Conservation ecology research, management and modeling often assume species-specific fixed traits ignoring intraspecific variation. Dispersal in animals is a heritable trait where intraspecific variation should be common, as it develops via interactions between landscape and behavioral processes. We conducted translocation-radio-tracking experiments and novel-environment tests on a Neotropical rainforest bird (Pyriglena leucoptera, Thamnophillidae) to assess whether dispersal success and exploratory behavior are determined by an individual's population of origin (i.e. fragmented or continuous forest). Based on a model for non-optimal animal movement in human-modified landscapes, we predicted that individuals that evolve or develop in fragmented landscapes, with daily exposure to risky boundary and matrix conditions, would have higher resistance to boundary-crossing and overall increased dispersal success than individuals from continuous habitats. We found that birds from fragmented landscapes were more resistant to cross boundaries and more successful at crossing the matrix relative to birds from continuous forest. Novel-environment tests detected reduced exploratory scores for birds from fragments, suggesting they were slower-explorers, and possibly more thorough in assessing their environment which, in turn, may have enabled more successful matrix transit. Observed behavioral differences can emerge by genetic adaptation or behavioral adjustments. In any case, because P. leucoptera is capable of adaptive behavioral adjustments to fragmentation, gradual landscape changes should be encouraged to minimize the potential for emergence of non-optimal dispersal behaviors in human-modified landscapes.
Dispersal is a complex process involving the interaction between animal behavior and landscape structure (Baguette and Dyck, 2007). This is because landscapes in which organisms move are heterogeneous and composed of elements with dynamically changing costs and benefits for dispersing organisms (Belisle, 2005). Spatial models of animal distribution, commonly assume that dispersal strategies are fixed and evolutionarily stable. That is, individuals move according to species-specific decision rules, for example to avoid inbreeding or low suitability habitat (Lima and Zollner, 1996; Bonte et al., 2012). But dispersal is a complex and multi-phase life-history process that is under selection at each of its stages (Clobert et al., 2009) and, therefore, should rarely be invariant.
Sex-biased dispersal, for example, is a well-documented form of intraspecific behavioral variation (Pusey, 1987) and is commonly assumed to be a near-universal avian trait. Though, it is rarely considered in conservation planning, empirical study designs or in models of landscape connectivity. A recent study tested for sex-biased movement in an understory forest bird species in fragmented landscapes finding that males indeed had a reduced inter-patch movement success when compared to females (Awade et al., 2017). This finding confirms that sex-biased movements occur in fragmented landscapes, and should be more widely considered in connectivity-conservation scenarios; but also, that inter-individual variation in general should be considered. Given especially that dispersal behavior is heritable and under selection, differences among traits related to the different stages of dispersal (i.e., emigration, transfer in the matrix and immigration) should be expected among populations that persist in different landscape contexts (e.g., continuous or fragmented forests).
Indeed, organisms that persist in fragmented landscapes do exhibit inter-population variation in dispersal traits related to landscape structure. Butterfly populations from fragmented or disturbed habitats differ in dispersal related traits when compared to populations from continuous or undisturbed habitats (Merckx and Van Dyck, 2006; Öckinger and Van Dyck, 2012). In a long-term capture–recapture study of forest birds in Amazonia, conspecific individuals exhibited differences in maximum dispersed distances depending on whether they occurred in forest fragments or continuous forest (Van Houtan et al., 2007). Studies in temperate forests demonstrate that birds hatched in isolated territories have greater natal dispersal distances (Pasinelli et al., 2004) and a delay of dispersal events (Lens and Dhondt, 1994).
Fahrig (2007) proposed a conceptual model for dispersal evolution in different landscape contexts. The model predicts that individuals evolving in continuous habitats, with little or no experience with habitat boundaries, would exhibit low resistance to cross boundaries that they do encounter. In fragmented landscapes, those individuals experience high mortality in the matrix due to the usually high-risk matrix encountered after crossing a boundary, and therefore have generally low dispersal success, which makes them maladapted in human modified landscapes. On the other hand, in young birds from fragmented landscapes that developed exposed to a risky matrix and its boundary conditions, there should be strong selection for appropriate risk-averse movement decisions including behavioral avoidance of crossing dangerous habitat boundaries. This model assumes selection and genetic adaptation over longer time scales as the main driver of boundary responses but behavioral variation in the short term can also emerge by behavioral plasticity (Stamps and Biro, 2016).
