biodiversity is a multifaceted concept that integrates taxonomic, functional, and phylogenetic diversity. Nonetheless, the effects of biodiversity on the carbon sink capacity, or net carbon change (the balance between carbon gains through productivity and recruitment and carbon losses through tree mortality (Phillips et al., 1998)) remain poorly understood (Poorter et al., 2017). For instance, local-scale studies in the subtropical BAF found no relationship between biodiversity and carbon sink capacity and stocks (Bordin and Müller, 2019; Capellesso et al., 2020). However, as forests in the BAF are very heterogeneous, varying in species composition (Bergamin et al., 2017), structure (Bordin et al., 2021), and biodiversity (Lima et al., 2020), local assessments may be unable to capture the regional picture. Thus, regional studies assessing the influence of biodiversity on net carbon change would allow deeper understanding of forest dynamics and their long-term implications for conservation (Ferreira et al., 2018) and restoration of both carbon sink capacity and biodiversity (Matos et al., 2019).
Taxonomic diversity is commonly used to evaluate the relationship between carbon and biodiversity (Ferreira et al., 2018; Mori et al., 2021). Nevertheless, taxonomic diversity is only one of the diversity facets, and may be unable to capture other aspects of diversity, such as functional and phylogenetic diversity. Functional diversity, for instance, quantifies the range of plant forms and strategies, and therefore is expected to capture ecosystem functions due to niche complementarity (Diaz and Cabido, 2001). Additionally, different plant lineages may have different roles in forest carbon cycling (Coelho de Souza et al., 2019), and closely related species often show similar capacity of carbon storage (de Aguiar-Campos et al., 2021). Thus, the presence or absence of certain species may imply in lower or higher phylogenetic diversity, and then affect carbon gains over time. Therefore, distinct diversity measures may have distinct relationships with carbon dynamics and may help understanding of such relationship and its implications for conservation.
Old-growth and successional forests are expected to differ in terms of their carbon dynamics and to show different relationships between their biodiversity metrics and carbon sink capacity. In the tropical region, successional forests frequently show larger carbon uptakes than old-growth forests (Heinrich et al., 2021; Poorter et al., 2016). In contrast, old-growth forests in subtropical BAF show higher gains in biomass over time than successional forests (Shimamoto et al., 2014; Souza and Longhi, 2019). These old-growth subtropical BAF are also able to stock large amounts of biomass, equivalent to many tropical forests (Bordin et al., 2021). The subtropical BAF is highly diverse (Bergamin et al., 2017), and is likely to have high carbon accumulation potential (Bordin et al., 2021; Bordin and Müller, 2019; Capellesso et al., 2021; Gross et al., 2018; Souza and Longhi, 2019; Vibrans et al., 2022), but it is currently underrepresented in global net biomass change estimations (Requena Suarez et al., 2019). The carbon sink capacity across successional and old-growth subtropical BAF and its correlation with biodiversity is a powerful and yet missing information to future conservation efforts across this region.
Here we investigated the relationships between the different aspects of biodiversity measured at different levels (i.e., taxonomic, functional, and phylogenetic diversity) and net carbon change across successional and old-growth forests in the subtropical BAF. We aim to quantify the carbon sink capacity of these forests, determine whether there is a correlation between biodiversity and carbon sink capacity and which biodiversity aspect most affects the net carbon change in this region. A positive relationship between biodiversity and net carbon change would imply a win–win scenario in forest conservation.
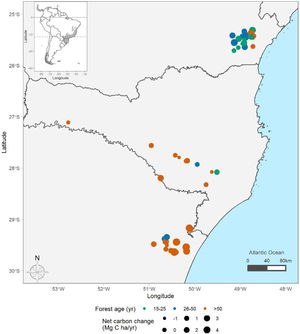
Material and methodsStudy area, forest dynamics, and trait dataThe Brazilian Atlantic Forest (BAF) is a biodiversity hotspot, with large latitudinal distribution (from latitude 5 ° to 30 °S) encompassing high heterogeneity of environments (Oliveira-Filho and Fontes, 2000). The mean temperature across subtropical BAF is 18 °C, and the mean annual precipitation is 1800 mm. We used vegetation data from 55 plots distributed across subtropical BAF (Fig. 1), where 31 are old-growth forests and 24 are successional forests, with regeneration age varying from 15 to over 80 years after disturbance from pasture or agriculture ceased. Each of these sites contain permanent plots with two censuses over time. All censuses occurred between the year 2000 and 2020. Most of the first census (t1) occurred between 2010 and 2016, and most of the second census (t2) occurred between 2016 a 2020. The mean sampled area per plot is 0.4 ha (0.06–1.2 ha) and mean census interval is 6 years (1–9 years) (Supplementary information 1).
