To preserve biodiversity, the Brazilian law postulates that rural properties must keep a percentage of native vegetation cover, denominated as “Legal Reserve” (LR). Recent political efforts are being made to disoblige the farmer to keep the LRs. In this study we evaluated the role of LRs in ensuring the ant biodiversity on LRs and soybean plantations. Ants were collected in 42 landscapes within Amazonian forests and Cerrado savannahs and the transition vegetation between them. In each landscape, sets of pitfall traps were placed in a paired design, in the Legal Reserve area and in the adjacent soybean cultivation matrix. As expected, the number of species was extremely lower on soybean plantations. Despite richness, we observed strong turnover between the LRs and plantations. The landscape types on which the Legal Reserve was inserted did not influence the number, but did influence the composition of species. Also, the similarity among samples decay with geographic distance only on LRs. Our results show the importance of the maintenance of Legal Reserve areas for the ants and associated biodiversity in agro-ecosystems. This fact reinforces the need to preserve the Legal Reserves as described in Brazilian law.
In a country with continental dimensions, such as Brazil, the responsibility for biodiversity and natural resources demands attention and is extremely important for worldwide biodiversity and environment safety (Metzger et al., 2019).The Brazilian Forest Code determines that rural properties preserve a minimum percentage of their area with native vegetation, the so-called Legal Reserves (LRs) (BRASIL, 2012). The LRs are responsible for housing most of the protected vegetation in Brazil, since they cover an area larger than the Conservation Units (Lewinsohn, 2010). Thus, despite of recent controversy and the fact that several farmers do not maintain the integrity of LRs in their properties (D’Albertas et al., 2021), the idealized function of the LRs is to ensure biodiversity and the various associated ecosystem services provided to rural properties such as to ensure climate sustainability and regulation, minimize erosion and soil loss, control the emission of greenhouse gasses, water provisioning, water quality regulation, preventing the silting up of rivers affecting water quality, pollination, biological control of pests and diseases (Dainese et al., 2019; Metzger et al., 2019). However, the preservation of LRs has been threatened by initiatives of the current Brazilian government, such as, for example, the attempt to approve Law no. 2362/19, which proposes to completely remove the requirement to maintain Legal Reserve areas (Abessa et al., 2019).
The large-scale conversion of natural areas for agricultural use, if legally authorized, will have numerous negative effects (Alves et al., 2020) such as: the extinction of endemic or already threatened species and the reduction of ecosystem services, including those that are directly beneficial for agricultural production, such as natural pest control, pollination, and maintenance of regional microclimate (Cividanes et al., 2018). Particularly, the native habitat in the two largest Brazilian biomes which border on each other, the Amazon forest and the Cerrado (a savanna-type vegetation) has been severely reduced (Marques et al., 2019). About 80% of the two million km² of original Cerrado savannah areas have already been transformed into pastures, annual crops and other human activities (Myers et al., 2012). Currently, it is in this biome where the largest Brazilian grain production occurs, mainly soybean (Glycine max L.) cultivation (EMBRAPA, 2018). The expansion of the agricultural frontier towards the north also threatens the Amazon, forming the region known worldwide as the Arc of Deforestation (Fearnside, 2017). As a consequence of this conversion process, the landscape of this region is currently composed of several remnants of native habitat, surrounded by an array of extensive monoculture areas (Oliveira-Júnior et al., 2015). The major part of these remnants was formed by a product of the demand to preserve the LR within farm properties.
In such a panorama, the study of the biodiversity preserved in the native habitat is urgent. Because of their great diversity of species, high abundance, wide terrestrial distribution, relatively well-known taxonomy and action at all trophic levels, ants are widely used in studies that assess environmental quality (Ribas et al., 2012). Ants provide numerous services for agriculture and natural ecosystems, such as soil aeration, seed transportation, herbivory, predation and various mutualistic interactions (Del Toro et al., 2012). Several studies have already used ants to measure the degradation of disturbed areas (Frizzo and Vasconcelos, 2013; Pacheco et al., 2017), as well as their recovery (Philpott et al., 2010) and at different spatial scales (Solar et al., 2016). Many species can coexist in natural environmental conditions, but only resistant species are able to maintain themselves under more stressful conditions (Pacheco et al., 2017). Thus, a large number of specialist species from forested habitat may not withstand the new conditions caused by agricultural activity, which in addition to the conversion of habitat, may include changes in luminosity, increased temperature and wind circulation (Frizzo and Vasconcelos, 2013).