Animals have the ability to change their behavior to reduce risk (Lima and Dill, 1990). During exploration and dispersal movements, risks to birds abound but the collection and use of information about those risks can make dispersal safer for individuals (Delgado et al., 2014). However, dispersing animals can only use information if they are able to correctly interpret available cues, for example those related to different kinds of predation threats (Huang et al., 2012). The capacity of using this information can be either inherited or obtained from direct experience with their environment (Spiegel and Crofoot, 2016). That is, movement behavior in fragmented landscapes might also emerge as some individuals adjust their movement behavior based on state-dependent conditions. For example, wild great tits (Parus major) became fast movers or fast explorers (i.e., exhibit increased risk taking behavior) when their probability of survival was experimentally decreased (Nicolaus et al., 2012).
Within birds and other vertebrates, exploratory behavior is a key trait underlying individual variation in use of space and resources because it provides the means to learn about and utilize the environment (Mettke-Hofmann et al., 2006). Thus, individuals that spend their juvenal exploratory phase in fragmented landscapes may be exposed to many different cues, information types and sources, and develop different decision-making strategies when compared to individuals that grow up in a continuous habitat without complex boundary conditions (Spiegel and Crofoot, 2016; Reader, 2015). Additionally, juvenal individuals that make poor decisions in gap and forest boundary crossing may get weeded out, leaving adults in fragmented habitat with better spatial decision-making regarding edges and their dangers (Cosentino and Droney, 2016).
We conducted a translocation experiment with radio-tracking to assess whether inter-patch dispersal success is different among individuals of populations belonging to contrasting landscapes (i.e. fragmented vs. continuous forest) in a Neotropical rainforest bird species, the White-shouldered Fire-eye (Pyriglena leucoptera, Thamnophillidae). Our main hypothesis, derived from Fahrig's (2007) framework and other empirical evidence reviewed above, is that lack of previous experience with a fragmented landscape should hinder successful inter-patch movement in White-shouldered Fire-eyes raised in continuous unbroken forest (and vice versa; birds living in fragmented habitat would disperse better in a fragmented landscape). Furthermore, we propose that this pattern is a consequence of higher emigration propensity in edge-naïve birds from continuous forest (i.e. low resistance to cross boundaries) with higher mortality in the matrix, while individuals from the fragmented landscape should show lower emigration propensity (i.e. high resistance to cross boundaries) but an overall higher dispersal success because once they leave a fragment they are more successful crossing the matrix. To help reveal differences in emigration propensity, we subjected birds to a short behavioral trial in a cage to determine their location on the fast-slow exploratory continuum, because there seems to be a correlation between ‘slow’ exploring in birds and higher survival in the face of risky decision-making (Hall et al., 2015).
Therefore, our specific predictions are as follows: individuals from fragmented landscapes (i) should take longer to leave a small forest patch, (ii) spend less time crossing matrix habitat when moving (thereby minimizing exposure to predators) and, consequently, (iii) exhibit greater success in arriving to a neighboring forest patch and, finally, in the behavioral trials, (iv) birds from the fragmented landscape should be slower explorers than birds raised in continuous forest. Our study design cannot disentangle selective genetic change from behavioral adjustments, but rather aims to test whether inter-population variation in dispersal traits can arise in response to human-driven landscape change. We also seek to provide information for better management practices to enhance connectivity in fragmented landscapes.
MethodsStudy site and model speciesWe conducted this study in the sub-tropical Atlantic Forest region located in the Atlantic Plateau of São Paulo, southeastern Brazil (47° 20′W–48° 40′W and 23° 43′S–24° 06′S). The area encompasses a fragmented landscape (ca. 30% forest cover) dominated by small habitat patches of secondary forest (<100ha) embedded in a matrix dominated by croplands and pastures (>80% of the matrix). The fragmented landscape is adjacent to a large protected forested area (>1,000,000ha of continuous forest; Ribeiro et al., 2009).
