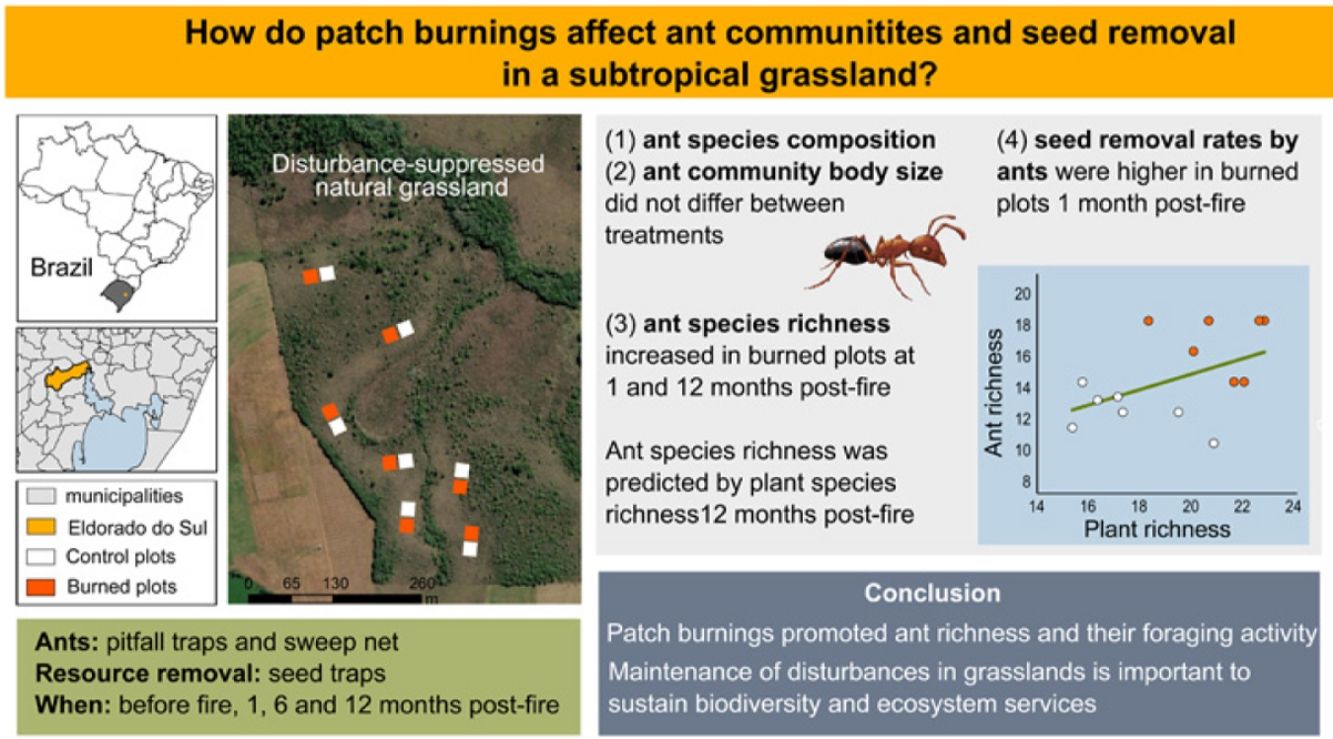
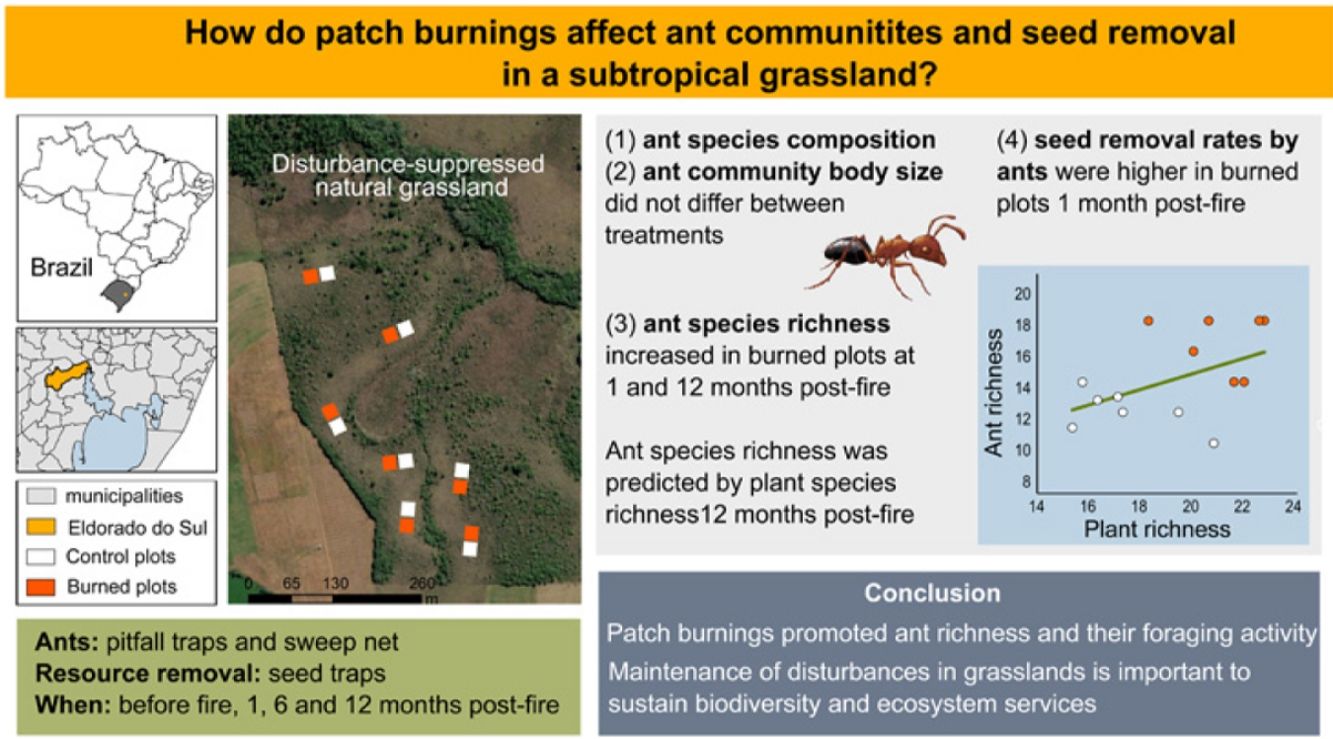
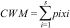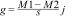Disturbances modify local abiotic properties, habitat structure and resource availability, shaping community assembly and ecological interactions. Open ecosystems have an evolutionary relationship with fire. We evaluated the effects of patch burnings on grassland ant communities and patterns of seed removal. We established 14 plots of 10 m2 in pairs in a disturbance-suppressed grassland in South Brazil. A random plot of each pair was burned, and another plot was the control. We accessed ant communities with pitfall-traps and sweeping net, and seed removal with seed traps in all plots prior the experimental fires, and then on three occasions following fires. We recorded 57 ant species belonging to 29 genera. Ant species composition did not significantly vary between treatments neither did ant body size. We detected significantly positive fire effects on ant richness after 1 month and 12 months, mediated by the increase in plant species richness in burned plots. Mean seed removal rates were increased in burned plots after 1 month. We showed that prescribed patch burnings in fire-prone grasslands promoted ant richness, and their foraging activity. Our study may serve as a basis for conservation decisions, showing the importance of maintenance of disturbances in grasslands.
Disturbances are natural or anthropogenic processes removing biomass from communities (Grime, 1977). They modify local abiotic properties, habitat structure and resource availability creating important environmental filters for biota, thus shaping community assembly, functional traits, ecological interactions, and functions (Bond and Keeley, 2005; Díaz et al., 2007). Open ecosystems worldwide such as savannas and grasslands have a close evolutionary relationship with grazing and fire, and therefore present not only disturbance-adapted but also disturbance-dependent communities (Veldman et al., 2015). In these ecosystems, plant conservation can be suitably coupled with traditional management (e.g., grazing, excepting intense forms, prescribed burnings), while disturbance suppression policies usually lead to typical plant diversity loss by competitive interactions (Abreu et al., 2017; Ferreira