Fragmentation alters landscape structure and its relationship with organisms, where movement is one of the most affected processes. Movement choices are influenced by a cost and benefit evaluation, associated particularly to risks of predation and access to new resources. We analyzed the effect of matrix type on matrix-transfer success of a bird, Pyriglena leucoptera. We used a translocation-monitoring approach and evaluated time and trajectories used to reach an adjacent forest patch when released inside the matrix. The risk of predation was estimated by quantifying the density of birds of prey in three matrices (pastures, cornfields, and Eucalyptus) with different degrees of exposure given by differences in their vegetation structure. This variation was perceived by individuals, as evidenced by changes in their movement patterns and differences in their matrix-transfer success. The Eucalyptus, a low-risk matrix with the lowest density of predators and with more resources and shelters, resulted in tortuous movements by translocated birds. The pasture, an intermediate-risk with a wide visual field, resulted in straight and fast movements. The cornfields, a high-risk matrix with the highest density of predators and an increased obstruction of the visual field (when compared with pastures), resulted in more tortuous movements and longer exposure to risk, and thus in lower successful arrivals to forest patches. Our results highlight the importance of quantifying the effects of matrix type on movement behavior and on the persistence of species in fragmented landscapes. The proper management of matrices appears as a cost-effective option for improving connectivity in modified landscapes.
In fragmented and human modified landscapes, different types of matrices (i.e. extensive non-habitat areas surrounding remaining habitat fragments, Fahrig, 2003) can have variable levels of quality for native habitat species because of their different structural and compositional characteristics (Ricketts, 2001). These characteristics result in different levels of permeability because each matrix type has a diverse set of risks and benefits for the survival of animals passing through it (Ries and Debinski, 2001; Rodríguez et al., 2001; Antongiovanni and Metzger, 2005). As a consequence, modifications of habitat configuration or matrix composition due to fragmentation and land-use expansion can lead to decreased connectivity (Fahrig, 2003).
Animals can perceive levels of matrix quality and then modify their movement behavior in order to minimize risks and maximize benefits according to each environment (Zollner and Lima, 2005; Cornelius et al., 2017). Matrices can have different degrees of permeability depending on their vegetation structure, acting as either a complete or a semi-permeable barrier, and they can also contribute to species persistence functioning as a habitat complement (Gascon et al., 1999; Castellón and Sieving, 2006; Driscoll et al., 2013;).
Among risks, predation is one of the main factors affecting animal choices when crossing a matrix. Individuals may be more or less vulnerable to predators depending on the structure of the matrix (Desrochers and Hannon, 1997; Bélisle, 2005; Zollner and Lima, 2005; Roth et al., 2006). In order to minimize risks and/or maximize benefits in each site, animals can modify their movement behavior, permanence in the matrix and spatial distribution (Powell, 1974; Schooley and Wiens, 2003; Zollner and Lima, 2005; Haynes and Cronin, 2006). In forested landscapes, open matrices may present high risk of predation. Thus, forest animals tend to move fast in the matrix and they generally use straight pathways toward the nearest forest fragment (Zollner and Lima, 2005). On the other hand, where the risk of predation is low (e.g. with higher or denser vegetation cover), animals can stay longer and do more tortuous (or randomic) pathways to explore the available resources (Powell, 1974).
There is an increasing knowledge about how different animals use different landscape elements, but some challenges remain. Several studies have shown that this relationship depends on the structure of each landscape and is species-specific (Turcotte and Desrochers, 2003; Uezu et al., 2005; Fahrig, 2007; Van Houtan et al., 2007; Lees and Peres, 2008; Turlure et al., 2011a), which makes obtaining general rules very difficult (Zollner and Lima, 2005; Moore et al., 2008). This emphasizes the need of studies that assess animal movement in different matrix types in order to generate more accurate data sets and develop assertive mitigation actions to improve connectivity in fragmented and human-modified landscapes.