The White-shouldered Fire-eye (P. leucoptera, Thamnophillidae) is an endemic bird of the Atlantic Forest found in the understory of primary and secondary forests and frequently close to edges in forest fragments (Hansbauer et al., 2007, 2008a,b; Sick, 1997). It is a 30g insectivorous bird with marked sexual dimorphism that forages while following army-ants (Sick, 1997). We selected this species because it is common in fragmented landscapes where forest cover exceeds 30%, but uncommon or absent where less than 15% forest cover remains (Boscolo and Metzger, 2011). It is not endangered and was categorized with medium sensitivity to landscape change (Hansbauer et al., 2008a; Uezu et al., 2005).
Experimental designWe used a translocation-and-radiotracking experimental design similar to that utilized by Castellón and Sieving (2006) and we followed the same field protocol as Awade et al. (2017). Birds were captured in continuous forest sites (>1,000,000ha) or in fragmented landscape sites and then released in forest patches located >10km away in the fragmented landscape. This design simulates a situation analogous to a characteristic phase of dispersal; that is, having to move through an unknown area with no previous information of that specific location (Castellón and Sieving, 2006). We controlled release site characteristics, as much as possible in order to provide a standardized dispersal stimulus for all individuals. For this study, we used only males of P. leucoptera, because males have lower inter-patch movement success than females (Awade et al., 2017) and represent the portion of the population that most limits dispersal. We captured males with mist nets and banded them with unique numbered metal-bands and radio tagged them following the glue on attachment procedure described in Hansbauer and Pimentel (2008; see for transmitter specifications). We translocated only adults because our goal was to understand how adult individuals would cope with landscape change (e.g., when forced to disperse after clear-cutting). We conducted translocations from January 2008 through April 2009. Capture sites in the continuous forest were >2.5km from any forest edge. Capture and release sites in the fragmented landscape were located >5km from the continuous forest edge and in areas with ca. 30% of forest cover. We translocated 19 birds from the fragmented landscape and 15 from the continuous forest (n=34).
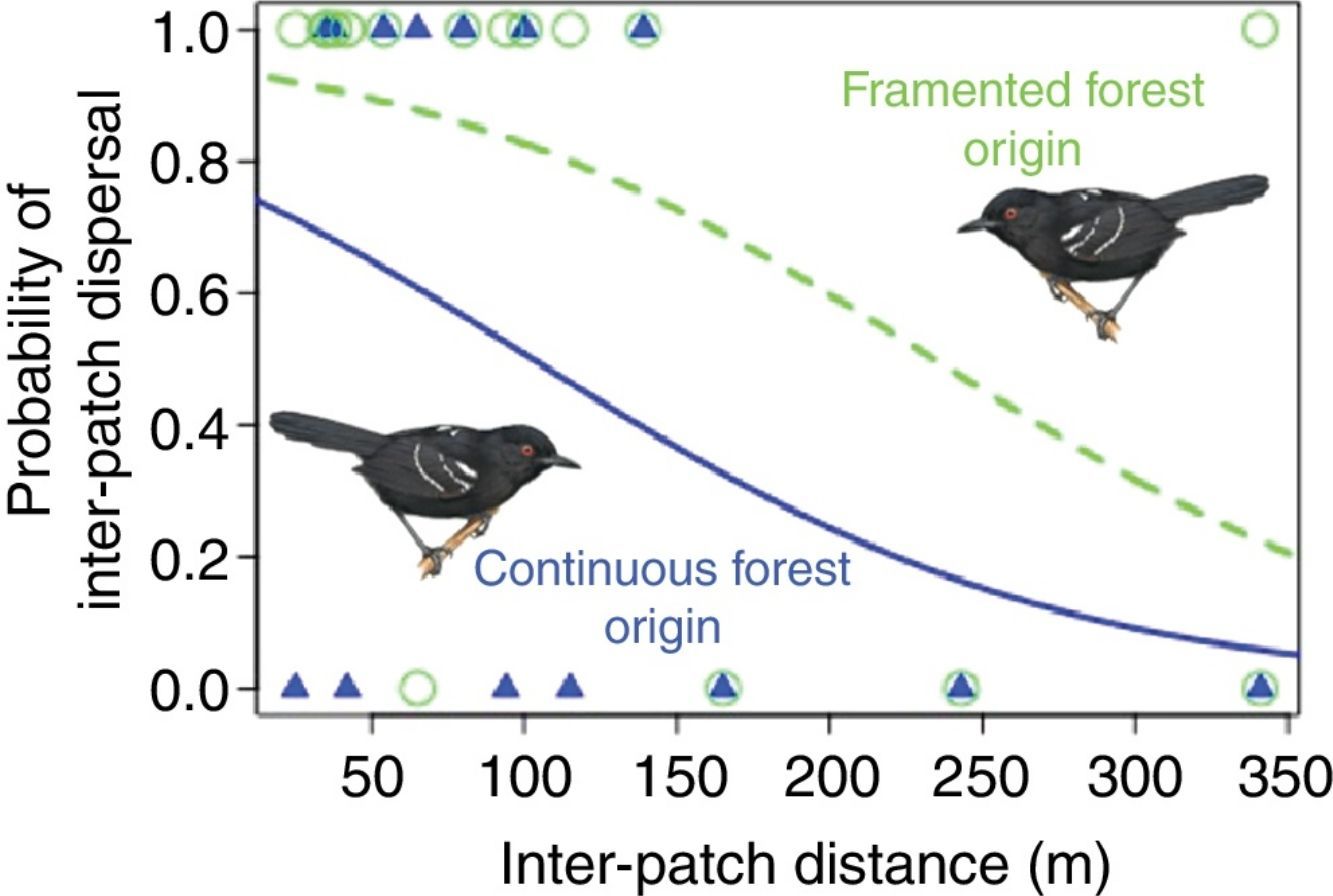