Changes in species composition between different environments can be quantified using β-diversity, spatial species turnover and nesting (Baselga, et al., 2010). β-diversity is highly context-dependent in terms of organism and habitat type (Solar et al., 2016). Different ant species show differences in home range size and dispersal capacity; particularly in relation to the habitat they occupy (Del Toro et al., 2012). Although some ant species have large foraging areas, such as ants of the genus Atta (Kost et al. 2005), most terrestrial ant species forage over small spatial distances, restricted to a few meters (Baccaro et al., 2015). In addition, several ant colonies can co-exist in small areas, with overlapping foraging areas on the ground (Hanisch et al., 2018). In biomes with vegetation so different in structure and climate, the conversion into plantations can cause a differential loss in the biodiversity of ants. However, regardless of structure and the identity of original species, LRs must be responsible for preserving a significant amount of original species. We expect that more than different species composition, the β-diversity patterns of turnover and nestedness of soybeans plantations and RLs should also be different, showing a higher loss of β-diversity in plantations.
Therefore, this work aims to evaluate the role of RLs areas in maintaining the ant assemblage that persists in agricultural areas, especially soybean cultivation (Glycine max L.) areas in the high diversity and understudied transitional vegetation between the Amazon forest and Cerrado savannah biomes. For this, we evaluated whether: I) There is a difference in the number of species and in the composition of ants between the LRs areas and the adjacent soybean matrix areas?; II) Do the patterns of ant community similarity change differently between LRs and soybeans along geographic distance?; III) Is the difference in the number of species and composition of ants between LRs and cultivation areas affected by the vegetation type in which the area is inserted? and finally; IV) Does the amount of native habitat and density of green vegetation (NDVI) affect the number of species and composition of ants in the interior of the LRs and in the adjacent soybean plantations areas?
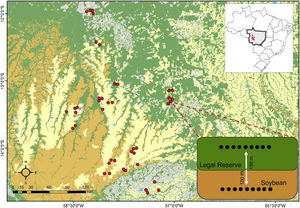
Material and methodsStudy areaThe determination of the study area was based on biological and geographic reasons. The farms studied here are all located in the State of Mato Grosso, located in the Midwest region of Brazil, which include three important Brazilian biomes (Amazon forest, Cerrado savannah and Pantanal) and host a high richness and almost unstudied ant fauna (Vicente et al., 2018). It is characterized by tropical savanna climate, according to the Köppen-Geiger classification, with average temperatures ranging from 24 to 36 °C and an annual precipitation of 1700 mm with well-defined periods between drought and precipitation (Alvares et al., 2013). Furthermore, the State of Mato Grosso is the largest grain producer in Brazil (EMBRAPA, 2021). Thus, the remnants of native habitat (the LRs) sampled are among the most advanced in monoculture and livestock, the main economic activities of the State (IMEA, 2017). The collections were carried out in 42 landscapes distributed in eight municipalities, inserted in transitional vegetation between the Amazon forest and Cerrado savannah biomes (Fig.1; Appendix Table A.1).
Location of the study area and the 42 landscape sampled in Amazonian forests (green), Cerrado savannahs (brown), and soybean cropland (yellow). At each sampled landscape (red circles), 9 pitfall traps were distributed 150-m apart along a 300-m core-edge-soybean transect including native vegetation, and soybean plantation. Grey areas in the map indicate cattle pasture. The sample design is shown int the inset.
At each sample site, two linear transects were established: one located in the native habitat (LR of the farm) and the other within the adjacent soybean plantations, both at a minimum distance of at least 150 meters from the edge that separates the two habitats. In each transect, nine pitfall traps were installed, ten meters apart (Fig.1). All pitfall traps were plastic containers with a diameter of 14 cm and a volume of 1000 ml, and filled with about 200 ml of a water, detergent and salt solution. The pitfalls were kept active for 48 hours at each site. As the time since planting is related to mechanical soil revolving and chemical treatments, the samples were standardized in order to minimize this source of variation in the ant community. Thus, all locations were sampled from November 2017 to February 2018, but were restricted between reproductive periods denominated as R1 the beginning of flowering to R7, the beginning of the maturation of the soybean pods. These stages occur in the middle of the rainy season, which also maximizes the number of species in natural environments (Neves et al., 2010).