Distribution of sampling points across subtropical Brazilian Atlantic Forest (BAF). The colours represent forest age (green = 15–25 years, blue = 25–50 years, and orange ≥50 years), and point size represents the net carbon change estimates per plot. Distribution of the BAF is detailed in grey. Points show the location of the sampling units at subtropical BAF. For visualization purposes, the position of points is slightly shifted when they overlap.
The forest inventories followed the RAINFOR protocol whenever possible (22 plots) (Phillips et al., 2001). In all plots, all stems ≥ 10 cm of diameter of breast height (dbh) had their diameter measured at 1.3 m above the ground, or 30 cm above buttresses or trunk irregularities using a diameter tape. Stems were tagged with a unique number, identified at species level, and had tree height (H, m) estimated by eye (0.8% of stems) or measured using telemetric and laser tapes (99.2 % of stems). This procedure was adopted in the first (t1) and second census (t2). In t2, all surviving stems tagged in t1 were re-measured, new trees (recruits) were measured, tagged, and identified, and dead or absent stems were accounted as dead stems. For more details, see (Phillips et al., 2001). We maintained palm species in the dataset to account for carbon gains through recruitment and losses through mortality, as palms do not present secondary growth (Supplementary information 2). Lianas and ferns were excluded from our analyses.
We collected information for the following plant traits: specific leaf area (SLA, cm2 g−1), leaf dry-matter content (LDMC, mg g−1), wood density (WD, g cm3), and species maximum height (Hmax, m). We used species leaf trait values for 90% of species; when species value was unavailable, we used genus or family means. These data are also available at TRY database and can be accessed under formal request (Kattge et al., 2020). All data collection followed the standardised protocol developed by (Pérez-Harguindeguy et al., 2013). For leaf traits, we collected at least five leaves per individual and at least three individuals per species within the sites. The leaf area values were calculated from scanned fresh leaves. All simple leaves selected were measured including petioles, and compound leaves had just the leaflet area measured, without rachis. Wood density collection also followed the standardised protocol, and species values were obtained from plot level (Missio et al., 2017), regional level (Oliveira et al., 2019), and global databases (Chave et al., 2009; Zanne et al., 2009). Details about the trait collection, measurement, and expected effect of each trait on species fitness and overall community is described at Supplementary information 3.
Carbon estimationAll living stems were used to estimate individual above-ground biomass (AGB) in both censuses, by applying a pantropical biomass equation. This equation considers tree dbh, height, and species wood density (AGB = 0.0673*(WD*dbh2*H)0.976, (Chave et al., 2014). We converted all AGB values to above-ground carbon by multiplying AGB by 0.456 (Martin et al., 2018). The tree-level above-ground carbon estimates were summed to obtain the above-ground carbon values for each plot.
Net carbon change was estimated as the balance between above-ground woody carbon productivity and above-ground carbon loss through mortality (Phillips et al., 1998). Total above-ground carbon stocks per plot was obtained as the sum of the above-ground carbon estimations of all living trees in t2. To obtain woody productivity and loss metrics, we used the information of all stems in both t1 and t2 censuses. Then, we obtained the annual rates of above-ground woody carbon productivity by combining carbon estimates for growth of surviving trees (meaning growth, t2–t1) and recruitment (recruited trees from t1 to t2). Carbon losses through mortality were estimated based on the carbon of dead trees between t1 to t2. We evaluated above-ground woody carbon productivity and loss by using the equations 3 and 4 recommended by (Kohyama et al., 2019), as it reduces potential bias due to unobserved recruits and growth of trees before death (Supplementary information 2). All carbon dynamics metrics were obtained as annual estimations (Mg C ha−1 yr−1).