Release landscapesWe translocated birds into 14 distinct release landscapes, each with a release patch, a nearest-neighbor patch, and an area of matrix that surrounded both patches. To standardize gap characteristics, matrix areas all had low growing herbaceous vegetation (either pasture or short crops like beans). Open areas without woody cover of any kind represents a high-risk dispersal matrix for P. leucoptera (a skulking understory bird; see Biz et al., 2017). Nearest-neighbor distance varied from 25 to 340m among release landscapes. This allowed us to simulate a dispersal event in which inter-patch distance is the main environmental variable affecting dispersal decisions.
Radio-trackingWe uniformly distributed releases (regarding nearest patch distance and population of origin) throughout the study period to avoid seasonal confounding effects. Each release patch received two individuals at different times, one from the continuous and one from the fragmented population. Each translocated individual was systematically tracked for a maximum of five consecutive days or until completion of an inter-patch movement event (see details in Appendix A). We obtained a complete systematic monitoring dataset for at least one bird per population at each release landscape (n=28). We repeated translocations when the transmitter detached prior to the end of the monitoring period (n=6); of these individuals we determined the fate of two (by the recovery of the banded bird) but the fate of four individuals was unknown (i.e. censored).
Exploratory behaviorTo quantify exploratory behavior we conducted tests in which each individual was exposed to a novel environment using a portable cage (300cm long, 150cm wide and 150cm high) with five wooden perches similar to that used by (Huang et al., 2015; see for details). The following variables were recorded: (1) number of flights and hops in the first 2min (‘exploratory score’ – ES; Dingemanse et al., 2002), (2) number of perches visited, (3) number of scanning events (SC), and (4) number of vocalizations (VOC) in 20min. Scanning is not only limited to “vigilance” for predator detection, scanning is also directly related to the acquisition of information about the landscape (Huang et al., 2015). Vocalizations are also an important way of obtaining information, especially (but not restricted to) from conspecific (Lima and Zollner, 1996; Sieving et al., 2010). We tested 28 males, 12 from the continuous forest and 16 from the fragmented landscape.