et al., 2020; Koch et al., 2016). Prescribed burning is a commonly applied management tool to improve forage value, to prevent wildfires, and ultimately to increase landscape heterogeneity and benefit biodiversity where grazing is low or absent (Bond and Keeley, 2005; Fernandes et al., 2013; Morgan et al., 2020) Nevertheless, to foster efficient conservation strategies in open ecosystems, it is fundamental to understand the effects of disturbances and their suppression on a wide-range of organisms, since, among animals for example, responses can be taxa dependent (Pastro et al., 2011).
Here we performed a controlled patch burning experiment to shed light on how prescribed burnings in long-term disturbance-suppressed grasslands affect ant communities and their functions in South Brazil. Ants are a globally dominant faunal group with sessile colonies, performing outstanding ecological roles (Folgarait, 1998). Ants display a large range of feeding strategies, removing widely dispersed aboveground resources such as plant diaspores, nectar, dead animal remains, and leaves. By transporting these resources to their nests, ants help redistribute nutrients in the ecosystems (Farji-Brener and Werenkraut, 2017). In grasslands, ants nest essentially belowground, where direct colony mortality by fires is negligible (Arnan et al., 2006; Debano, 2000). Nevertheless, ant communities are commonly affected by fire-induced habitat changes, such as in structure, microclimate, and resource availability (Andersen, 2019). For example, post-burnt grassland sites usually present higher habitat openness (e.g., suppressed vegetation, high sun exposure, soil temperatures, and low moisture retention) and simpler substrate (e.g., suppressed litter, high bare soil proportion) for ant locomotion than unburned sites (Podgaiski et al., 2014). Such conditions could eventually trigger species and trait-based community assembly through an environmental filtering process (Bishop et al., 2021; Wiescher et al., 2012), besides changing the community foraging patterns (Parr et al., 2007). Also, as plant diversity has been widely proposed as a proxy of food diversity for ants (Dröse et al., 2021), it is expected that the pace of post-fire vegetation regeneration would drive the diversity patterns of foraging ant communities (Bishop et al., 2021).
