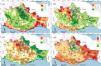
The greatest challenge that we currently face is generating integrative conservation strategies to guarantee the preservation not only of species richness, but also of ecological functions and evolutionary history. We propose to assess these different dimensions to identify areas of high multidimensional diversity (AMC), and exemplify this approach with cricetid mice, in a region of Mexico with high diversity that is considered a priority for global conservation. We elaborate models of the potential geographic distribution of 49 species of mice to predict their communities; we then used those predictions to calculate the number of rare species, and taxonomic, functional, and phylogenetic diversity. Subsequently, we identify AMC and evaluate their ecological integrity and the level to which they are protected by government and indigenous communities. We found a high spatial incongruity between the different dimensions of biodiversity, which indicates that in situ diversification processes and dispersal limitation drive spatial heterogeneous patterns of diversity. The AMC overlapped very little with the governmental (6.4%) and indigenous communities (15.2%) conservation areas, the Payments for Environmental Services initiative is the most overlaps. Half of the AMC had an intermediate ecological integrity. Protected areas systems protect few sites with high multidimensional biodiversity values, and half of the priority areas we identified requires restoration programs. The proposed methodology is an integral way to evaluate current protected areas systems and will be useful to guide effective and efficient conservation priorities involving evolutional and ecological dimensions of the biodiversity.
Conservation strategies focus on protecting taxonomic diversity (i.e., species richness), endemism, and species rarity, but ignore other dimensions of biodiversity (Devictor et al., 2010; Dreiss et al., 2015). Using taxonomic diversity directly or through substitutes (e.g., species richness) as the only way to assess conservation may omit areas with high biodiversity values based on other dimensions (Díaz and Cabido, 2001). Functional diversity considers the ecological roles of species and reflects their ecological, morphological and physiological strategies, which could have an effect on the functioning of ecosystems (Petchey and Gaston, 2006). Phylogenetic diversity reflects the evolutionary history of species capturing the uniqueness of lineages through time (Isaac et al., 2012) and can influence the susceptibility of species to extinction (Fritz and Purvis, 2010). Finally, the rarity is an important topic in conservation, and is evaluated using local population size, geographic range, and habitat specificity (Rabinowitz, 1981).
A comprehensive strategy for conserving biodiversity should consider more than just one dimension of biodiversity (Winter et al., 2013). However, this is challenging because different dimensions of biodiversity (e.g., taxonomic, functional, and phylogenetic diversity) do not always occupy the same geographic space—that is, areas with high taxonomic diversity do not present high levels of functional or phylogenetic, and vice versa—or the same time. This makes conservation management more complex (Mazel et al., 2014; Martín-Regalado et al., 2019). For this reason, it is essential to identify integrative approaches that consider multiple dimensions to effectively plan for biodiversity conservation, while a multidimensional approach would increase the probability of preserving different ecological and evolutionary properties of ecosystems (Devictor et al., 2010; Brum et al., 2017).
Biodiversity is inherently multidimensional; despite this, only a few recent studies have considered the potential benefits associated with measuring multiple dimensions of biodiversity (Strecker et al., 2011; Brum et al., 2017; Xu et al., 2019; Brumm et al., 2021). However, in these works, the areas with high diversity were not evaluated for their ecological quality, nor were the contributions of social conservation initiatives addressed.
A worldwide analysis of protected areas showed that at least 11% reserves have habitats that are still affected by human activities (Geldmann et al., 2019), and human disturbances alter the ecological integrity of landscapes (Parrish et al., 2003; Mora, 2019). Ecological integrity is measured according to the organization of its elements, the quantity and strength of interactions between species, the diversity and complexity of its components (structural complexity), and the connectivity between habitats. In Mexico, maps of ecological integrity have been estimated using this information and changes in areas with natural vegetation (Mora, 2019), and they are beginning to be used to make decisions about conservation and ecosystem services (Munguía-Carrara et al., 2019). One of those decisions could be to use fragmented ecosystems with good ecological integrity, as well as the sites that interconnect them, in such a way that they could serve as areas or islands conservation.