Biodiversity metricsWe estimated taxonomic, functional, and phylogenetic diversity to evaluate their effects on the forest carbon sink capacity. These metrics were obtained based on the community matrix per site described by species stem density (number of stems ha−1; Supplementary information 2) using the data from second census in each plot.
Taxonomic diversity was calculated using the Inverse Gini-Simpson index, which gives a greater weight to the common species across the communities. Functional diversity (FD) was estimated using Rao’s quadratic entropy to give a greater weight for the functional contribution of the common species across communities. This FD metric is equivalent to the chosen taxonomic diversity metric. Phylogenetic diversity (PD) was evaluated via Mean Pairwise Distance, to obtain the mean phylogenetic relatedness among species. To calculate PD, we constructed an ultrametric phylogeny for tree species using a complete, time-calibrated phylogeny developed by (Smith and Brown, 2018) as a backbone. As our phylogenetic tree does not contain polytomies, no corrections for phylogenetic uncertainty were considered.
Data analysisPlots differed in their area and the length of time they were monitored for (Supplementary information 1). This is likely to affect carbon estimations, as smaller areas are more sensitive to mortality of larger trees, for instance. To control for these potential biases, we conducted two analyses to detect the effect of plot size and census interval on carbon estimations, following (Lewis et al., 2009). This diagnosis showed the need to account for the effect of monitoring length and plot size in our analyses (Supplementary information 4). Here, we used the ((cubic root of plot size) + (cubic root of census interval) – 1) as a weighting variable in the models (see Lewis et al., 2009 for details).
We tested for the correlation among the biodiversity variables, which are not correlated (Pearson’s correlation < 0.6). We fitted linear mixed models to evaluate the relationship between different biodiversity metrics and net carbon change, accounting for forest age as a random effect (three groups: 15−25, 26−50 and >50 years). We separated forest age into three categories: 15−25 and 26−50 years since clear-cut are characterised as successional forests, and >50 years since clear-cut as late successional/old-growth forests (Chazdon, 2008), as the 50 years’ time-frame is enough to recover almost 90% of the forest functioning along succession (Poorter et al., 2021). As we do not intend to test causality among the variables, but their relationship, the Pearson’s correlation among the diversity metrics and net carbon change is also shown. To control for spatial autocorrelation detected in our data, we included an exponential correlation structure (corExp) in the model. The model accounting for spatial structure had significantly lower Akaike Information Criterion (AIC) (Burnham and Anderson, 2002) when compared with models not accounting for it (ΔAIC = 5 without accounting for spatial variation). All predictor variables were standardised prior to the analyses to allow direct comparisons, and all the mean values are presented as weighted means. All analyses were conducted at R statistical programming language, version 4.1.1 (R Core Team, 2021). All the functions and packages used to conduct data analysis are available at Supplementary information 2.