The samples were transferred to the Entomology Laboratory of the University of the State of Mato Grosso (UNEMAT), Tangará da Serra, where the species were identified for the lowest possible taxonomic level using several dichotomous keys present in the literature on neotropical ant species and photos of types in AntWeb (AntWeb, 2019). The vouchers were deposited in a collection of the Community Ecology Laboratory, Biodiversity Center- UFMT, and in the Entomological Collection of Tangará da Serra of the University of the State of Mato Grosso (UNEMAT - CEnTg), Brazil.
Landscape CharacterizationThe metrics of the landscape for each sampled location were extracted using Sentinel satellite images from the Modis sensor (July 2018), with 10 meters of spatial resolution. We generated multiple buffers (500, 1000, 2000 and 5000 meters) at each collection point. For each buffer, we calculated the NDVI (Normalized Difference Vegetation Index), which ranges from 1 to -1, where values close to 1 corresponded to areas of dense vegetation and -1 areas with scarcity of vegetation. The mean of the NDVI inside the buffer was used to represent the complexity of the vertical vegetation structure. In the same buffers, using the Bhattacharya classifier, we quantified the land use in the agricultural area and native habitat (Amazon forest or Cerrado savannah) classes through supervised classification.
The landscape scale for ant assemblages is a geographic space in which species coexistence is determined by their dispersion capacity between different types of habitats (Schmidt et al., 2017). According to Spiesman and Cumming (2008), this geographic space to analyze the structure of ant communities must be a circular area of at least 500 meters in radius. Due to the high correlation between the variables in the buffers established in this study, we decided to use the metrics referring to the 1000 m scale for the delimitation of the landscapes.
In addition to the IBGE classification observed prior to collection (IBGE, 2020), we established a classification for the local definition within the transition environment. We considered the vegetation types predominant within the 1000-meter buffer to categorize the locations as: 1) Amazon forest - landscapes that presented more than 80% of the native habitat composed of forested vegetation; 2) Transitional vegetation - landscapes with Amazon forest vegetation and Cerrado savannah vegetation (with at least 20% of the native habitat composed of Amazon forest vegetation or Cerrado savannah vegetation); 3) Cerrado - landscapes that presented more than 80% of the native habitat composed of savannah vegetation. In all sampled points we avoided riverside vegetation of the metrics. Also, these metrics were validated in the field by the researchers.
Of the 42 landscapes, ten were inserted in Cerrado savannah areas, 22 in Amazon forest areas and ten in transitional vegetation. The difference in the number of collection points in the different vegetation types was caused by particularities of this study: 1) a minimum distance of 3.5 km between the sample points to guarantee independence; 2) the need of soybean crops being in the reproductive stage during the collection period and 3) the need for permission from rural producers to access property. We performed the procedures of mosaic, clipping, segmentation and classification of the images using Software SPRING 5.3 and the extraction of the metrics of the landscape using Software ArcGis 10.5.
Data analysisWe constructed a Mixed Generalized Linear Model (GLMM) using the glmer function of the lme4 package, to assess which variables affect the number of ant species. We used the landscape sample site as a random variable and the sample site identities (LR or soybean plantations), average NDVI within the 1000 m buffer, amount of habitat and landscape types as explanatory variables in the model. We also built Generalized Linear Models (GLM), to assess whether the number of species was being affected by the type of vegetation, amount of habitat or NDVI when we evaluated the type of habitat (LR or soybean plantations) separately. The family of errors used in all models were Poisson. However, as overdispersion was detected, we employed a Quasi-Poisson distribution. All models were checked for spatial correlation. The significance of the tests was assessed by the x2 test. Additionally, rarefaction curves were generated using the iNEXT function of the iNEXT package, to standardize the sampling effort (n = 22) and enable the comparison of the number of species between habitats (LR and soybean plantations) and between the landscape types of native vegetation (Amazon forest, Cerrado savannah and transitional vegetation) (Appendix Fig.A.1).