We propose hypotheses and predictions on how fire would indirectly affect ant species composition, community body size, species richness, and seed removal by ants.
- (1)
Fire-induced habitat openness trigger ant species composition changes. We predict a turnover of ant species post-fire. Species more prone to forage under open habitat will be favored, while those with preferences for more shady environments will be disfavored in burned grassland (Andersen, 2019; Farji-Brener et al., 2002; Lessard and Andersen, 2019).
- (2)
Due to the more simplified and sun-exposed environment, burned grasslands benefit ant species with larger size. Among functional traits, ant body size is related to mobility and desiccation resistance (Farji-Brener et al., 2004; Kaspari and Weiser, 1999). Large-bodied foragers present faster mobility in less complex habitats relative to more complex ones (Farji-Brener, 2004b; Parr et al., 2003), and also show more heat tolerance (e.g., UV radiation incidence) than small ants (Kaspari et al., 2015; Kaspari and Weiser, 1999). Therefore, we predict a higher ant community mean body size in burned than unburned grasslands.
- (3)
The high plant richness of burned grasslands favors the coexistence of a great number of ant species. Fires usually increase grassland plant diversity by reducing competition for light and space among plants, favoring growth, reproduction and early flowering of plant species with less competitive capacities (Fidelis et al., 2012; Fidelis and Blanco, 2014; Joner et al., 2021; Overbeck et al., 2005). By considering plant richness as a proxy of resource diversity for ants and that resource diversity allow coexistence of foraging ant species (Dröse et al., 2021), we predict a positive association of plant and ant richness, and a higher ant richness in burned than unburned grasslands.
- (4)
The simplification of the habitat post-fire makes it easier for ants to forage and find seeds. The suppression of vegetation cover by fire opens the habitat, facilitating ant locomotion and activity, usually resulting in greater resource finding (Dolabela et al., 2020; Gibb and Parr, 2010; Radnan et al., 2018). Thus, we predict higher rates of seed removal by ants in burned than unburned grasslands, and that such rates would be predicted by ant activity.
We conducted the study in disturbance-suppressed grasslands (no cattle and no fire) in the Agronomic Experimental Station of the Universidade Federal do Rio Grande do Sul (UFRGS), Eldorado do Sul County, RS, Brazil (30°06′58″ S; 51°41′05″ W) between September 2009 and February 2011. The area has a Cfa climate type (Peel et al., 2007). Mean annual precipitation is 1440 mm, well distributed along the year, and mean temperatures range from 9 °C in winters to 25 °C in summers (Moreno, 1961).
Sampling designWe established 14 experimental plots of 10 × 10 m each in pairs, six meters apart, each pair constituting a block. In December 2009, a random plot of each block was burned, and another plot was taken as a control. We sampled ant communities and seed removal in all plots prior to experimental fires (September/November 2009) to assess their homogeneity and current status, and then on three occasions following fires: about 1 month (January–March 2010), 6 months (June/July), and 12 months (December 2010). More details in Podgaiski et al. (2013, 2014). This randomized block design standardizes small-scale local factors potentially influencing ant communities within the blocks (e.g., soil type, slope, moisture, solar incidence, vegetation composition). Thus, differences in sampled ant communities between paired plots are expected to be due to fire-induced habitat changes. Due to the small size of the burned plots, we assumed that there were no restrictions for ants nesting in the unburned matrix to access and forage within burned patches. The smaller the experimental plots, the more the effects of fire can be related to habitat preferences for foraging by ants (Swengel, 2001).
To sample ground-dwelling ants, we used five pitfall-traps per plot (9 cm in diameter, 200 mL of 70% alcohol + detergent drops) open for four days each. To complement pitfall-traps, we swept herbaceous plants in four transections within the plots (sweeping net with a 0.1 m² opening), for one morning and one afternoon period per occasion. Ant communities sampled from pitfalls and sweeping nets were pooled to provide plot-level data. Ants were sorted and mounted on entomological pins in the Laboratório de Ecologia de Interações (LEIN) of UFRGS. Species or morphospecies (hereafter species) were determined by specialists (Dr. Rogério Silva and Dr. Rodrigo Feitosa) in comparison with specimens deposited in the scientific ant collection of Museum of Zoology of the Universidade de São Paulo (MZUSP, Brazil). Vouchers were deposited at LEIN. We measured Weber's length (distance from the anterodorsal margin of the pronotum to the posteroventral margin of the propodeum) of at least one minor individual per species in each plot and sampling date. Then we averaged values as a descriptor of species body size (Gibb and Parr, 2013; Ruzi et al., 2021).