In order to clarify the influence of matrix characteristics on animals’ movement, this study aims to test how permeability, as a function of different levels of predation risk and vegetation structure, changes among different matrices. We chose the risk of predation as a main variable to be tested and used an understory forest bird to investigate its movement patterns and behavior in three matrix types (clean pastures, cornfields and Eucalyptus plantations). Here we assessed three questions: I) How much does the density of predators vary among the three matrix types? II) Do bird trajectories across the matrix differ among the three matrices? and, if so, III) Do these differences result in different capacities to cross the matrix?
We expect different density of predators among matrices because different vegetation types may offer different amount of resources or even facilitate or hinder access to prey (Jullien and Thiollay, 1996; Rodríguez et al., 2001, 2014). As a consequence, forest-birds should move accordingly when crossing the matrix. In the pasture birds should move fast and straight toward the nearest forest fragment (Powell, 1974), which is facilitated by the open vegetation structure (Zollner and Lima, 1997; Schooley and Wiens, 2003; Zollner and Lima, 2005). In the matrices with closed vegetation, Eucalyptus and cornfields, we expect a slower and more tortuous movement, because birds have more protected sites provided by vegetation structure (e.g. they should be less noticeable for predators; Fahrig, 2007; Williams et al., 2012). Also, the Eucalyptus matrix can have some structural similarity with the natural forest, which could provide cues that are familiar for forest birds (Laurance and Yensen, 1991; Forman, 1995; Mesquita et al., 1999; Caryl et al., 2012; Magrach et al., 2012).
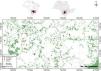
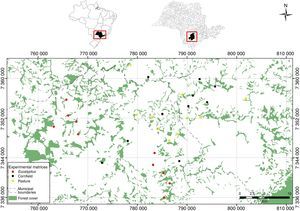
Material and methodsThis study was conducted from October 2009 to February 2010 and from November 2010 to February 2011 on the Atlantic Plateau of São Paulo, in the southeast of Brazil, where pastures, agricultural fields and Eucalyptus plantations have replaced extensive areas of the original Atlantic Forest. In the past, Atlantic Forest covered a vast area of southeastern Brazil (Joly et al., 2014), but approximately only 11–16% of the original forest remains today, which is mostly distributed in isolated small forest fragments (1–280ha) (Ribeiro et al., 2009).
We used Pyriglena leucoptera (Passeriformes: Thamnophilidae), an understory insectivorous bird (Sick, 1997) that is endemic to the Atlantic forest (Stotz et al., 1996) as study species. Understory birds are generally considered adequate organisms to study the effects of fragmentation on movement patterns (Castellón and Sieving, 2006), as they depend on forest habitat, but some may be able to explore the matrices that make up the mosaic of fragmented landscapes (Uezu et al., 2005; Hansbauer et al., 2008a).
Pyriglena leucoptera is a forest specialist and has medium sensitivity to fragmentation (Anjos, 2006; Hansbauer et al., 2008a, 2008b, 2010), thus occurring also in secondary forests and forest fragments (Parker et al., 1996; Stotz et al., 1996; Uezu et al., 2005). It prefers environments with vine-tangles and bamboos, and it forages moving actively through the forest understory (Lopes et al., 2006) and along edges in fragmented landscapes (Hansbauer et al., 2008b). Their territories do not seem to exceed 2ha (Duca et al., 2006), but their home range is estimated at 15.4ha (Hansbauer et al., 2008b).
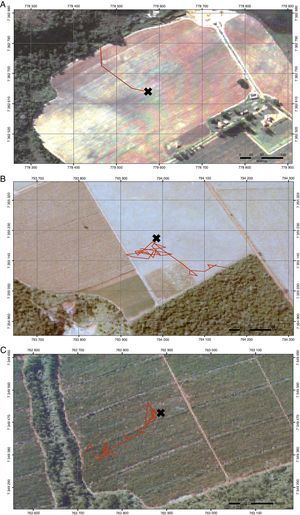
We described predation risk and movement patterns of P. leucoptera individuals in three types of matrices: clean pastures, cornfields and Eucalyptus plantations. We selected 30 experimental landscapes (Fig. 1), 10 for each type of matrix, with at least 2km of distance among them. The pastures were areas in use, covered by grass with no more than 10cm high; the cornfields were areas with plants that had 1.5m high at minimum; and Eucalyptus were plantations with 8–10m high trees and poor understory. The understory density was estimated visually by the researchers. All the selected landscapes were composed by a matrix area adjacent to a forest fragment with at least 150m of contact between the matrix and the focal fragment.