The dissimilarity decay of the ant community was calculated separately between LRs and soybeans plantations. First, we ran a Mantel test with the entire community using Sorensen index and the geographic distance between sample sites. Geographical distances were calculated as Euclidean distances, ranging from 3.5 to 315 km. After, we ran separate Mantel tests, for the ant community sampled on LRs and soybean plantations, using Simpson Index and the Matrix of Euclidean geographic distances. Once we partitioned the biodiversity, we used Simpson Index in order to represent the turnover component of the Sorensen index, decreasing the effect of the difference on the number of species between sites (Baselga, 2010). To visualize the decay in similarity within distance, we calculated the similarity (instead of dissimilarity) based on the Simpson index and built a scatter plot of the similarity decaying with the geographic distance. Also, we extracted the dissimilarity between LR and soybeans plantations for each sample site and built a GLM to see if the dissimilarity was influenced by the vegetation types that the collection point was inserted into.
To assess whether there was a difference in total diversity-β between the LR areas and soybean plantations and between landscapes types, we performed a Permutational Multivariate Analysis (PERMANOVA) (Anderson, 2017) using the Sorensen Index. The effects of the amount of habitat and NDVI on species composition were included as predictor variables. Due to the observed relationship in the dissimilarity among points and geographic distance (described above) which implies spatial autocorrelation (Mantel = 0.31 p < 0.001), we also included a predictor variable in the models representing the spatial variation of the collection points. This variable was obtained from a geographic distance of plantations, through the function DBMEN from the Adespatial package. Once interaction was detected, a posteriori GLMs were done, analyzing diversity-β patterns in the LRs and soybean plantations, separately. To test the dispersion homogeneity within the landscape types and habitats, we used the betadisper test of the vegan package. The total diversity-β was thus partitioned into Turnover and Nestedness components (Baselga, 2010) using the Betapart package. The contribution of each one of the Nestedness and Turnover components to