Seed removal by ants was measured through seed traps experiments, a standard approach in ant ecology (Fontenele and Schmidt, 2021; Palfi et al., 2020; Paolucci et al., 2016). We used five seed traps per plot. The traps were made of plastic petri dishes (4.8 cm wide; 1.5 cm high) with a cover, and four side perforations of 5 mm² per trap (Supplementary Material 1), allowing ants to enter but excluding large granivores such as birds and rodents. Inside the dishes, we set a standardized mixture of 35 seeds from four species: Phalaris canariensis (grass; Poaceae; 10 seeds), Paspalum notatum (grass; Poaceae; 10 seeds), Solanum viarum (shrub; Solanaceae; 10 seeds) and Schinus molle (tree; Anacardiaceae, 5 seeds). This mix of seed types was chosen to mimic potential availability of seed resources in grasslands (e.g., higher proportion of grass seeds, but also contribution of other plant life forms), and to attract a large variety of ants. A smaller amount of S. molle seeds was used because they are large, and thus occupied a larger volume than the other seed types. After six days of exposure, we retrieved the traps and calculated the proportion of disappeared seeds. Apart from ants, we did not observe other invertebrates interacting with seeds. Thus, we are assuming ants as the main vectors of seed removal. These experiments were performed a few days before ant sampling on the plots.
Statistical analysesAll analyses were developed in R (R Development Core Team, 2021), and performed independently for each sampling date (i.e., ‘pre-fire’, and 1, 6 and 12 months ‘post-fire’).
Species compositionTo test if ant species composition varied between burned and control plots, we performed PERMANOVA. We used a matrix of incidence of ant species distributed in the 14 plots, and the Jaccard index as a dissimilarity measure between plots. The analysis was conducted with the Adonis function, from the package vegan, with 999 permutations, considering blocks as a random variable (Oksanen et al., 2019).
Community mean body sizeTo evaluate whether ant community mean body size changed between burned and control plots, we calculated Community Weight Mean (CWM) as
where pi and xi are the relative abundance and body size of the ant species, respectively (Garnier et al., 2004). The relative abundance of ant species was calculated based on an incidence matrix, and thus each species is weighted equally in each plot.To evaluate fire effects on CWM body size, we used the Hedges' g standardized and impartial effect size estimator:
where M1 is the CWM in burned plots, M2 is the CWM in control plots, s is the combined standard deviation, and jj is a correction to reduce bias in small samples (Hedges, 1981). We controlled for block effects by subtracting CWM values of each block from the observed values before estimating the effect size (Ferreira et al., 2020; Podgaiski et al., 2018). To test if the observed fire effect was different from expected values at random, we calculated 95% confidence intervals with 10,000 iterations with the package BootES (Kirby and Gerlanc, 2013). Treatment effect is significantly positive or negative when intervals do not overlap zero (Nakagawa and Cuthill, 2007).Species richnessTo evaluate fire effects on ant species richness, we used the effect statistic with 95% confidence interval, as already described for CWM. To test the hypothesis that ant richness is influenced by the diversity of resources, we performed structural equation modeling (SEM). For that, we used data on plant communities sampled 9 months post-fire available from Podgaiski et al. (2013)) and data of ant communities sampled 12 months post-fire. We used plant species richness as a proxy for food resource availability/diversity for ants (da Silva et al., 2020; Dröse et al., 2021; Monteiro et al., 2019; Vasconcelos et al., 2019). We included direct effects of fire (0, 1) on plant species richness (average per m2), and direct effects of plant richness on ant richness. The goodness-of-fit test was performed with Fisher’s C and p-value. A p-value higher than 0.05 indicates consistency between observed data and the fitted model. We ran SEM with the piecewiseSEM R package (Lefcheck, 2016), including blocks as random effects.
Seed removalTo test fire effects on the proportion of removed seeds, we again used the effect statistic with 95% confidence intervals, as described for CWM above. For those sampling times that presented significant variation on removal rates between treatments, we used SEM to test the hypothesis that it is affected by ant activity. As a proxy of ant activity, we used the abundance of ant species known to interact with plant diaspores and harvest seeds based on scientific records from the literature, based on species or genus level (Supplementary Material 2). We included direct effects of fire on ant abundance (log transformed), and direct effects of ant abundance on seed removal in the model.