The state of Oaxaca, in Southern Mexico, has various conservation initiatives that are meant to maintain the biological richness of the region. These initiatives have been proposed by the government (Protected Natural Areas, PNA) and by social and indigenous communities (Community Conservation Areas, CCA). Here we propose the use of multidimensional criteria to identify Areas for the Multidimensional Conservation (AMC) of biodiversity and illustrate this approach using cricetid rodents as a focal group. It is important to maintain mouse diversity because a loss of diversity could lead to higher zoonotic risks. Recent analyses show that habitat loss changes the structure of animal communities, favoring tolerant species that can increase in abundance, and increases the pathogen amplifier potential; in contrast, more diverse communities show dilution effects of pathogens (Garrido et al., 2021).
Therefore, the objectives of this study were: (1) to identify AMC of cricetid mice in Oaxaca, and within its 12 physiographic subprovinces, (2) to contrast AMC with the PNA and CCA, and (3) to relate the AMC with the ecological integrity of ecosystems. We measured three dimensions of biodiversity—taxonomic, functional, and phylogenetic diversity—and other important aspects of conservation—endemism, rarity, and risk categories of species. We then established AMC and evaluated the coincidence between their spatial distribution within existing protected areas, and ecological integrity. This allowed us to suggest concrete conservation or management actions. Given the spatial incongruences found in previous studies among different dimensions of biodiversity (e.g., Martín-Regalado et al., 2019), we expected areas with very high multidimensional biodiversity values to be rare.
MethodsThe Oaxaca regionThe study area is the state of Oaxaca, a region of high biodiversity located in southern Mexico, between the geographic coordinates 15°39′, 18°39′N and 93°52′, 98°32′W. It has an area of 95,364 km2, which represents 4.8% of the national territory (García-Mendoza, 2004). The topography is heterogeneous, with elevations ranging from sea level up to 3600 m a.s.l. Oaxaca has 26 climate types, from warm and dry on the Pacific coastal plain to cold and humid on the mountain tops. Due to its complex orography, the territory has been divided into 12 physiographic subprovinces that are distinguished by their particular geomorphological features (Ortiz-Pérez et al., 2004; Fig. 1).
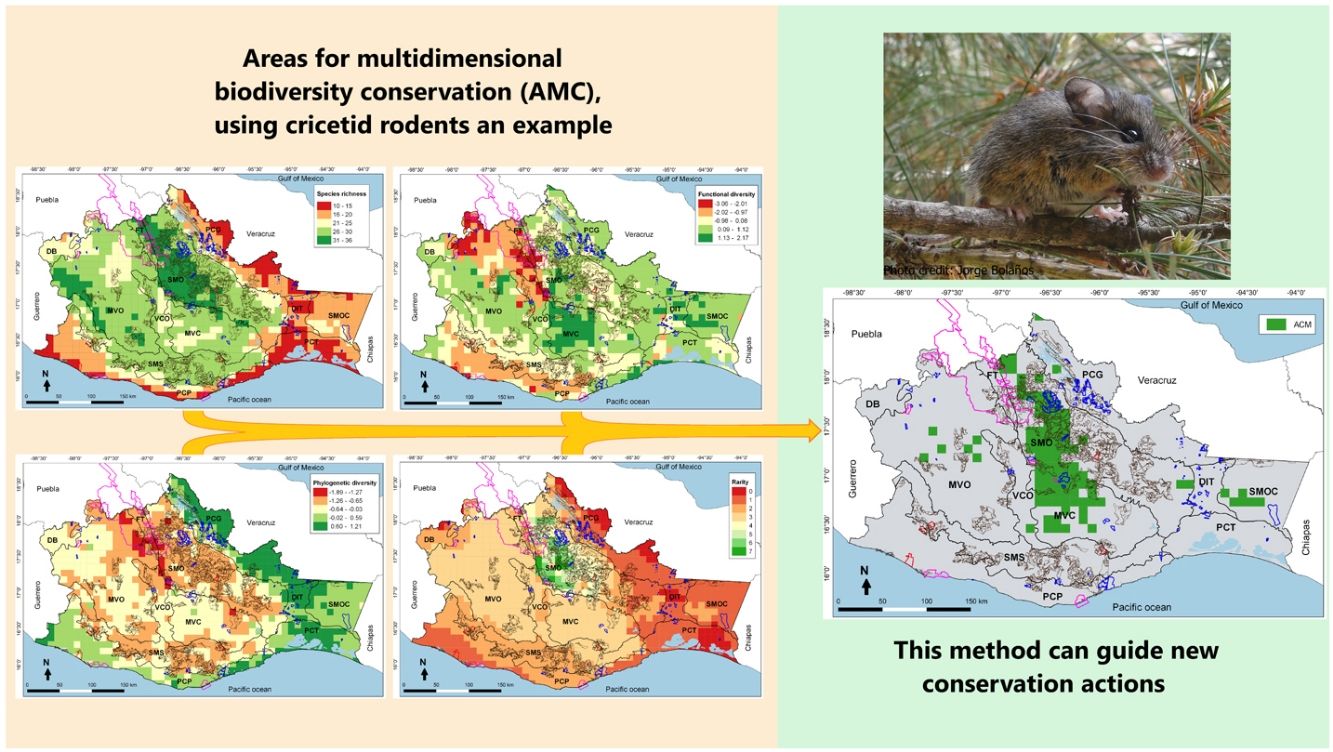