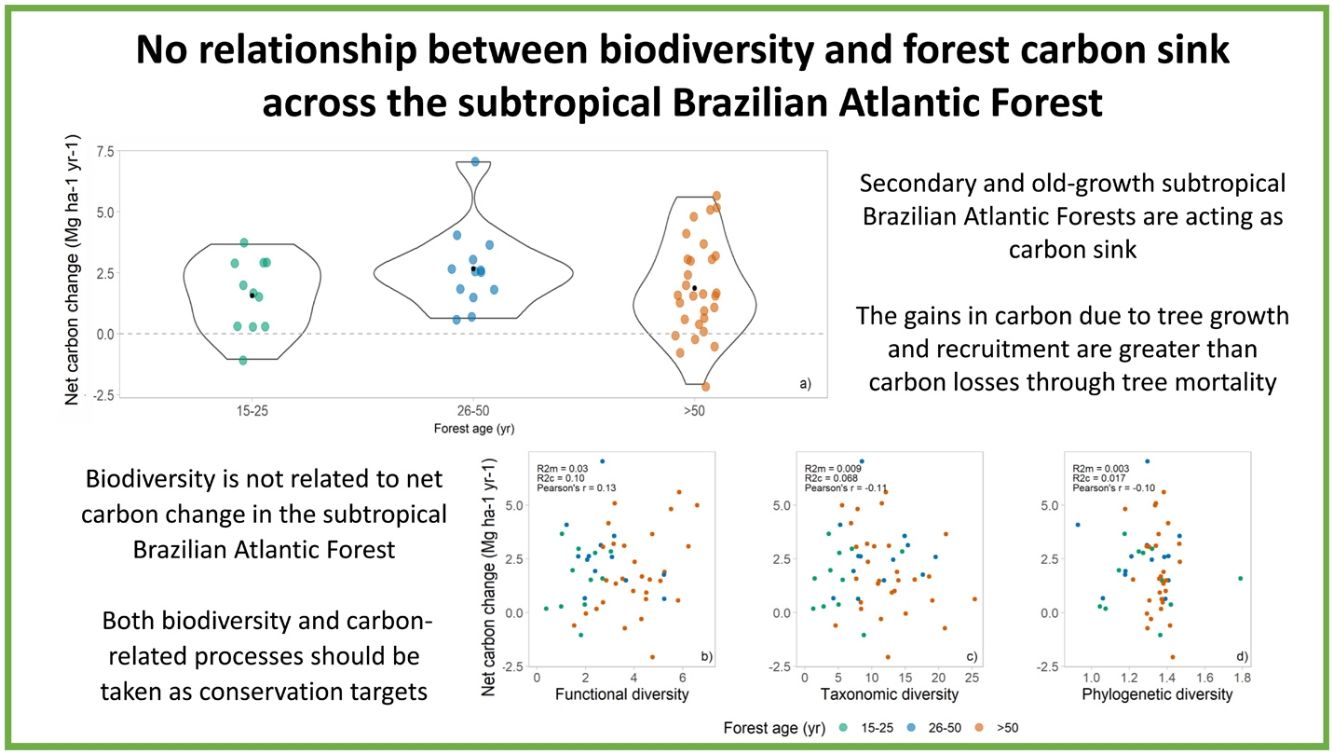
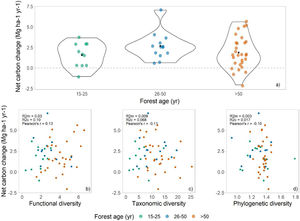
ResultsThe above-ground woody carbon stocks in the subtropical Brazilian Atlantic Forest are on average 98.5 Mg C ha−1 (± 45.3) across all forest ages. The subtropical BAF are acting as carbon sink (in the period from 2009 to 2020), with a positive net carbon change of 1.8 (±1.75) Mg C ha−1 yr−1. This is the balance between the mean annual above-ground woody productivity of 3.3 (±1.73) Mg C ha−1 yr−1, and carbon losses of 1.5 (±1.04) Mg C ha−1 yr−1 (Table 1, Fig. 2a). Net carbon change does not vary significantly among different successional stages, but we observed a great variation in the net carbon change across the late successional/old-growth forests (Table 1, Fig. 2a).
Mean (± standard deviation) values of carbon dynamics metrics and biodiversity variables for forest communities in the subtropical Brazilian Atlantic Forest grouped into different successional stages (15–25 years, 26–50 years and >50 years). Overall results are highlighted in bold.