We assessed risk of predation by estimating the density of birds’ predators in each matrix type. Because P. leucoptera is a diurnal species (Sick, 1997) and most dispersal events should occur during the day, we used density of birds of prey to assess risk of predation. Birds of prey are the most representative group of predators that consume birds and forage during the day; other predators that commonly consume birds and their eggs, such as snakes and small mammals (Sick, 1997), are mostly nocturnal.
We used matrix-specific detection functions to estimate the density of birds of prey (in individuals/ha) with the Distance Sampling method (Buckland et al., 2001) (See supplementary material S1). An 800m transect was established in each matrix, approximately 100m distant from the focal fragment edge. Transects were sampled during 1h along the morning (between 9am and 12pm) by three observers. Each transect was sampled twice in different days and times, totalizing 60h of sampling (Whitacre et al., 1992). Only potential predator species of P. leucoptera were included in the analyses, excluding all the species with no literature records of feeding on small birds (Sick, 1997). All detections that occurred at more than 500m orthogonally distant to the transect line were excluded to avoid identification errors (Buckland et al., 2001).
We tested the differences of movement behavior of P. leucoptera in the three matrix types, using a capture-translocation-monitoring approach. All individuals were captured with mist-nets in forest fragments at least 8km away from the experimental matrix, avoiding translocations inside their well-known own territories (e.g. Bélisle et al., 2001). Besides, we chose capture areas with the same matrix of the released area, ensuring that the experimental individuals had previous experiences with the considered matrix. The time between capture and release varied from 1 to 2.5h, regardless of the matrix type.
For our translocation experiment, we used only adult males of P. leucoptera. Bird species commonly have sex-biased dispersal (Johnson and Gaines, 1990). The main reason for this pattern is that males in most bird species show resource defense (e.g. territories). Thus, the movement costs are different for each sex, with the sex that keeps the resources (in most cases males) being the less dispersive one (Greenwood, 1980; Clobert et al., 2004). A recent study demonstrated sex-bias dispersal for P. leucoptera, with females having a higher success when dispersing through fragmented landscapes (Awade et al., 2017). Thus, males are more prone to be affected by changes in the landscape and may limit overall dispersal. Also, by translocating only adult males we avoided behavioral variations between sex and ages (Johnson and Gaines, 1990; Paradis et al., 1998).
We selected only healthy individuals and we marked them with numbered aluminum bird-bands. After that, we attached a radio transmitter on the uropygium area (Telenax model TXB-002G, 0.9g, <5% of bird weight). The attachment was performed with non-toxic eyelash latex glue, allowing free movements of wings and legs and causing no damages to the uropygial gland (Raim, 1978; Kenward, 2001). The transmitters were not removed because the frequency of recapture is low. However, the transmitters most likely fell down after 3 to 4 weeks since the glue is water-soluble.
We radio-tagged and monitored 30 individuals that were separately translocated to the release point in each experimental matrix (i.e. N=10 for each matrix type), 150m distant from the focal fragment and more than 150m distant from any other possible fragment. We used this distance based on the observation that males from the fragmented landscape have ca. 75% probability of completing successfully an open inter-patch dispersal event at this distance (Cornelius et al., 2017). Before being released, individuals were maintained for 10min inside an opaque box with wholes for breathing, in order to decrease stress. The release method, proposed by Forero-Medina and Vieira (2007), consisted in maintaining the animal in a box that is removed vertically and manually by pulling a 50m rope attached to the box top. This method allowed equal chances to move toward any direction.
Once released, we monitored birds using radio-tracking triangulation (Kenward, 2001). To describe pathways as accurately as possible, three observers equipped with receptors and Yagi antennas, collected simultaneous positions every 5min during the first 2-h and then after every 15min or when necessary (e.g. highly moving individuals). Monitoring was performed while animals were moving on the matrix until reaching the focal fragment (or other adjacent fragments), with observers located as far as possible (from 50 to 150m, depending on the matrix structure) and never crossing the bird's trajectories.
