Fragmentation of the world's most intact forest landscapes will likely increase the severity of Human Wildlife Conflict (HWC). The way these threats affect top predators involves a series of complex social and ecological relationships, which are not completely understood, and thus require socio-ecological studies. The aim of this study is to examine the socio-ecological factors that affect the tolerance of local people towards the endangered Black-and-chestnut Eagle (Spizaetus isidori) in rural villages of the eastern Andes of Colombia. We conducted 172 interviews in 20 rural villages and estimated the proportion of forest cover (i.e. amount of remaining native forest), human density, the yearly losses of domestic fowl by the Black-and-chestnut Eagle, and socio-demographic parameters (i.e. economic activity, domestic fowl ownership, age, education, gender). The likelihood of villagers being tolerant towards the Black-and-chestnut Eagle decreased when the forest cover, human density and yearly losses of domestic fowl were higher. The integration of socio-ecological information allowed us to identify key areas with increasing HWC. Our findings were in consonance with the most recent evidence indicating that declines of top predator populations, as well as other vertebrate biodiversity, can be severely affected by the exacerbation of HWC on the border of intact native habitat and deforested areas.
Human Wildlife Conflict (HWC) is a growing problem that is often exacerbated in areas with an abrupt ‘wilderness’ interface (i.e. from intact native habitat to agriculture; Betts et al., 2017; Di Marco et al., 2018; Woodroffe, 2000). HWC are situations in which the behaviour of a wild animal species poses a direct and recurrent threat to livestock, domestic animals, or game species used by people and, in response, persecution of the “threatening” species ensues (Inskip and Zimmermann, 2009; Zimmermann et al., 2010). Deforestation within intact landscapes opens up human access to wilderness areas, increasing contact between people and wildlife (e.g. improving hunter access, poaching and eliminating prey), and thus triggering situations where humans come into conflict with wildlife (Betts et al., 2017; Di Marco et al., 2018; Graham et al., 2005; Teixeira et al., 2020). These situations worsen with increasing human densities near intact landscapes and usually end with the extirpation of species considered problematic by people (Betts et al., 2017; Di Marco et al., 2018). For instance, historic increases in human density had a strong association with the historic loss of populations of large carnivores in North America (Woodroffe, 2000). The extinction risk of terrestrial mammal species worldwide was also positively associated with human density (Di Marco et al., 2018). Fragmentation of the world's most intact forest landscapes – such as the tropics – is predicted to increase over the coming five decades (Taubert et al., 2018), thus increasing the probability of occurrence and severity of HWC in these wilderness landscapes (Betts et al., 2017; Frank et al., 2019).
Raptors provide an interesting model for analysing how an increase in HWC, as a consequence of the increasing human pressure on natural habitats, is leading to declines of threatened top predator species worldwide (McClure et al., 2018). HWC involving raptors were recorded as early as the sixteenth century with the officially encouraged killing of millions of raptors in many parts of Europe as a control measure to avoid losses in livestock and game species (Broun, 2000; Newton, 1979). Since the early 20th century, HWC has become a threat with significant impact on several raptor populations, leading to local or regional species extinctions in Europe and North America (Bildstein, 2001; Bildstein and Keith, 2008; White et al., 1994). The most remarkable example of the consequences of HWC for raptors was the extinction of the Guadalupe Caracara (Caracara lutosa), an endemic species from Guadalupe Island in Mexico, which was persecuted (in the context of a HWC) to extinction at the beginning of the 20th century (White et al., 1994). Raptor persecution is, nowadays, far from being the widespread activity it used to be in the past in Europe and North America, although conflicts involving raptors are still present and threaten these species in many countries around the world (Donázar et al., 2016; Madden et al., 2019). Effects of raptors on livestock and on populations of game species have been quantitatively well-studied suggesting low incidence of predation, rarely reaching values above 3% in both the proportion of the raptor diet as well as among the livestock mortality causes, suggesting low impacts to people's economies (Aguiar-Silva et al., 2014; Ballejo et al., 2020; Davies, 1999; Kenward, 1999; Madden et al., 2019; Restrepo-Cardona et al., 2019; Sarasola et al., 2010; Valkama et al., 2005). Despite this, as happens in many HWC involving carnivores, conflicts with raptors are widespread and hunters and farmers perceive predatory (and in some cases scavenger) species as harmful (Ballejo et al., 2020; Davies, 1999; Kenward, 1999; Madden et al., 2019; Sarasola et al., 2010; Valkama et al., 2005). This implies the existence of subjacent factors not related to material or monetary losses behind these conflicts (Thondhlana et al., 2020; Zuluaga et al., 2020).