Four-dimensional spatial patterns of cricetid biodiversity in 100 km2 cells: (a) Species richness, (b) Functional diversity, (c) Phylogenetic diversity, and (d) Species rarity. The polygons with a pink line correspond to the Protected Natural Areas (PNA), the blue lines to the Voluntarily Conservation Area (VCA), the brown lines to the Payments for Environmental Services (PES) and the red lines to the Environmental Managements Units (EMU) for wildlife conservation in Oaxaca. We studied the following Physiographic subprovinces in Oaxaca: Depresión del Balsas (DB), Montañas y Valles del Occidente (MVO), Fosa de Tehuacán (FT), Sierra Madre de Oaxaca (SMO), Valles Centrales de Oaxaca (VCO), Montañas y Valles del Centro (MVC), Sierra Madre del Sur (SMS), Planicie Costera del Pacífico (PCP), Planicie Costera de Tehuantepec (PCT), Depresión del Istmo de Tehuantepec (DIT), Sierra Madre del Sur de Oaxaca y Chiapas (SMOC), and the Planicie Costera del Golfo (PCG).
Oaxaca has two main biodiversity conservation instruments: (1) government initiatives, represented by Protected Natural Areas (PNA) and (2) social initiatives, namely Community Conservation Areas (CCA), established and managed voluntarily by indigenous, rural, or other communities (Martin et al., 2011; Briones-Salas et al., 2016). The CCA complement the objective of conservation, since many of them have been integrated into the PNA system under the concept of Voluntary Conservation Area (VCA), which allows non-governmental groups to maintain ownership, management, or governance of the lands. There are also other CCA: areas that use Payments for Environmental Services (PES) and Environmental Managements Units (EMU, named UMA in Spanish) to conserve wildlife. The objective of the PES is to preserve natural areas without transformation through economic incentives, while EMU are designed so people can use their wild species in a sustainable way and conserve the habitat and its resident species. In both cases, the beneficiaries or users can be indigenous, rural, or other. Currently, 12.02% of the state of Oaxaca’s surface is protected through 13 PNA (3.65%), 868 VCA (1.45%), 337 communities supported by PES (6.69%), and five EMU (0.23%) (Briones-Salas et al., 2016).
Cricetid communitiesGlobally, Mexico stands out for its high richness of mouse species and the presence of threatened species, with fully identified priority areas (Kennerley et al., 2021). For example, Oaxaca is a region with the highest richness of cricetid rodents (Cricetidae, 49 species), half of which are endemic to Mexico and more than a quarter have a very restricted distribution (less than 10 km2) (Briones-Salas et al., 2015). Furthermore, one third of mouse species is in national or international risk categories (Lavariega et al., 2017). Due to their high taxonomic diversity, distribution patterns, evolutionary history, ecological importance, and have been well collected in the study area since there are many collecting efforts in databases and scientific collections, therefore cricetid rodents are an ideal group to address comprehensive conservation issues.