Data analysesWe considered three stages that describe the dispersal process and that represented our response variables: (1) emigration propensity – measured as the time individuals spent in the release patch before leaving the patch (n=34); (2) matrix transfer – for this stage we measured two response variables, time individuals spent in the matrix before arriving a neighboring patch (n=16, for the remaining individuals we could not obtain a complete history while in the matrix), and the number of individuals that died while crossing the matrix (i.e. mortality in the matrix); here we included only birds that emigrated and had known fate (n=21); (3) inter-patch dispersal success – the number of individuals that successfully reached a neighboring patch out of those that were released in the patch (n=30; four unknown fate individuals were excluded); here we define dispersal as a successful inter-patch movement event. Predictor variables were distance to the nearest patch (25–340m) and population of origin (continuous or fragmented). For the exploratory behavior analyses we used the behavioral response variables described above and population of origin as predictor variable.
We built a unique set of models to explain the source of variation in each response variable. We used the second-order Akaike information criterion (AICc) to evaluate the plausibility of competing models. We based our inferences on the best model of the set (with the lowest AICc value), but considered every model with ΔAICc<2.0 as equally plausible (Burnham and Anderson, 2002). The strength of evidence in favor of a model being the best one was given by its Akaike weight (wi).
To analyze emigration propensity and time spent in the matrix we used parametric survival models (Collett, 2003) using day-light hours as a measure of time. We modeled the survival function as a Weibull or Exponential distribution. The first allowed us to evaluate whether the hazard rates changed over time (i.e. the instantaneous probability that an individual emigrates at time t, given that it stayed in the release patch until t) while the second is a particular case in which the probability of emigration does not depend on the time spend in the release patch. We used generalized linear models (GLM) to analyze mortality in the matrix and inter-patch dispersal success with binomial distributions, and exploratory behavior with poison or negative binomial distributions when data presented over-dispersion (see Appendix A for details). The sets of models we used to analyze emigration propensity, mortality in the matrix and inter-patch dispersal success were composed by five models with the following general structure: (i) a global model, including the interactive effects of origin and distance; (ii) an additive model, considering the effects of distance and origin without their interaction; (iii) a distance model only; (iv) an origin model only; and (v) a “constant” model, in which the response variables are not affected by any of these predictors. For exploratory behavior analyses we contrasted (i) a model including the effect of population of origin against a (ii) “constant” model. All analyses were run using R 3.2.1 software (R-Core-Team, 2015) with the bbmle (Bolker and Team, 2016) and survival (Therneau, 2015) packages.
ResultsEmigration propensityFive individuals from the continuous population (out of 15) and seven from the fragmented population (out of 19) did not leave the release patch in less than five days (ca. 65 daylight hours). Two models explained emigration propensity; the first model included only distance as a predictor (AICc=201.2, wi=0.595) and the second model, with ΔAICc=1.8 considered an additive effect of distance and population of origin (AICc=203, wi=0.253). The evidence in favor of these two models was very strong compared with the constant model (ΔAICc=4.9, wi=0.052), and together, the strength of evidence in favor of these two models sums to 0.848. All other models had very weak support (ΔAICc≥4, wi≤0.083; Table S1). Based on the parameters of the best models, inter-patch distance had a positive effect on the time spent in the patch before emigrating; but males from the fragmented population remained longer in the release patch than males from the continuous population (Table 1). In addition, there was no evidence supporting constant hazard rates since the Weibull models were always selected over the Exponential models (Table S1). The hazard rates ρ were <1 (Table 1) meaning that the probability of emigration decreases as individuals stay longer in the release patches.