the total biodiversity was calculated. To visualize the results, we built graphs using the first two axes generated by the Principal Coordinate Analysis (PCoA). In both cases, the data of presence and absence of species were used employing the diversity-β (Sorensen index). All graphs were made using the ggplot2 package to build the graphs and all statistical analyzes were performed using Software R version 3.4.2 (Team R Core 2018).
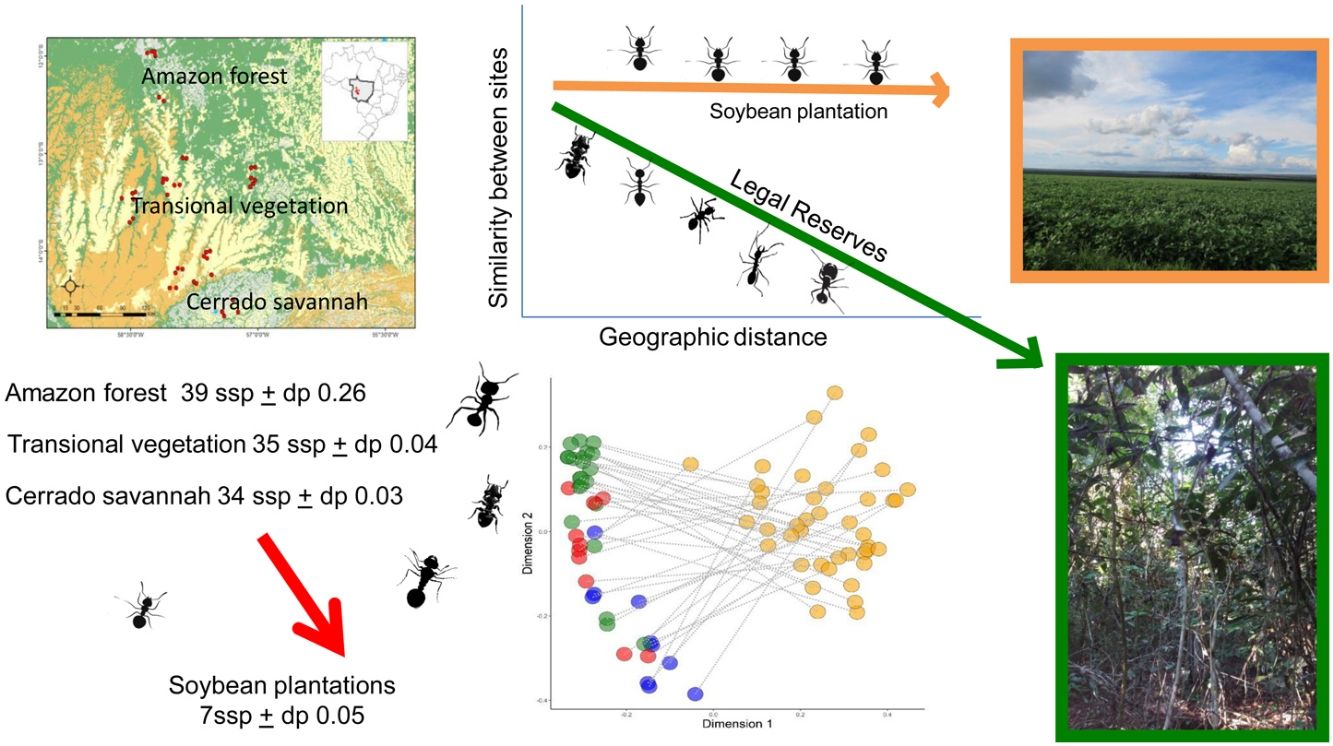
ResultsOf the 3537 occurrences, 177 species of ants were identified, distributed in eight subfamilies and 42 genera (Appendix Table B.1). Myrmicinae was the subfamily with the largest number of species (93), followed by Formicinae (28) and Ponerinae (20). The most frequent species were Camponotus sp1 (189) and Pheidole aff radoszkowskii (Mayr, 1884) (159). Among the species collected, 110 occurred exclusively in the areas of LRs and only ten were exclusive in soybean plantations. When separated by the landscape types, we obtained 47 species exclusively for the Amazon forest, eigth for the Cerrado savannah and only six for the transitional vegetation. In addition, we obtained 72 species that are widespread in the three landscape types.
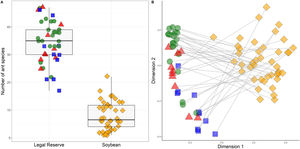
The number of species differed significantly between LRs areas and soybean plantations (x2 = 26.90; p < 0.001), but did not differ between landscapes types: Amazon forest, Cerrado savannah and transitional vegetation (x2 = 1.48; p < 0.13) (Fig. 2). The quantity of native habitat and NDVI did not have a significant effect on the number of ant species. When we separated the types of habitat, the variable landscape types (Amazon forest, Cerrado savannah or transitional vegetation), had an effect for the areas of LRs (x2 = 1.54; p < 0.02), but it was not significant for soybean plantations (x2 = 1.48; p < 0.12). The variables representing the amount of native habitat and NDVI were both not significant for either habitat type (Table 1).
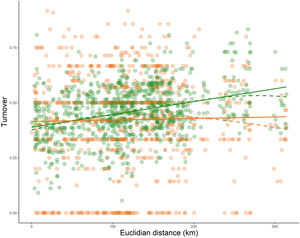
Community similarity between Legal Reserve and soybean plantations for geographic distance each sites sampled and dissimilarity of ant species captured with pitfall traps within Legal Reserves (green), and soybean plantations (orange) in 42 systems productive units distributed in eight municipalities within the State of Mato Grosso, Brazil.