Diversity studies have been based on expert-based maps (IUCN Red List Threatened Species). These maps are adequate for coarse-scale macroecological studies (1-degree resolution), but at finer resolutions (∼1 km2) these maps may give imprecise spatial patterns (Peterson et al., 2018). Therefore, we did species distribution modeling to construct species range maps with a finer resolution, appropriate to the objective of this study. The potential distribution models were combined in QGIS (Quantum GIS Development Team, 2012) to obtain a general species richness map, which was later superimposed on a grid with cells of 100 km2 (a total of 1092 cells) with physiographic subprovinces polygons of Oaxaca (Ortiz-Pérez et al., 2004). We selected this spatial resolution because it covers the geographic range of microendemic species and this cell size has been used previously in studies with small mammals (e.g., González-Maya et al., 2016), and because conservation in the study area is mainly at small local scales. Each cell was considered a local ecological community in which different cricetid species could potentially coexist. Complete and detailed information of distribution models is provided in Martín-Regalado et al. (2019). The diversity measures and the number of species rarity were calculated in entire cells, regardless of whether the cells had irregular borders. Only, for the presentation of the figures the cells that are on the edges of the polygon of the state of Oaxaca were cut.
Taxonomic, functional and phylogenetic diversityThe number of species in each cell was considered as the taxonomic diversity. The index of functional diversity (FD) of Petchey and Gaston (2006) was calculated for each cell; this measures the total length of the branches that unite all the species on a functional dendrogram of the community. In addition, the PD phylogenetic diversity index was calculated (Faith, 2016). This index measures phylogenetic richness as the total amount of evolutionary history between all the species in a community. More detailed information of diversity analyses is provided in Martín-Regalado et al. (2019, 2020).
Species raritySpecies rarity was classified as demographic, ecological, and geographic. A species was considering as demographically rare if its litter size was less than three, and it has been reported with low abundance according to experts’ monographs (Ceballos and Oliva, 2005). For the ecological rarity, information related to the species’ habitat restrictions was used. We consider a species with habitat rarity when is restricted in a specific habitat (Meffe and Carroll, 1997). Finally, geographic rarity was determined using information related to the geographic distribution of the species in the state of Oaxaca (Amori et al. (2013). We consider a species with geographical rarity when its distribution is restricted was less than 3500 km2 according to endemic species of Oaxaca state (Amori et al., 2013). The information used for these classifications came from Ceballos and Oliva (2005), Amori et al. (2013), the IUCN (2019), and experts in the study group (see Supplementary data). For the cricetid species list, we used a binary matrix for every type of rarity, where a 0 was assigned to species not classified as rare and 1 for the species classified as rare for every type of rarity. Therefore, every species could have values ranging 0–3 of rarity.
Areas for multidimensional biodiversity conservation (AMC)The four dimensions of biodiversity (rarity, taxonomic, functional, and phylogenetic diversity) were rescaled in values ranging from 0 to 100, to weigh them equally and give the same importance to all dimensions. Consequently, the multidimensional diversity value for each cell ranged from 0 to 400. The analyses were done at two spatial scales: (1) for all cells in Oaxaca, and (2) independently for each of the 12 physiographic subprovinces, due to the high topographic, climatic, and biogeographic heterogeneities of Oaxaca (e.g., Briones-Salas et al., 2015; Calderón-Patrón et al., 2013).
The AMC were identified as the 17% of cells with the highest multidimensional diversity values. This percentage follows the Convention on Biological Diversity (COP, 2010), which set a goal for 17% of terrestrial areas to be protected for 2020. We also made a histogram of the frequency of the multidimensional diversity values for the cells. We identified AMC at both the state level (17% of cells in Oaxaca) and physiographic subprovince level (17% of each subprovince).