Parameter estimates of the two selected survival models on the source of variation in emigration propensity (ep) (i.e., time remaining in the release patch before emigration) of P. leucoptera as a function of distance and population of origin (F=fragmented population). Positive estimates imply that the probability of remaining in the patch increases with the effect covariate and ρ represents the shape parameter of the Weibull distribution (hazard rate).
| Effect | Estimate (β) | S.E. | ρ |
|---|---|---|---|
| ep∼distance (Weibull) | 0.526 | ||
| Intercept | 2.514 | 0.669 | |
| Distance | 0.014 | 0.006 | |
| Log (scale) | 0.644 | 0.170 | |
| ep∼distance+origin (Weibull) | 0.535 | ||
| Intercept | 2.036 | 0.812 | |
| Distance | 0.014 | 0.006 | |
| Population (F) | 0.755 | 0.603 | |
| Log (scale) | 0.625 | 0.170 | |
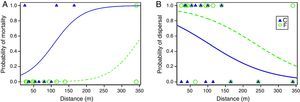
We obtained complete matrix transfer histories for 16 individuals (9 from the continuous and 7 from the fragmented population). Three survival models were selected to explain the time spent in the matrix, but the best model was the constant model (AICc=57.1, wi=0.3124), indicating that the most parsimonious explanation for the time remaining in the matrix is the model that does not consider an effect of any variable (Table S1). There was evidence, however, supporting different mortality in the matrix among populations. Three out of 9 males from the continuous population that emigrated died in the matrix, while only one out 12 from the fragmented population that emigrated died in the matrix. The best single model selected that explained mortality in the matrix included an additive effect of distance and population of origin (AICc=19.9, wi=0.520). The constant model was not selected among the candidate models, but the evidence in favor of the best model was only moderate compared with the constant model (ΔAICc=2.7, wi=0.130; Table S2). Parameter estimates of this model indicate that there was a positive effect of distance on mortality and that at equivalent distances individuals from the fragmented landscape had a lower probability of mortality than individuals from the continuous population (βIntercept=−2.69, SE=1.632; βPopF=−5.863, SE=4.639; βdistance=0.025, SE=0.017; Fig. 1A).
(A) Probability of mortality for P. leucoptera males during transfer in the matrix as a function of inter patch-distance and population of origin (C=continuous forest, F=fragmented forest); (B) probability of dispersal (i.e. probability of reaching successfully a forest patch after release) for P. leucoptera males as a function of inter-patch distance and population of origin. Curves were fitted based on the estimated parameters of the best models that included and additive effect of distance and population of origin (Table S2).
We obtained inter-patch dispersal data for 30 translocated birds (16 from the fragmented and 14 from the continuous population). Out of these, 12 males from the fragmented population (75%) were able to arrive in another patch, whereas only seven males from the continuous population (50%) were successful. The best model to explain inter-patch dispersal success was the model including the additive effects of distance and population of origin (AICc=38, wi=0.423), the second model selected with ΔAICc=0.5, included only the effect of distance (AICc=38.5, wi=0.330). The evidence in favor of these two models was very strong compared with the constant model (ΔAICc=3.6, wi=0.07) and together, the strength of evidence in favor of these two models summed to 0.753 (Table S2). Per the estimated parameters of the best model (βIntercept=1.194, SE=0.762; βPopF=1.523, SE=0.939; βdistance=−0.012, SE=0.005), distance had a negative effect on inter-patch dispersal success and at equivalent distances males from the fragmented population had higher inter-patch dispersal success than males from the continuous population (Fig. 1B).
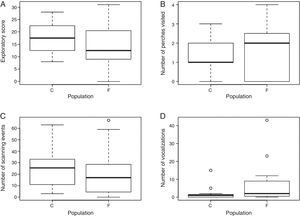
Exploratory behaviorResults of exploratory variables are shown in Fig. 2. The evidence in favor of the models including a population effect on the exploratory score (ES), number of scanning (SC) and vocalization (VOC) events was strong compared with their respective constant models (constant models had ΔAICc>4.4, wi<0.1, Table S3). According to the estimated parameters of the population effect models, ES and SC were lower for males from the fragmented landscape than from the continuous forest (ES: βIntercept=2.89, SE=0.07; βPopF=−0.25, SE=0.09; SC: βIntercept=3.28, SE=0.06; βPopF=−0.26, SE=0.08), while VOC was higher for males from the fragmented landscape than from the continuous forest (VOC: βIntercept=0.77, SE=0.59; βPopF=2.61, SE=0.77). We found no evidence for a population effect on the number of perches visited in 20min. The evidence in favor of the constant model (AICc=88, wi=0.72) was strong compared with the population effect model (ΔAICc=1.9, wi=0.28). Most individuals (ca. 80%) visited only one or two perches and none visited five during the observation time, but 5 males from the fragmented landscape visited zero perches and only two males from the fragmented landscape visited four perches (Table S4 and Fig. S1).