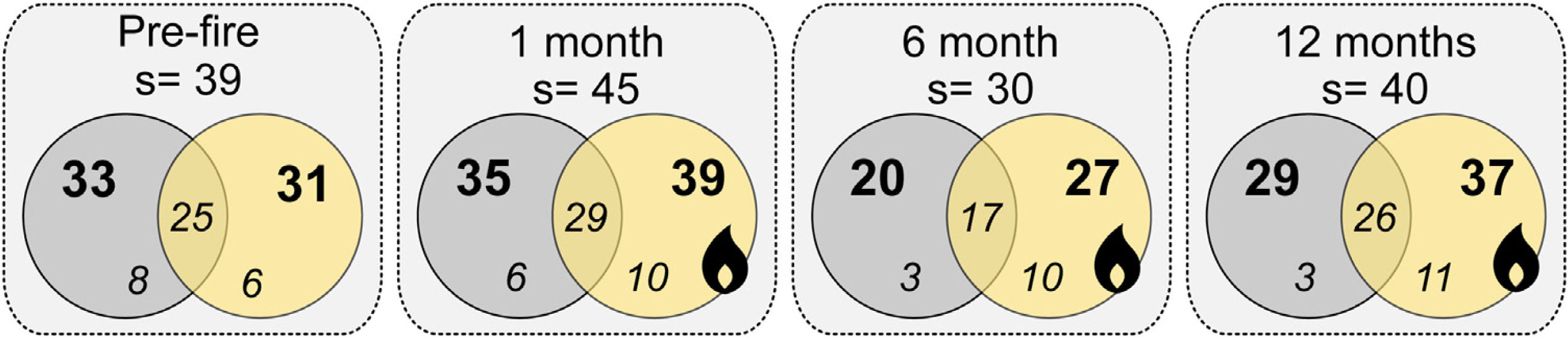
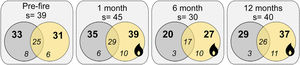
ResultsWe sampled a total of 4316 ants belonging to 29 genera, and 57 species. Camponotus crassus Mayr, 1862, Brachymyrmex sp., Camponotus fastigatus Roger, 1863, Holcoponera striatula Mayr, 1884, and Ectatomma brunneum Smith, 1858 were the most frequent species in the study (Supplementary Material 2). The total number of sampled species varied with season of sampling, with lower richness accumulated in the wintertime (6 months post-fire; Fig. 1). Plots from different treatments shared a large proportion of species (i.e., about 60% of the total community of each time), but burned plots accumulated higher total species richness than controls (i.e., 11.4%, 35% and 27.6% more species at 1-, 6- and 12-months post-fire respectively; Fig. 1). Burned plots also accumulated more unique species than controls.
Venn diagrams showing the total number of ant species (s), the number of species on each treatment (bold numbers), and the number of exclusive and shared species (italic numbers) between treatments for each sampling occasion (Pre-fire, 1-, 6- and 12-months post-fire). Burned plots in yellow, controls in grey.
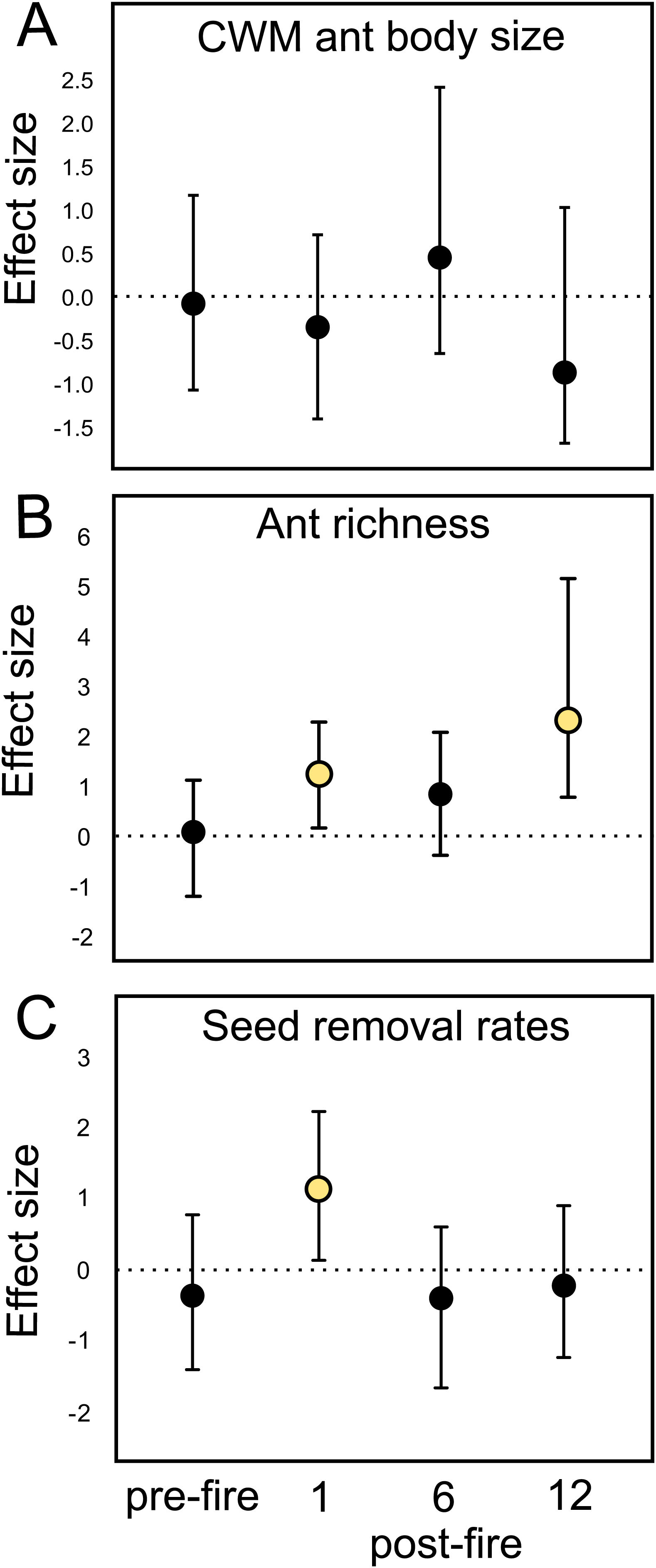
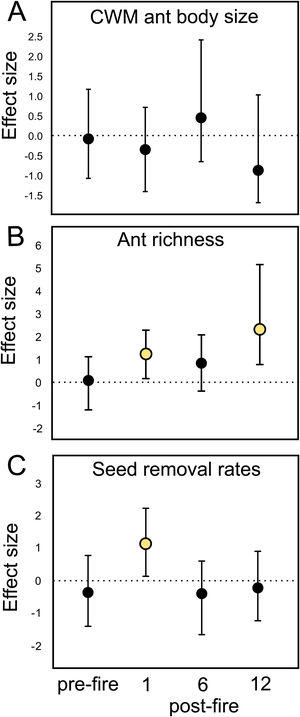
Ant species composition did not significantly vary between treatments: either pre-fire (F1,18 = 0.63; p = 0.84), 1 month (F1,18 = 0.92; p = 0.55), 6 months (F1,18 = 0.5; p = 0.92), and 12 months post-fire (F1,18 = 0.6; p = 0.86). Ant community body size also did not vary (Table 1A). Confidence intervals on the effect size value greatly overlapped with the null effect value (Fig. 2A), indicating no fire effects on CWM body size at any sampling occasion.