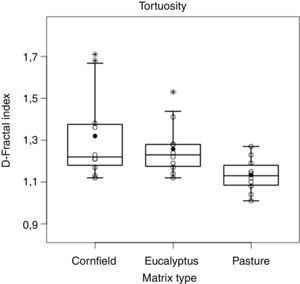
Movement patterns were compared among matrices using fractal geometry (D-fractal; Mandelbrot, 1967; Dicke and Burrough, 1988), with Fractal 5.2 software, in which higher indices indicate more tortuous trajectories. We used analysis of variance (ANOVA) to test for an effect of matrix type on density of birds of prey and tortuosity (D-fractal index), and a Tukey HSD (honest significant difference) as a posteriori test to estimate pairwise differences (Gotelli and Ellison, 2004). For these analyses, we log-transformed density data and used de box-cox transformation for the D-fractal index to meet better the model assumptions, but reported results in the original units (Gotelli and Ellison, 2004). We also used a G-test to compare the frequencies of successful matrix-transfer events among matrix types, and a regression analysis to relate tortuosity (independent variable) with time spent in the matrix (response variable) in each matrix type. The permanence time was compared among matrices using Kaplan–Meier survival analyses (Kaplan and Meier, 1958). These analyses were performed using R 3.2.1 software (R-Core-Team, 2015).
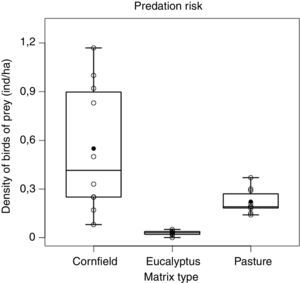
ResultsPredation riskWe registered 255 birds of prey from at least 15 species (Table S1) that are potential predators of P. leucoptera. We used these data for estimating densities using the matrix-specific detection functions (Figure S1, Table S2).
Densities of predators were different among the matrices (F2,27=16.27, p<0.001) being lower in Eucalyptus when compared either to pasture (p<0.05) or cornfields (p<0.001), and lower in pasture than cornfields (p<0.05) (Fig. 2). The cornfield matrix had the largest variation (mean±SD: 0.55±0.39 ind./ha), when compared to the pasture (0.22±0.07) and the Eucalyptus matrix (0.03±0.01).
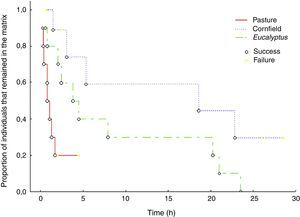
Movement patternsTranslocated birds ability in reaching the forest fragment (i.e. completing successfully a matrix-transfer event) was different among the matrices (G=8.72, p<0.05). In the Eucalyptus matrix all ten individuals were successful (100%), in the pasture matrix eight out of ten (80%) reached the fragment and in the cornfield only five out of ten individuals (50%) successfully reached the nearby fragments.
Permanence time of P. leucoptera individuals varied among matrices (Kaplan–Meier Survival χ2=10.34, df=3, p<0.05) (Fig. 3). Translocated individuals stayed longer in Eucalyptus and cornfields, while permanence in the pasture matrix was shorter. The two birds that stayed longer in the pasture died: one was consumed by a bird of prey (Caracara plancus, Falconidae), and the other was found dead with no predation mark. In the cornfields, we found an evidence of predation (feathers in the site where we found the detached transmitter) and one translocated bird was found dead with no predation marks. In two other cases, we observed indirect evidences of predation. For one individual signal quickly disappeared (even the audible signal), in the second the signal moved around 300m, stayed inconstant and disappeared.
Trajectory patterns of the translocated individuals were different among matrices (F2,27=5.27, p<0.05, Fig. 4). The most tortuous trajectories, quantified by the Fractal Dimension index, were found in the Eucalyptus and cornfield matrix, but there was also a large variation within matrices. In the pasture, trajectories were straighter, being different from cornfields (p<0.05) and Eucalyptus (p<0.05), with no differences among trajectories in Eucalyptus and cornfields (p=0.81) (Fig. 5).