Exploratory behavior variables: (A) exploratory score, (B) number of perches visited, (C) number of scanning events and (D) number of vocalizations recorded during the novel environment tests for P. leucoptera males from the continuous (C; n=12) and fragmented forest population (F; n=16). In the number of vocalizations graph one observation was excluded for better visualization of data (one fragmented forest individual with 363 vocalizations; see Appendix A and Table S4).
Utilizing the rationale set out in Fahrig's (2007) conceptual model for non-optimal animal movement in human-modified landscapes we investigated whether individuals of P. leucoptera taken from different populations of origin (i.e. with or without previous experience with fragmented landscapes) would exhibit (a) different behavioral patterns when moving freely through a fragmented landscape, (b) different inter-patch dispersal success and (c) different exploratory behavior in a novel captive environment. In agreement with model predictions we found that individuals taken from continuous habitat, presumably with no previous experience of fragmented boundaries or non-forest habitats, had a higher emigration propensity (i.e. reduced boundary response), lower success when crossing the matrix, and consequently, exhibited an overall reduced dispersal success in human-modified landscapes.
The central implication of this study (and central prediction of Fahrig's model) is that sudden landscape changes may result in non-optimal spatial behaviors that can lead directly to reduced population persistence in fragmented landscapes. On the other hand, our findings (as predicted) revealed that P. leucoptera individuals living in older fragmented landscapes had an overall higher dispersal success than individuals from the continuous forest population, suggesting that species can adapt to life in fragmented landscapes with time (Cheptou et al., 2017).
Larger inter-patch distances result into reduced dispersal success as Awade et al. (2017) demonstrated in the same system. But individuals from the fragmented population crossed longer distances more successfully than individuals from the continuous forest. Interestingly, time spent in the matrix was similar among populations, contrary to expectations, suggesting that once past the boundary individuals from both populations moved quickly to minimize exposure to risk (Lima and Dill, 1990). However, individuals from the fragmented population, moving equivalent inter-patch distances as individuals from the continuous forest, experienced reduced probability of mortality in the matrix. Specifically, the distance at which individuals from the fragmented landscape had a 50% chance of dispersing successfully (ca. 250m) is about 2.5 times larger than the distance for individuals from the continuous forest (ca. 100m). This suggests that their previous exposure to the dangers of fragmented landscapes helped them move more safely (Reader, 2015).
The differences in emigration propensity, or boundary response, observed in individuals from the different populations may be explained by the differences in their exploratory behavior that we detected (i.e., reduced exploratory score in individuals from the fragmented population). Fast versus slow exploration, assessed via activity levels in novel environment tests, is one of several traditional axes of personality syndrome (Carter et al., 2013) that drive inter-individual variation in many types of behaviors, including dispersal propensity (Dingemanse et al., 2003; Debeffe et al., 2013). Individuals (and species) that move more frequently, visit a larger volume of the cage and visually scan their environment more, fall within a “fast explorer” behavioral profile (Huang et al., 2015). But these fast explorers explore their environment more superficially when compared to slow explorers (Van Oers et al., 2004). Fast exploration is therefore commonly used as a proxy for risk-taking behavior (faster explorers take more survival and reproductive risks; Nicolaus et al., 2012).
On the other hand, slow exploration can predict better performance at some problem-solving tasks, including discrimination of vocal cues (Guillette et al., 2011), or a higher perception of incident predation risk (Huang et al., 2012) and presumably lower chance of attack by avian predators. Many such slow versus fast exploration functional differences could be related to the differences we observed in dispersal success and movement patterns in P. leucoptera. Individuals from the fragmented landscape were overall slower explorers when compared to the continuous forest individuals. If birds from fragmented landscapes are risk-averse and more accurate in interpretation of information cues, then these behavioral patterns could indeed enable more successful dispersal.