Mean and standard error (se) values of (A) CWM ant body size values, (B) species richness, and (C) seed removal rates by ants in burned and control plots at different sampling times in grassland ecosystems. se = standard error.
| Sampling time | (A) CWM body size | (B) Ant richness | (C) Seed removal (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burned plots | Control plots | Burned plots | Control plots | Burned plots | Control plots | |||||||
| mean | se | mean | se | mean | se | mean | se | mean | se | mean | se | |
| Pre-fire | 1.40 | 0.06 | 1.41 | 0.09 | 13.14 | 0.75 | 13.00 | 1.34 | 20.40 | 3.74 | 24.73 | 6.79 |
| 1-month post-fire | 1.44 | 0.06 | 1.47 | 0.04 | 16.28 | 1.14 | 13.71 | 0.96 | 81.87 | 5.32 | 68.40 | 4.07 |
| 6 months post-fire | 1.71 | 0.16 | 1.64 | 0.10 | 8.57 | 1.10 | 7.00 | 0.47 | 22.20 | 6.82 | 28.00 | 7.80 |
| 12 months post-fire | 1.40 | 0.04 | 1.47 | 0.07 | 16.00 | 0.75 | 12.71 | 0.96 | 50.04 | 7.80 | 53.38 | 7.07 |
Mean fire effect size (Hedges' g) and bootstrapped 95% confidence intervals on (A) CWM ant body size, (B) ant species richness, and (C) seed removal rates in each sampling occasion (pre-fire, 1-, 6-, and 12-months post-fire). Treatment effect is significantly positive or negative when intervals do not overlap zero.
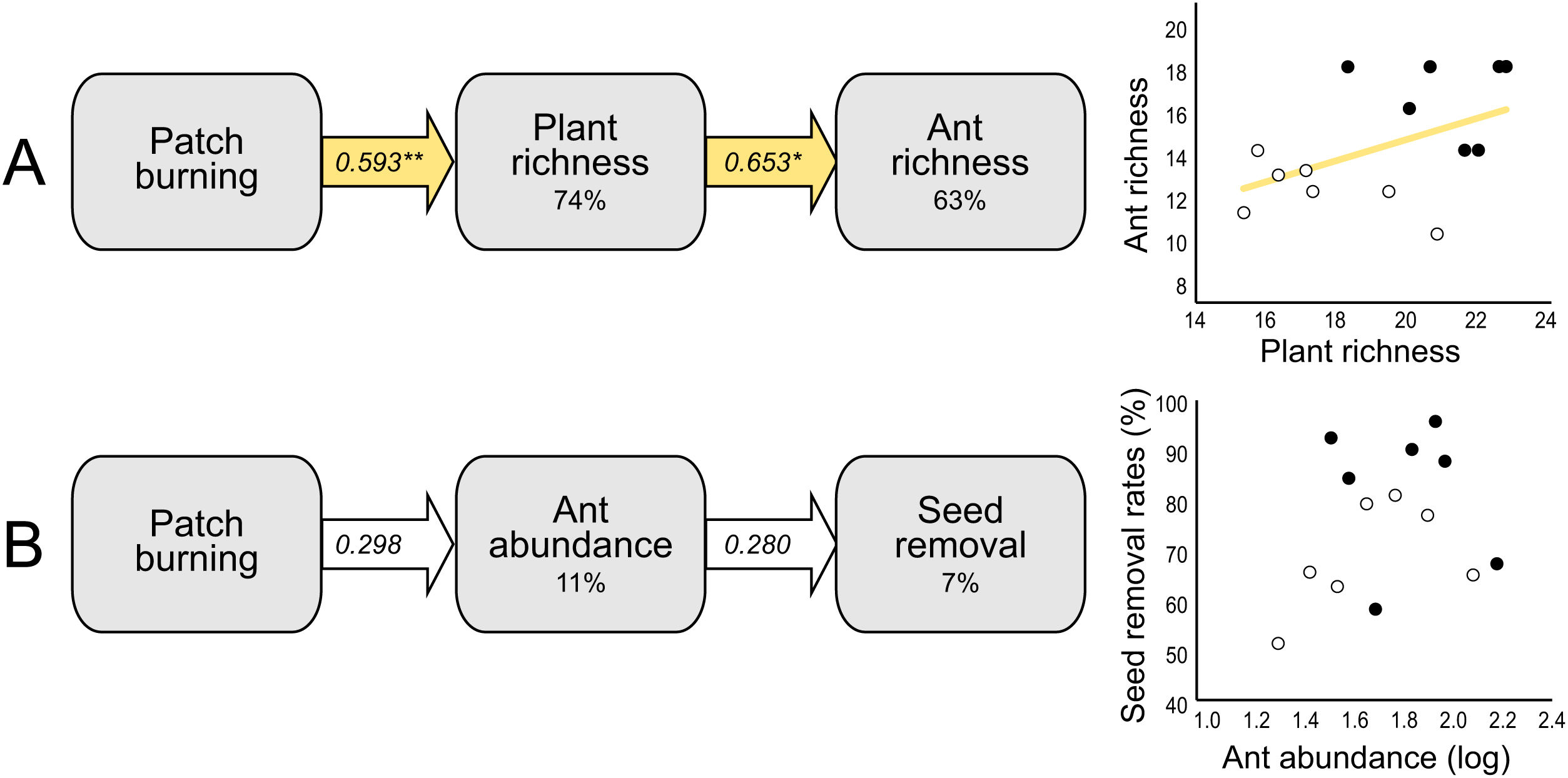
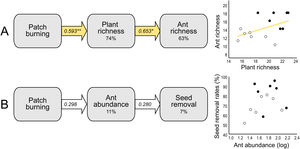
At the local scale, mean species richness varied between burned and control plots (Table 1B). We detected significantly positive fire effects on ant richness after 1 month (Hedges' g = 1.20) when burned plots accumulated 10% more species. At this time, each burned plot presented on average 2.57 (18.7%) more species than controls. We detected stronger fire effects after 12 months (Hedges' g = 2.28) with 22% more species at total. At this time, burned plots had on average 3.3 (25.9%) more species per plot. No significant fire effects were detected for 6 months post-fire (Fig. 2B). The SEM testing the hypothesis that ant richness increased after fire (12 months) due to direct effects of the diversity of resources was validated (Fisher's C = 4.085; df = 2; p = 0.13), and all single relationships were significant (p < 0.05; Fig. 3A).
Structural equation models (SEM) testing indirect fire effects on (A) ant species richness through changes in plant species richness at 12 months post-fire, and (B) seed removal rates (%) through changes on ant abundance of selected species (see Supplementary Material 2) at 1-month post-fire. The percentage number within the boxes indicate the conditional R-squared of the relationship. Yellow arrows represent positive relationships (**p < 0.01; *p < 0.05), and white arrows non-significant relationships (p > 0.05). In the scatterplots, black circles represent burned plots and white circles control plots.