Effects of predictor variables on number of species of ants in Legal Reserves and adjacent soybean plantations. Habitat type = Legal Reserve or soybean plantations; Landscape types = Amazon forest, transional vegetation or Cerrado savannah and Structural complexity of vegetation = NDVI.
| NUMBER OF ANT SPECIES - GLMM | |||
|---|---|---|---|
| Habitats | Variables | p-value | χ2 |
| Legal Reserve + Soybean plantations | Type of habitat | <0.001 | 26.90 |
| Landscape types | 0.13 | 1.48 | |
| Amount of Native Habitat | 0.59 | 0.52 | |
| NDVI | 0.87 | 0.15 | |
| NUMBER OF ANT SPECIES - GLM | |||
| Habitats | Variables | p-value | χ2 |
| Landscape types | 0.02 | 63.38 | |
| Legal Reserve | Amount of Native Habitat | 0.22 | 61.51 |
| NDVI | 0.51 | 62.96 | |
| Landscape types | 0.13 | 136.75 | |
| Soybean plantations | Amount of Native Habitat | 0.24 | 133.04 |
| NDVI | 0.12 | 134.38 |
The ant community sampled on LRs changed with the geographic distance among samples (Mantel = 0.34; p < 0.001). But this geographic change was not observed on soybean plantations (Mantel = 0.04; p = 0.23) (Fig.3). The species composition differed significantly between the areas of LR and soybean plantations (R2 = 0.23; p < 0.01), independently of the landscape types they were inserted in (F = 0.26; p = 0.26) (Fig.4). The composition also differed between landscape types (R2 = 0.04; p < 0.01). We also observed an interaction between habitat type and landscape types (R2 = 0.03; p = 0.006). Assessing the patterns of beta diversity, 97% of the total diversity was due to turnover and only 3% due to nesting. Even when we evaluated only the points of the soybean plantations, we found that the turnover component captured 96% of the total dissimilarity between crops. Therefore, we did not run a posteriori test because we considered as the total diversity-β was explained almost entirely to spatial autocorrelation and turnover of species among samples.
Number of ant species collected within Legal Reserves separated by the respective landscape types of Amazon forest (green), transional vegetation (blue) and Cerrado savannah (red) and areas of soybean plantations (orange) for each sites sampled in 42 systems productive units distributed in eight municipalities within the State of Mato Grosso, Brazil.
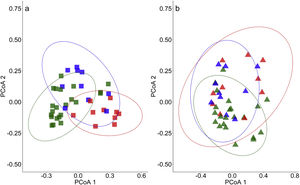
Composition of ant species captured with pitfall traps within (a) Legal Reserves and (b) soybean plantations in the Amazon forest (green), Cerrado savannah (red) and transional vegetation (blue) landscape types in the 42 sampled sites distributed in eight municipalities within the State of Mato Grosso, Brazil.
When assessing habitat types separately, spatial autocorrelation was observable between the LR points (Mantel = 0.35; p < 0.001) but not between the points of soybean plantations (Mantel = 0.08; p = 0.09). However, when we separated LR from soybean plantations, species composition differed significantly among Amazon forest, Cerrado savannah and transitional vegetation both within the LRs (R2 = 0.53; p < 0.01) (Fig.5A) and in soybean plantations (R2 = 0.38; p = 0.02) (Fig. 5B). We found heterogeneity between the groups in the LRs areas (F = 4.96; p = 0.01) but not in soybean plantations (F = 2.10; p = 0.14), indicating that there is a higher diversity-β inside the LRS, and a lower diversity in plantations. Any other variable (amount of native habitat, NDVI and spatial distance (DBMEN) did not show influence on species composition for soybean plantations (Table 2).
Effects of predictor variables on composition of ants in Legal Reserves and adjacent soybean plantations. Habitat type = Legal Reserve or soybean plantations; Landscape types = Amazon forest, transional vegetation or Cerrado savannah; Structural complexity of vegetation = NDVI and Spatial variable = DBMEN.