Individuals from the fragmented landscape vocalized more also, raising several possible factors. The birds could have been seeking conspecific social information (King and McGregor, 2016) because birds raised in riskier fragmented habitat may rely more heavily on social cues about risks and rewards (Griffin, 2004). For example, individuals seeking suitable habitat, under a model of conspecific attraction, will use vocalizations of conspecifics to orient toward suitable, and minimize time in unsuitable habitat (Fletcher, 2006). Alternatively, higher calling rates may simply signify an increased level of anxiety and perception of greater predation risk that birds from continuous forest are too naïve to sense (Sieving et al., 2010).
Our study was not designed to disentangle the relative importance of genetic adaptation versus behavioral plasticity (or individual behavioral adjustments) in determining the observed inter-population behavioral variation in dispersal patterns and success. We argue, however, that this variation is likely a consequence of individual behavioral adjustments that result from state-dependent decisions in different landscape contexts. In fragmented landscapes P. leucoptera individuals may be adjusting their behavior as they gain experience and encounter edges and the risky matrix. As juvenile birds they may learn to be slow, and careful explorers with greater risk-aversion (Reader, 2015). This is more likely than a landscape-level difference in genetic frequencies of slow-exploring individuals in fragmented areas for two reasons. First, fragmentation in this area is only ca. 150 years old (Ribeiro et al., 2009) and P. leucoptera has too a long generation time to have changed that much. Second, this species can probably disperse far enough to insure at least some gene flow among continuous and fragmented populations within our study region, as evidenced by high haplotype sharing among southern populations (Maldonado-Coelho, 2012); even a few dispersal events among populations may counteract gene frequency changes due to selection (Lenormand, 2002). It certainly is likely that given enough isolation, an evolutionary signature could emerge quickly because our data suggest that the mortality rate of non-optimal dispersers could represent hard selection (Wade, 1985). Future studies on similar systems should not discount the simultaneous occurrence of both mechanisms in attempts to discern underlying mechanisms.
Our findings have important implications for connectivity management and conservation of populations in fragmented landscapes. Observed behavioral adjustment as reported for P. leucoptera may explain in part why fewer species have gone extinct than expected in the Atlantic forest (Brooks et al., 1999). This, however, should not be interpreted as a high resilience of forest bird species to landscape change because behavioral adjustments may occur only under a small range of conditions. For P. leucoptera, distances above 350m result in very low probability of dispersal success regardless of their population of origin. At such distances corridors may be a more effective measure to increase connectivity. In fact, probability of occurrence of P. leucoptera and other forest-dependent species is very low in landscapes with less than 30% of forest cover (Banks-Leite et al., 2014; Boscolo and Metzger, 2011). Therefore, our results should be rather interpreted as a call for caution for sudden and abrupt landscape changes that reduce the chances of adaptive selection of species traits, or of individuals to adjust their behavior to the new environment. But when landscape change is unavoidable, a gradual transformation should be preferred coupled with movement-friendly matrix management (see Biz et al., 2017) and considering habitat configuration when forest cover is below 50% (Villard and Metzger, 2014) to increase connectivity in fragmented landscapes.
Conflicts of interestThe authors declare no conflicts of interest.
This study was funded by FAPESP (Project number 2007/5649-6). CC was funded by a post-doctoral fellowship granted by FAPESP (2007/55642-6). J.P.M. was funded by the National Council for Scientific and Technological Development (CNPQ, process number: 307934/2011-0). Field work and behavioral trials were performed under the permit for conducting scientific activities granted by the Brazilian Government – license number ICMBio/SISBio 14568-2 and bird-banding permit IBAMA/CEMAVE 3127 issued to CC. We would like to thank all researchers and colleagues that provided insights at different stages of this research whose contributions greatly improved the design and analyses of this study. We also thank two anonymous reviewers for their valuable comments. We are very grateful to assistants that helped us in the field, especially to Soizic Le Saout, Gregório dos Reis Menezes and Osorio Nascimento Neto. We also would like to give special thanks to the many owners of field sites who allowed us to enter their properties and that in many cases provided logistical support.