Mean seed removal rates varied between burned and control plots (Table 1C). We found positive fire effects on removal rates after 1 month (Hedge' g = 1.09), when burned plots presented 19,76% more seeds removed per plot than controls. No fire effects were detected for other sampling occasions (Fig. 2C). The SEM testing the hypothesis that seed removal at this time was influenced by ant activity was validated (Fisher’s C = 3.80; df = 2; p = 0.15), but none of the single linear relationships were significant (p > 0.05; Fig. 3B).
DiscussionWe experimentally burned patches in a long-undisturbed grassland to quantify fire effects in ant communities and their foraging behavior. Our results suggest that patch burnings do not harm grassland-adapted ant communities. On the contrary, by reducing aboveground biomass and reassembling plant communities, fire creates temporary resource-rich habitats for these communities that subtly increase species richness and resource use.
We had initially hypothesized that patch burnings would change local ant composition and community body size by altering grassland openness. Nevertheless, our experimental results did not show changes in overall ant species occurrences across burned and control sites. This probably happened because ant colony mortality directly caused by fire was negligible (as in Arnan et al., 2006; Frizzo et al., 2012), and the resprouting and recover of the grassland vegetation after fire was extremely fast (Joner et al., 2021). Community body size also did not shift in the direction we had predicted, as would be expected if habitat simplification and higher sun exposure had locally selected groups of ant species with larger body sizes. While larger ants may indeed have advantages against desiccation in a harsh post-fire environment (Bujan et al., 2016), they can also be more detectable to visual predators, such as birds, that are usually very abundant post-fire (Beal-Neves et al., 2020). This may be potentially masking the trait-based patterns, which should be clarified in further studies. Moreover, Farji-Brener, 2004b also emphasize that inconsistent associations between the rugosity condition of an environment and ant body sizes may happen in manipulative experiments because such associations operate better at evolutionary rather than ecological scales. Nevertheless, our results concur with other studies in open ecosystems worldwide (Calcaterra et al., 2014; Lopes and Vasconcelos, 2008; Hosoishi et al., 2015; Izhaki et al., 2003; Parr et al., 2003) indicating a highly resilient, or resistant, ant fauna associated to fire disturbances in these types of ecosystems, even after long-term absence of disturbances (Parr and Andersen, 2008).