Overlap with protected areas and ecological integrityFor both the state level and individual subprovinces, the polygons of the government and social initiatives protection (Briones-Salas et al., 2016) were superimposed on the AMC and the percentage of the territory being conserved by these was calculated. To determine the current conditions in each cell, the AMC were superimposed onto the ecological integrity map of Mexico proposed by Mora (2019). Integrity values were divided into five classes: very low (0–18), low (19–37), intermediate (38–55), high (56–74) and very high integrity (75–92). In the map, the cells with higher values represented areas with greater natural vegetation. The ecological integrity map and the areas with government and community protection were downloaded from the Geoportal of the National System of Information on Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO; http://geoportal.conabio.gob.mx). The resolution all maps were 1:250,000 and geographic analyses were performed in QGIS v.2.8 (Quantum GIS Development Team, 2012).
ResultsDifferent biodiversity dimensions showed different spatial patterns. The greatest taxonomic diversity (Fig. 1a) and species rarity (Fig. 1d) were recorded in mountainous ecosystems and temperate climates, mainly in the Sierra Madre de Oaxaca (SMO); functional diversity (Fig. 1b) was highest in the subtropical plains and semi-mountainous, especially in the Montañas y Valles del Centro (MVC); and phylogenetic diversity (Fig. 1c) was highest in the low and warm lands, such as the Planicie Costera del Golfo (PCG). No cell yielded the maximum diversity value (400). The largest number of cells had intermediate values (249–300); there were no cells with low values (<200) and only a few (5%) with high values (>300). The top 17% of cells had values >277 (see Supplementary data).
We identified AMC mainly in the central and northern areas (Fig. 2a). Within each of the physiographic subprovinces, the AMC were distributed following a pattern of environmental heterogeneity, most AMC in mountain sites (Fig. 2b). At the state level, AMC overlapped very little (3 cells) with government conservation initiatives (PNA). Interestingly, there was greater overlap (49 cells) with community strategies (i.e., PES, VCA and EMU), mainly in the south and center of the state (Fig. 2a).
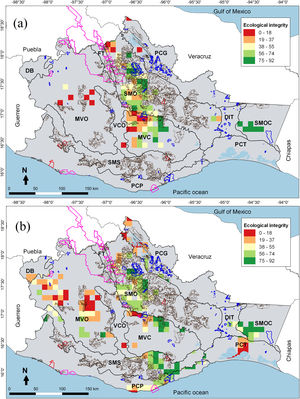
Areas for multidimensional conservation (AMC) of cricetid mice and ecological integrity values in 100 km2 cells: (a) state level and (b) by for physiographic subprovinces. Polygons with pink lines correspond to Protected Natural Areas (PNA), blue lines to Voluntary Conservation Area (VCA), brown lines to Payments for Environmental Services (PES), and red lines to Environmental Managements Units (EMU) in Oaxaca. See the “Methods” section for the names of the physiographic subprovinces.
At the subprovince level, the AMC coincided very little spatially with the governmental (6.4%) and social (15.2%) conservation initiatives (Fig. 2b). Seven subprovinces exhibited AMCs completely outside of government conservation areas. The AMC detected in the Depresión del Balsas was the only one that did not overlap with community conservation initiatives (see Supplementary data). Finally, the AMC in the Fosa de Tehuacán and the Sierra Madre de Oaxaca were the best represented in the PNA, VCA, and PES. The Sierra Madre de Oaxaca had the largest AMC area protected by government and community conservation initiatives. On the other hand, the Depresión del Balsas contained the AMC with the least protection (see Supplementary data).
Most of the AMC presented an intermediate ecological integrity, both at the state level (≈65%) and by physiographic subprovinces (≈70%). At the state level, 18.4% of the AMC presented very high ecological integrity values; these were located mainly in mountainous and semi-mountainous subprovinces (e.g., Sierra Madre de Oaxaca and Sierra Madre del Sur de Oaxaca y Chiapas). On the other hand, 14.3% of the AMC showed very low ecological integrity values; these were found in tropical sites (e.g., Montañas y Valles del Centro and Montañas y Valles del Occidente) (Fig. 2a). When assessing physiographic subprovinces, 15.3% of the AMC presented very high ecological integrity values; these areas with the highest integrity were in mountainous sites (>1000 m above sea level, e.g., Sierra Madre del Sur de Oaxaca y Chiapas and Montañas y Valles del Centro). On the other hand, 12.4% of the AMC showed very low ecological integrity values; these were found in subprovinces with a flat topology (e.g., Planicie Costera de Tehuantepec and Montañas y Valles del Occidente) (Fig. 2b).