In pasture, individuals that used more tortuous trajectories stayed longer in the matrix (F1,8=7.341, R2=0.413, p<0.05), while in the cornfields (F1,8=0.77, R2=0.087, p=0.41) and in the Eucalyptus (F1,8=0.02, R2=0.002, p=0.91) no relationship between tortuosity and permanence time was observed (Figure S2).
DiscussionHere, we show that different human-modified matrices exhibit variable levels of risk for a forest dependent species, with birds showing different movement patterns and hence different levels of matrix-transfer success. Our results corroborate those of other studies that showed variable types of movements in matrices with different vegetation structure or predation risks (e.g. Desrochers and Hannon, 1997; Bélisle, 2005; Zollner and Lima, 2005; Roth et al., 2006), and highlight the importance of considering matrix quality (and thus permeability) for dispersing organisms.
As expected, we found differences in density of birds of prey among matrices. In pasture and cornfield, the density of birds of prey was higher than in Eucalyptus. Pastures usually favor species of natural open areas providing a wide visual field and also wide flight space (del Hoyo et al., 1994; Sick, 1997; Ferguson-Lees and Christie, 2001). Cornfields on the other hand, that had the highest density of birds of prey, may provide increased food resources (e.g. small mammals; Umetsu and Pardini, 2007) and even facilitate access to prey (Rodriguez et al., 2014). Overall vegetation structure seems to play an important role in affecting foraging habitat of birds of prey.
The three matrix types we studied here have different vegetation cover and degrees of risk. These variations may be perceived by individuals of P. leucoptera, as indicated by changes in their movement patterns and differences in matrix-transfer success. Pasture and cornfield matrices, that have high density of predators and none or short vegetation cover, entail higher risk of predation for dispersing birds when compared to the Eucalyptus matrix. In addition, the observed difference in P. leucoptera movement patterns in pastures and cornfields can be explained by the prominent differences in vegetation structure resulting in movement patterns that attempt to minimize the costs in the matrix, with straight flights (in the pasture) or more tortuous movements because of the obstruction of the visual field (in the cornfield; Lima, 1998; Lima and Bednekoff, 1999; Bélisle, 2005; Fahrig, 2007). Simulations and empirical data have been shown that the risk of predation, the perceptual range and environmental characteristics have relevant roles in the patterns of movement of individuals through the matrix (Desrochers and Hannon, 1997; Bélisle, 2005; Zollner and Lima, 2005; Roth et al., 2006). Therefore, it is important to consider multiple factors when evaluating movement patterns across the matrix.
Movement tortuosity analyses provide valuable information, assuming that individuals prioritize short and straight movements under a stress condition (Zollner and Lima, 1999; Fahrig, 2007; Hodgson et al., 2011; Turlure et al., 2011b). In pasture, the straight movement of P. leucoptera probably results from the perception of the higher risk and an open visual field. In the cornfield, even with a high risk of predation, we observed more tortuous movements, which may be a consequence of the visual obstruction caused by vegetation rather than feeding activity, since pauses for feeding would result in a higher exposure time to risks (Zollner and Lima, 2005). We can consider that in this matrix the animals move randomly, which increases the path length, the permanence time and also elevates the costs, since just half of tested individuals reached the fragment (Serrano and Tella, 2003; Bowler and Benton, 2005). On the other hand, in the Eucalyptus matrix, where the vegetation structure is more similar to the native forest coupled with the low density of predators allowed more time to use the resources provided by this habitat, idea reinforced by the high success in reaching the fragment. In this case, matrix exploration (Antongiovanni and Metzger, 2005) coupled with intermediate visibility (Zollner and Lima, 2005) resulted in movements of considerable tortuosity.
More tortuous trajectories, however, do not always result in a greater permanence time in the matrix. Only in pastures, individual that used routes that were more tortuous stayed longer in the matrix. This is because in some cornfields and Eucalyptus areas, animals that performed long pauses but more straight pathways also resulted in longer stays in the matrix. While in the Eucalyptus matrix these pauses were probably to use provided resources, in cornfields they were probably related to protection against predators (Lima and Dill, 1990) or caused by stress. Moreover, longer matrix permanence time (i.e. potentially longer exposure to risk) in less open vegetation types (cornfields and Eucalyptus) is also explained by visual obstruction caused by vegetation.