| COMPOSITION OF ANT SPECIES – PERMANOVA | |||
|---|---|---|---|
| Habitats | Variables | p-value | R2 |
| Legal Reserve + Soybean plantations | Type of habitat | <0.01 | 0.23 |
| Landscape types | <0.01 | 0.04 | |
| Amount of Native Habitat | 0.19 | 0.01 | |
| NDVI | 0.64 | 0.00 | |
| DBMEN (spatial) | 0.09 | 0.01 | |
| Landscape types | <0.01 | 0.17 | |
| Legal Reserve | Amount of Native Habitat | 0.98 | 0.06 |
| NDVI | 0.29 | 0.02 | |
| DBMEN (spatial) | <0.01 | 0.04 | |
| Landscape types | <0.01 | 0.08 | |
| Soybean plantations | Amount of Native Habitat | 0.26 | 0.02 |
| NDVI | 0.19 | 0.03 | |
| DBMEN (spatial) | 0.2 | 0.03 | |
| Interaction | Type of habitat + Landscape types | 0.006 | 0.03 |
Our results clearly support that Legal Reserves ensure local biodiversity within agro-ecosystems regardless of the landscape types in which they are inserted. The determining factor in the difference in the number of species and composition is the type of habitat, native or soybean plantations. The dissimilarity (β-diversity) between the LRs and soybean plantation ant communities at each sampled site was high, thus the replacement of the natural habitat causes a change on the ant assemblages of the agro-ecosystems independently on the landscape types they are inserted. This change may have been driven by an increase in the frequency of ants, usually rare on RLs, or the colonization of soybeans plantations by new species. We also observed that the ant composition in the RLs change along geographic distances. However, in the soybean plantations this β-diversity pattern is disrupted, since in soybean plantations the impoverished and modified ant communities do not change across space. These results suggest not just the expected local species extinctions associated with habitat changes, but also spatial homogenization of community structure due to the high dominance of habitat-generalists species widespread in the soybean plantations across the studied landscape (Martello et al., 2018).
We got to mention that few species (∼5%) were collected exclusively in soybean plantation, and all of them show generalist habitat and preference for anthropized environments, such as Pheidole aff radoszkowskii (Mayr, 1884), P. gertrudae (Forel, 1886), Pseudomyrmex termitarius (Smith, F., 1855) and Dorymyrmex brunneus (Forel, 1908) (Baccaro et al., 2015; Paolucci et al., 2017). On the other hand, ∼60% of the total species recorded were found exclusively on the LRs. In general, these are typical species of tropical forests such as Neoponera verenae (Forel, 1922) and N. apicalis (Emery, 1901) (Solar et al., 2015; Vicente et al., 2018). These findings support the fact that a relaxation in the Forest Code that would allow for the removal of Legal Reserve areas would threaten the biodiversity of ants at local and on a landscape scale by wiping out the endemic and specialist species.
We cannot overlook the fact that 35% of the total species were registered both in the Legal Reserve areas and in soybean plantations. In fact, even the studied farms show apparently healthy LRs, it is not necessarily true, once the history of the LRs were not known. In this case, it is expected that some generalist species can also establish populations on LRs. Among these species found in both habitats, some had generalist habits and provided important ecosystem services for agriculture, such as species of the genera Ectatomma, Gnamptogenys, Odontomachus and Pachycondyla that are predators and can contribute to the natural biological control of agricultural pests (Stein et al., 2014). Their presence in the anthropized environment ensures ecosystem services for soybean plantations. So, our study adds evidence that Legal Reserves actually fulfill their role established by law, not only promoting biodiversity conservation, but also guaranteeing services for agricultural production (Metzger et al., 2019). On the other hand, these genera host species of large body sizes and some with large nests (Baccaro et al., 2015), thus more sensitive to intensive soil turning. We cannot say how stable some populations of species collected on plantations are, or if the colonies found in plantations are able to reproduce and whether they are not dependent on constant colonization from LR propagules in a metacommunity approach (Nunes et al., 2020).
The composition recorded in the transitional vegetation areas had similarities to Amazon forest and to Cerrado savannah, but the composition of species observed in Amazon forest LRs was notably different from the Cerrado savannah. The species registered in the Amazon forest correspond to typical species of forested environment, especially forest with dense vegetation (Vicente et al., 2018). The observed species that occurred in the Cerrado savannah, on the other hand, are typical of environments with more spaced vegetation, species that tolerate conditions such as high temperature, increased incidence of winds and greater luminosity (Ribeiro and Walter, 2008). Indeed, the similarities and differences among LR habitats crops explain the interaction in the model: Cerrado LRs has more species in common to those found in crops, particularly the not common (rare) species, which has a low leverage on composition and dissimilarity measures (Franklin et al., 2013).