DiscussionSpatial inconsistency in the dimensions of biodiversityAs expected, our maps revealed incongruity or spatial non-coincidence between rarity and taxonomic, functional, and phylogenetic diversity, pointing the great challenge to adopt an integrative approach to biodiversity conservation. However, the identification of conservation sites must take into account different dimensions of biodiversity (Brum et al., 2017) to ensure the preservation of species, functional and evolutionary processes. The incongruity found does not appear to be specific to cricetids in the study area, it has also been observed in other regions and taxonomic groups—e.g., birds from France (Devictor et al., 2010) and plants in South Carolina, USA (Brumm et al., 2021), suggesting that the high spatial congruence of multiple dimensions of biodiversity is the exception, not the rule. If the different dimensions of diversity present a spatial congruence, then the concerns would be less relevant and a conservation scheme focused on a facet would be the optimal strategy to prioritize all others.
Areas for multidimensional conservation (AMC)Three AMC overlap with priority areas for mammal conservation in southern Mexico obtained with the number of species, for example, the sites that Escalante (2003) identified in the Sierra Madre del Sur, the Planicie Costera del Pacífico, and the Sierra Madre de Oaxaca; similarly, Illoldi-Rangel et al. (2008) indicated areas in the Sierra Madre de Oaxaca, Sierra Madre del Sur, and the Planicie Costera del Pacífico; and Briones-Salas et al. (2016) suggested sites in the Sierra Madre de Oaxaca. It should be noted that all these areas detected in previous studies were proposed based on taxonomic diversity, endemism, and rarity but they ignored other dimensions of biodiversity such as functional and phylogenetic diversity. Therefore, the multidimensional view of biodiversity proposed in this study can help guide new conservation actions. For example, we could propose areas for the conservation of functional diversity where environmental services are most required, or areas for the conservation phylogenetic diversity, where some rare lineages should be preserved, or a combination of them.
Representation of multidimensional biodiversity in protected areasOur results suggest that the protected areas are not very effective at protecting different dimensions of cricetid diversity, because the majority (78.4%) of the cells that we found with high diversity values are outside the government and community conservation polygons; of course, none these protected areas was designed to protect cricetid rodents. Instead, areas are protected because they provide water, have a scenic value, a high degree of plant endemism, or even for spiritual reasons. Therefore, exist an underrepresenting multidimensional diversity in protected areas risks losing species with important functional roles in the ecosystem and a very unique evolutionary history. Poor multidimensional representation of biodiversity in natural areas has been reported in other countries and taxonomic groups (e.g., Strecker et al., 2011; Xu et al., 2019; Brumm et al., 2021), begging the question of whether it is effective and efficient to protect these areas, since the loss of biologically unique species can reduce the potential for communities to respond to changing conditions and loss of ecosystem services (Devictor et al., 2010).
At the finer, physiographic subprovince scale, community initiatives appear to be better at conservation. Similar results have been reported for the species richness of terrestrial vertebrates in the region (Monroy-Gamboa et al., 2019). This suggests that social initiatives, mainly those emanating from indigenous and rural communities, are a good strategy for conserving biodiversity in Oaxaca. Most of these have a territorial planning, which indicates forested areas to be protected, avoiding logging, firewood extraction and hunting, among other activities that contribute to carbon and water capture. The state of Oaxaca may be a good example of how social conservation initiatives contribute to the conservation of biodiversity and therefore these should be promoted to achieve conservation goals with a more comprehensive perspective (Martin et al., 2011).