Our results suggest that P. leucoptera can differentiate risks and benefits in different matrices, which can affect the probability of moving successfully through the matrix. Similar results have also been observed for temperate forest birds that use matrices differently (Aben et al., 2012), butterflies that fly faster in low quality matrices (Turlure et al., 2011a), mammals with perceptual capacity that is inversely proportional to vegetation visual obstruction (Prevedello et al., 2011), and movement of amphibians that is conditioned to matrix quality (Cline and Hunter, 2016). Only the number of predators in each environment was considered in our study. However, other characteristics that may vary among matrices (e.g. food resource and abiotic conditions), could also affect movement (Stratford and Robinson, 2005; Turlure et al., 2011b; Ockinger et al., 2012; Santos-Filho et al., 2012).
In sum, we provided evidence that Eucalyptus is the matrix with the highest quality, that is, with lower risk and with higher permeability. The similarity with the natural habitat of P. leucoptera certainly makes Eucalyptus plantations the most favorable environment, with low risk because of lower density of predators and vegetation cover, and more benefits because of food availability (Laurance and Yensen, 1991; Forman, 1995; Mesquita et al., 1999; Caryl et al., 2012; Eycott et al., 2012; Magrach et al., 2012). This makes animals less resistant in leaving the matrix (Castellón and Sieving, 2006). Even though the pasture is a matrix with high density of birds of prey and physiologically stressful conditions for forest animals, we suggest this is a medium-risk matrix, because the absence of visual obstruction allows individuals to fly straight to the fragment, resulting in a lower exposure time to risks. At last, the cornfield is the matrix with lowest quality, with high risk provided by the highest density of predators, and with high exposure time to risk (due to the visual obstruction caused by vegetation) and to different microclimatic conditions, which results in low matrix-transfer success.
In conclusion, our study contributes to a better understanding of the movement process across non-habitat areas in fragmented landscapes, which is essential to ensure persistence of populations in these landscapes (Taylor et al., 1993; Hanski, 1999; Zollner and Lima, 2005; Santos-Filho et al., 2012). Planning land-use and the management of matrices in a way that favors landscape connectivity (e.g. planning for a mosaic of matrices in which high quality matrices are interspersed among low quality ones), is recommended, especially in areas where it is not possible to change habitat configuration or in key areas for biodiversity conservation (Franklin and Lindenmayer, 2009; Caryl et al., 2012). Maintenance or creation of matrices similar to native forest habitats seems to be an efficient strategy to both increase connectivity in fragmented landscapes (Aberg et al., 1995; Gascon et al., 1999; Barbaro et al., 2005; Lantschner et al., 2008). In this way, understory suppression in tree plantations must be avoided, given that the maintenance of understory vegetation may results in more resources and better microclimate conditions, increasing overall matrix quality and the abundance of understory-dependent species (Lindenmayer and Hobbs, 2004; Atauri et al., 2005). Therefore, our results stimulate Atlantic Forest managers to incorporate matrix quality and permeability in their conservation plans, recommending areas with greater permeability, such as Eucalyptus plantations, around isolated native vegetation remnants in order to improve connectivity.
Conflicts of interestThere is no conflict of interest.
This study was funded by FAPESP (Project number 2007/5649-6 and 2009/04626-6), and by a master's fellowship (FAPESP) granted to MB, approved by the Ethics Committee of Biosciences Institute, University of São Paulo (protocol n. 096/2009) and by SISBIO (protocol n. 19600-2/2009). CC was funded by a post-doctoral fellowship granted by FAPESP (Project number 2007/55642-6). J.P.M. was funded by National Council for Scientific and Technological Development (CNPQ, process number: 307934/2011-0). We would like to thank all researchers that provided insights at different stages of this research, especially Marco Granzinolli, Marcelo Awade and Carlos Cândia-Gallardo, whose contributions greatly improved the study design and analyses of this study. We also would like to give a special thanks to the owners of the field sites who allowed me to use their areas, particularly Fibria Company.