Even sharing some species, mainly in the Cerrado LRs, we cannot say that species in soybean areas are significant and well-defined subsets of species from those found in Legal Reserve (Pacheco et al., 2013). Instead, when assessing beta diversity, we found that the largest component of ant biodiversity was the species turnover associated with the difference between soybean plantations and LRs. We also found that geographic distance causes decay on the similarity among LRs sites, but not in the soybean plantations. Thus in LRs some differences between landscape types were maintained. On the other hand, the composition seems to be spatially homogeneous in soybean plantations. Indeed, in soybean plantations, we observed a high substitution of habitat specialist species to widespread species, specialized in anthropized environments. This indicates that regardless of the biome in which it is inserted, the cultivation of soy alone (without the presence of the Legal Reserve) does not effectively contribute to maintaining local diversity. These results, associated with other studies with different taxa, point out that the expansion of crops and habitat disturbance limits and modifies biodiversity in all biomes (Van Meerbeek et al., 2014; Ribeiro-Neto et al., 2016), inducing the loss of specialists and the homogenization of the ant biodiversity in large scale.
The observed effect of landscape modification is so pervasive on the number of species and composition that the amount of native habitat is indifferent to the ant community present both in the LRs and in the cultivated areas. In general, larger areas of native habitat have a greater number of ant species. It can be explained by their greater structural complexity of vegetation, usually associated with greater availability of resources and shelter for most species (Fischer et al., 2005). However, in our study we observed that the LRs protect biodiversity, but their amount of habitat and structural vegetation complexity (NDVI) do not influence the ant community of the cultivated areas. Therefore, it is possible that these predictor variables are not associated with the most important process in determining the diversity of these habitats, so that this pattern can be better explained by the conversion and simplification of areas (Ortega et al., 2018). Also, even apparently healthy, we do not know the history of use of the LRs we visited. Particularly, we do not have data on the pesticide employed on farms and if it could occasionally be blown to the LRs during windstorms, maybe breaking some ecological patterns but maintaining a large part of the biodiversity. Finally, we need to admit that the permits to visit the farms and their LRs were provided by honest farmers who follow the Brazilian Forest code, which is not necessarily the same for some other farms (Vecchiano et al., 2018).
ConclusionThe Brazilian Forest Code requires that rural properties preserve areas of native habitat such as a Legal Reserve. Our results show that these areas are extremely important for keeping ant biodiversity in a region that suffers the impacts of advancing the agricultural frontier, such as the Amazon forest and Cerrado savannah. The LR, the species composition is different between the forest types studied and varies with the spatial distance among sampling points. On the other hand, even being different among the landscape types they are inserted in, the ant composition in the crops do change along the distance. Based on our findings, we suggest the maintenance of the Legal Reserves in rural properties as described in the current Brazilian Forestry Code. Furthermore, the structure and integrity of the LR should be frequently inspected by Brazilian environmental inspection offices in order to safeguard their purpose.
Declaration of competing interestThe authors have no conflicts of interest to declare.
FundingThis work was supported by Fundação de Amparo à Pesquisa do Estado de Mato Grosso - Biotechnology Network Project Applied to Biodiversity Services and Disservices to Agriculture in the Cerrado and in the Amazon (BioAgro Network) (Redes de Pesquisa em MT FAPEMAT - nº 037 / 2016) and scholarship granted by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to KRP. TJI is supported by CNPq (309552/2018-4).
We are grateful to the field team for the data collection, especially to Carlos Barbosa de Andrade. We also thank Jéssica Pereira Machado and João Victor Garcia de Freitas for the laboratory assistance. We are grateful to all landholders for logistical support. REV thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) of the Ministério da Ciência, Tecnologia e Inovações (MCTI) by the support provided by the Programa de Capacitação Institucional (PCI/INMA). TJI is supported by CNPq (312684/2021-5). We are grateful to Research Networks at MT FAPEMAT for financial support. Finally, we would like to express our thanks to the Universidade do Estado de Mato Grosso and the Graduate Program in Environment and Agricultural Production Systems.
The following are Supplementary data to this article:
Rarefied curves of Hymenoptera species: Formicidae collected in Legal Reserve areas (a) and soybean plantations (b) in the Amazon forest (green), transional vegetation (blue) and Cerrado savannah (red) vegetation within productive systems in 42 sites distributed in eight municipalities within the State of Mato Grosso, Brazil.