However, we think that the dimension to be emphasized will depend on the needs and objectives of the actors involved. It would not be the same proposal for indigenous communities that experience a particular problem daily, or for government (political) initiatives that experience applying reserves could be applied that aim to protect biodiversity in several patches or islands (Halffter, 2007, 2020), as it occurs in several regions of Oaxaca.
In some countries, efforts to improve biodiversity conservation succeed when the federal government and indigenous communities work together (Ross et al., 2009). For example, the Mexican government recently (2008) legally recognized the Voluntary Conservation Areas (VCA) (Martin et al., 2011). We highlight that, in Oaxaca state, community initiative areas overlap more with AMC than government initiative areas do, and both areas combined overlap with 21% of the AMC.
The results of this study suggest that the Payment for Environmental Services (PES) protects the most cricetid AMC. Therefore, this initiative plays a large role in protecting areas for the multidimensional conservation of cricetids, mainly in the mountains of the Sierra Madre de Oaxaca. These sites safeguard environmental services, such as carbon dioxide capture and water retention. These results coincide with those reported by Briones-Salas et al. (2016), who found that the areas with PES presented the highest numbers of mammal species.
Ecological integrity and conservation suggestionsBased on the results of this study, most of the AMC presented an intermediate ecological integrity; both at the state level and physiographic subprovinces, the highest and lowest values of ecological integrity occurred in less than a quarter of the AMC. For the above, we suggest two strategies; first, prioritize habitat restoration in AMC with low ecological integrity. In Mexico, this approach to environmental restoration is common, but remains underutilized (López-Barrera et al., 2017; Calva-Soto and Pavón, 2018), and in some states, social problems and a lack of economic resources seem to be preventing ecological restoration efforts from becoming widespread (Carabias et al., 2007). A nationwide restoration program could be implemented to support indigenous communities found at these sites; other conservation programs have been successful in the region, so proposing a program of this nature would have a high chance to succeed.
The second strategy focuses on AMC with high ecological integrity; we suggest that conservation efforts in these areas prioritize protection through social conservation schemes and minimizing human impacts. For example, in some areas of the Sierra Madre del Sur de Oaxaca y Chiapas (SMOC), despite land tenure and agrarian problems, local communities have unwritten agreements for conserving their territory (Lira-Torres et al., 2012).
Conclusions and recommendationsThe methodology proposed in this study can guide new conservation actions through an efficient way of examining the multiple dimensions of biological diversity, if the necessary information is available. As we illustrate this approach using cricetid rodents (a biological group well known in the state and with importance ecological and zoonotic), we recommend for further studies the inclusion of others biological groups, different spatial scales, socio-cultural aspects, and consider the objectives of conservation of the indigenous communities and government.
According to the differences found in spatial patterns of the dimensions (taxonomic, functional and phylogenetic diversity and species rarity) we propose that multidimensional conservation efforts use a scheme of archipelago reserves, a new type of protected areas proposed by Halffter (2007, 2020), which aims to protect biodiversity in several patches or islands, instead of at large single reserve. It would be very interesting to analyze the effectiveness of archipelago reserves in Oaxaca, a sites with high biological and cultural diversity, where indigenous communities have a very important role in conservation.
Declaration of competing interestThe authors declare no conflict of interest.
We thank M.C. Lavariega, J.M. Pech and J. Sosa for their comments on this manuscript, and F. Mora for their help in explaining ecological integrity maps. CNM-R thanks the scholarship (#622396) granted by the Mexican National Commission of Science and Technology (CONACyT) to pursue her Ph.D. in Sciences in Biodiversity and Conservation at the Universidad Autónoma del Estado de Hidalgo. Special thanks to the researchers who allowed access to the scientific collection visited. This work was supported by the Secretaría de Investigación y Posgrado at the Instituto Politécnico Nacional (SIP; 20170981). CNM-R thanks Sistema Nacional de Investigadores (SNI) for its recognition and support. MB-S thanks the Comisión de Operación y Fomento a las Actividades Académicas (COFAA) and the Programa de Estímulos al Desempeño a la Investigación (EDI) at the Instituto Politécnico Nacional for their support, as well as the Sistema Nacional de Investigadores (SNI) for its recognition and support.