| Forest age (yr) | Productivity (Mg C ha−1 yr−1) | Mortality (Mg C ha−1 yr−1) | Net carbon change (Mg C ha−1 yr−1) | Carbon stocks (Mg C ha−1) | Taxonomic diversity | Functional diversity | Phylogenetic diversity |
|---|---|---|---|---|---|---|---|
| 15−25 | 2.87 (1.07) | 1.31 (1.06) | 1.55 (1.37) | 50.66 (22.74) | 5.37 (3.79) | 1.78 (0.79) | 1.29 (0.20) |
| 26−50 | 3.80 (1.57) | 1.42 (0.86) | 2.38 (1.50) | 85.30 (25.72) | 10.97 (4.89) | 2.85 (1.22) | 1.27 (0.15) |
| >50 | 3.29 (1.85) | 1.62 (1.08) | 1.67 (1.85) | 109.06 (44.16) | 12.68 (4.96) | 3.97 (1.24) | 1.35 (0.06) |
| Overall | 3.3 (1.75) | 1.5 (1.04) | 1.8 (1.75) | 98.5 (45.3) | 10.82 (5.45) | 3.27 (1.44) | 1.32 (0.12) |
Net carbon change across successional stages and its relationship with different biodiversity metrics. (a) Net carbon change estimates across different forest ages (green points = 15–25, blue points = 26–50, and orange points ≥50 years) in the subtropical Brazilian Atlantic Forest. The black point in a) shows the mean value across the data. The scatterplots show the relationship of (b) functional diversity (RaoQ), (c) taxonomic diversity (Simpson index), and (d) phylogenetic diversity (Mean Pairwise Distance) and forest net carbon changes across the subtropical Brazilian Atlantic Forest.
We found no relationship between diversity metrics and net carbon change (Fig. 2a–c). Both functional, taxonomic, and phylogenetic diversity were not associated with forest carbon sink, and the models presented very low R²m (i.e., fixed effects). The Pearson’s correlation coefficients between biodiversity are low as well: 0.13, −0.11, and −0.13 for FD, taxonomic diversity, and PD, respectively (Fig. 2b–d).
DiscussionHere we provide the first regional analysis of carbon dynamics across the subtropical Brazilian Atlantic Forest (BAF). We show that these forests are acting as carbon sink. However, we did not find significant relationships between biodiversity and carbon capture capacity across the region. This implies that conservation efforts only focusing on carbon sink capacity will fail to protect distinct biodiversity facets (taxonomic diversity, FD, and PD of trees) across these forests. Therefore, management and conservation actions should consider carbon and biodiversity as independent targets to allow both the biodiversity and carbon-related processes conservation.
These subtropical forests have an important contribution to carbon cycling even across different successional stages, acting as carbon sink from initial secondary forests (15−25 years) to late successional and old-growth forests. Local studies previously showed the carbon sink of 0.4 Mg C ha−1 yr−1 in old-growth forests (Bordin and Müller, 2019). Here, with a broader coverage of evaluation with forest sites of different successional stages, we revealed a greater sink capacity of the subtropical BAF, on average of 1.8 Mg C ha−1 yr−1. Considering the old-growth forests (1.67 Mg C ha−1 yr−1), the estimated sink is higher than Andean (Duque et al., 2021), African (Hubau et al., 2020), and Amazonian forests (Brienen et al., 2015), but in a very smaller and fragmented area in comparison to these tropical forests. This positive carbon sink is potentially related to the forest recovery from past disturbances. Additionally, if this carbon sink is maintained over time, this region may represent an important refuge for carbon sequestration, particularly considering the weakening in carbon sink across tropics due to increasing tree mortality after drought periods or deforestation (Brienen et al., 2015; Maia et al., 2020). Thus, it indicates the potential of subtropical forests in capturing atmospheric CO2, to maintain carbon uptake and storage in the future (Duque et al., 2021), therefore making subtropical BAF an important region for forest restoration programs.
Contrary to our expectation, there is no relationship among biodiversity measures and net carbon changes (R2 and Pearson’s correlation near to zero). It means that higher diversity of species, functions, and lineages did not imply in higher carbon sink capacity in this region, and the absence of relationship was independent of the forest age (Supplementary information 5). In tropical forests, the relationship of biodiversity and carbon-related process are scale-dependent, and no relationship is observed across large spatial scales (i.e., larger grains such as one hectare plots) (Chisholm et al., 2013; Sullivan et al., 2017). This contrasting patterns in different scales may be occurring in subtropical BAF, where there is a positive effect of taxonomic diversity in carbon gains at smaller scales (i.e., smaller grains such as 0.05 ha plots) (Bordin and Müller, 2019), potentially due to local variation in stem density across the plots (Chisholm et al., 2013). Therefore, to protect biodiversity in subtropical BAF is crucial, and if managers and policy makers only target carbon-related processes in conservation actions, biodiversity conservation will be neglected (Ferreira et al., 2018; Reside et al., 2017), as these components are not correlated in this region.