As predicted in our hypothesis, burned patches supported higher local ant richness (at the patch-scale), and also accumulated more species (at the study site-scale) than unburned sites. While a recent meta-analysis did not show global significant fire effects on ant diversity in open ecosystems worldwide (Vasconcelos et al., 2017), several studies like ours have indeed found ant diversity to increase after fires, such as in the Brazilian, African, Australian and Mediterranean savannas/grasslands (Andersen et al., 2014; Bishop et al., 2021; Maravalhas and Vasconcelos, 2014; Parr et al., 2004). Shortly after fire (1 month) this pattern was weaker and probably influenced by an increased detectability of resident species foraging in a simplified environment (Melbourne, 1999) since despite a significant increase, we found only 10% more species. Nevertheless, one year after fire the effect was more robust, when burned patches reached about 25% more species at both scales (patch and site). Ant species aggregations on burned patches at this later time could be a result of increased nesting processes of different species along the former year. Moreover, this may also indicate that species that nest in the unburned matrix are foraging within the burned patches. Although most ground ant species forage just in the immediate vicinity of their nests (some centimeters to a few meters; (Peeters and Ito, 2001)), some medium and large-bodied species can indeed present high foraging ranges, and directly select for high-quality food spots, as the burned patches (Ronque et al., 2018). Ant richness clearly mirrored the increment in plant richness in burned patches, a possible source of resource diversity. The species-rich regenerating vegetation can be very attractive to ants by offering a suit of exploitable resources both in a direct (floral and extrafloral nectar, fruits, seeds, palatable leaves) and indirect way (honeydew from trophobionts and insect prey living on the vegetation), benefiting generalist species, and also those with specific feeding habits. High resource availability could also relax interspecific competition among ants fostering resource partitioning and coexistence of more local ant species (Ribas et al., 2003; Senior et al., 2013).
Seed removal by ants was about 20% higher in freshly burned patches in comparison to unburned sites. Ant foraging efficiency has been frequently linked to habitat simplification at the ground level, where a simpler and warmer environment could enable higher mobility to ants, which would find and remove resources more quickly (Beaumont et al., 2011; Gibb and Parr, 2010; Paolucci et al., 2016). For example, da Silva et al. (2020) found ants interacting with more individuals of a plant species bearing extrafloral nectaries (Chamaecrista repens) in recently burned grasslands in comparison to grasslands longer without disturbances, and ant-visiting densities were positively predicted by bare soil proportions. The removal of seeds, although increased after fire, was not linked to the abundance of potential seed harvesters, as expected (Fig. 3). Similarly, Parr et al. (2007) detected higher rates of seed removal post-fire in an Australian tropical savanna, which was primarily driven by some key ant species (Iridomyrmex) with highly patchy distributions. Probably, our removal rates were also influenced by particular key species (e.g., Pogonomyrmex, Pheidole). However, our experimental approach did not allow us to assess this directly. Nevertheless, here we do show that fire-induced habitat changes facilitate seed removal by ants, indicating an efficient use of the available resources, especially under highest fire-induced habitat simplification.
We showed that prescribed patch burnings in fire-prone grasslands promoted ant richness, its foraging activity, and potentially fostered the maintenance of ant-mediated ecosystem processes. Our experimental approach, considering small-scale patches distributed within an unburned matrix resembles a mosaic landscape as it occurs in some regions in South Brazil, where fire spreads rapidly and heterogeneously through a wavy relief with dry/humid areas that vary regarding available flammable biomass. Our study, combined with others, may serve as a basis for conservation management decisions, endorsing the importance of disturbances in grasslands to keep and increase plant and ant patch diversity. Future studies should complement our results by investigating fire-induced environmental filtering on ant community assembly and their associated roles under varying burned patch size, fire intensity and frequency in subtropical grasslands, to help more fully subsidize management actions.
Author contributionsLRP and MSMJ contributed to the study conception and design. Material preparation and data collection were performed by LRP and CPRF. Data organization and analysis were performed by GGB and LRP. The first draft of the manuscript was written by GGB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingThis work was supported by CAPES, Brazil (Finance code 001) that provided Master scholarships to GGB, and Post-doc grants to LRP. CNPq (Brazil) provided Productivity Grant to MSMJ (grant number 311298/2019-2).
Data availabilityAll data will be made available by the authors by request.
Compliance with ethical standardsConflict of interestThe authors declare that they have known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participateAll persons who meet the criteria for authorship are listed as authors, and all authors certify that they have participated sufficiently in the work to assume public responsibility for the content.
We thank CAPES, Brazil (Finance code 001) for the Master Scholarship granted to the first author. We are very grateful to the Feitosa Lab group, specifically to R. Feitosa, and R. Silva, for their help in ant identification to the species level. We thank all the people involved in the field work.