The subtropical BAF are highly productive, irrespective of forest ages (Supplementary information 6). As productivity is an important component to obtain net carbon changes, this result emphasizes the needs of preserving old-growth forests as well restoring young and degraded forest ecosystems. By restoring ecosystems and allowing the secondary succession, subtropical forests may achieve the mature stage, which will be crucial for carbon fluxes in the future (Capellesso et al., 2021; Heinrich et al., 2021; Souza et al., 2021). Additionally, by conserving old-growth forests we will be maintaining not only carbon sink, but a great amount of carbon stored in the large-sized trees across this region (Bordin et al., 2021). Finally, investments in conservation are crucial to maintain both the biodiversity and carbon sink provided by the subtropical Brazilian Atlantic Forest and all the related ecological processes across this important region for carbon mitigation.
ConclusionsThere is an urge for efforts to conserve highly productive forests, with a great carbon sink capacity as observed in the subtropical Brazilian Atlantic Forest. These conservation efforts should target both biodiversity protection and carbon storage and fluxes, as efforts focusing solely in one of these may neglect the other. This is especially important when considering the threats from future climatic changes and consequentially carbon losses through tree mortality. For instance, global change is likely to have been causing the decline of the carbon sink capacity in Amazonian forests (Brienen et al., 2015), and potentially leading forests to a shift from a carbon sink to a carbon source (Maia et al., 2020). Subtropical BAF have been suffering with higher precipitation rates (Souza and Longhi, 2019) and extreme climatic events (Bordin and Müller, 2019; Liebsch et al., 2021) due to global changes. These climatic changes are expected to enhance mortality rates and carbon losses in the near future, thus limiting the currently observed carbon sink capacity (Aleixo et al., 2019). Therefore, to maintain long-term records of forest dynamics from permanent forest inventory plots is crucial to understand the trends of carbon dynamics for this region (ForestPlots et al., 2021) and its potential as a carbon sink and its contribution to the global carbon cycle.
FundingThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001, through Portal de Periódicos, and scholarships granted to KMB, JK, and RCP under the Programa de Excelência Acadêmica (PROEX). The fieldwork was supported by Rufford Foundation [grant 27666-1], Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul [FAPERGS grant #19/2551-0001698-0], and Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq, Process 480738/2013-0]. KMB and SCM are supported by the Instituto Nacional de Ciência e Tecnologia (INCT) in Ecology, Evolution and Biodiversity Conservation, from MCTIC/CNPq [grant #465610/2014-5], and FAPEG [grant #201810267000023]. ESC and MCMM thank the Society for Wildlife Research and Environmental Education – SPVS and Fundação Grupo Boticário [Grants FGB 0801_20082, FGB A2012009]. AE-M is supported by the Royal Society (RGSR1221115), the ERC TreeMort project (758873) and the CESAB SynTreeSys project. RSB and AE-M are supported by the NERC UKRI TreeScapes MEMBRA (NE/V021346/1). SCM, ACS, PH, and MCMM are supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq; grants 309659/2019-1, 305916/2017-3, 304902/2019-5, and 303356/2019-7, respectively].
Conflicts of interestThe authors declare no conflict of interests in this work.
Data availabilityPart of the original data is available at ForestPlots.net database (ForestPlots et al., 2021). The processed data and R codes are available at https://doi.org/10.5281/zenodo.7521384.
Authors’ contributionsKMB, AE-M and SCM designed the research. KMB, JK, RCP, RSB, ACS, PH, ESC, MCMM, AFS collected the data. KMB analysed the data. KMB led the writing of the manuscript with great inputs and supervision from AE-M and SCM. All authors read and commented on the manuscript, giving final approval for publication.
Many thanks to all the ones involved in data collection and curation for the great effort in maintaining long-term plots, and then allowing studies on forest dynamics. Muito obrigada a todos os envolvidos com a coleta e processamento dos dados pelo enorme esforço em manter parcelas permanentes e assim permitir estudos de dinâmica florestal. A Ciência Brasileira resiste!