Eagles, one of the most threatened group of raptors in the world, are frequently involved in conflicts with humans due to their large size and their food requirements (McClure et al., 2018; Meyburg, 1986; Newton, 1979). The Black-and-chestnut Eagle (Spizaetus isidori) (hereafter BC Eagle), for instance, is one of the most endangered eagles of the world (BirdLife International, 2020). It is distributed across montane rainforests in the Andes from Venezuela and Colombia to north-western Argentina (Ferguson-Lees and Christie, 2001). This large raptor is globally listed as Endangered, with an estimated population size between 250 and 999 mature individuals (BirdLife International, 2020). The BC Eagle is considered to be very sensitive to habitat loss and fragmentation (Thiollay, 1991), and to human persecution (BirdLife International, 2020; Echeverry-Galvis et al., 2014; Lehmann, 1959; Restrepo-Cardona et al., 2020). BC Eagle is a large forest raptor of 63–74cm in body length, 1500–3500g of weight, and up 180cm of wingspan (Authors unpublished data). The species feeds mostly on arboreal mammals and large-medium sized wild birds, however, domestic fowl (Gallus gallus) are present in almost every nest where the species’ diet has been studied (Aráoz et al., 2017; Lehmann, 1959; Restrepo-Cardona et al., 2019). Retaliation of farmers against the eagles because of livestock losses has been reported as the cause of mortality in most of the 80 BC Eagles found dead in the last 80 years in Colombia (Restrepo-Cardona et al., 2020). Over 30% of these cases occurred in the eastern Andes of Colombia, where a small BC Eagle population resides, seemingly isolated from the northern and southern populations of the species in the country (BirdLife International, 2020; Restrepo-Cardona et al., 2020). This suggests that the conflict between BC Eagle and humans exists and that it may be intense in some areas of Colombia.
In Colombia, around 60% of the original vegetation cover in mid and high montane Andean forests has been lost (Etter et al., 2006). These high rates of deforestation have changed native prey availability and increased the proximity between BC Eagle and human rural populations, thus likely increasing the conflict between local farmers and BC Eagle (Echeverry-Galvis et al., 2014; Restrepo-Cardona et al., 2019; Zuluaga and Echeverry-Galvis, 2016). Historical and current hunting of native prey by farmers, as well the rise of free-range domestic fowl, could also be altering BC Eagle food availability and consequently increasing livestock (i.e. domestic fowl) predation (Lyamuya et al., 2014; Restrepo-Cardona et al., 2019; Woodroffe et al., 2005). Recent studies suggested that the variation in levels of human–BC Eagle conflict in several localities of Colombia may be explained by a negative relationship between the percentage of forest cover and domestic fowl predation by BC Eagle (Restrepo-Cardona et al., 2019, 2020). However, this conclusion is based on a small sample of nests and localities, which could thus have some bias (Restrepo-Cardona et al., 2019). In contrast, other HWC studies in the Neotropical region involving wild top predators with similar habitat requirements as the BC Eagle, indicated that most forested landscapes were positively associated with wildlife attacks on livestock, and thus with higher levels of conflict (Michalski et al., 2006; Soto-Shoender and Giuliano, 2011; Teixeira et al., 2020).
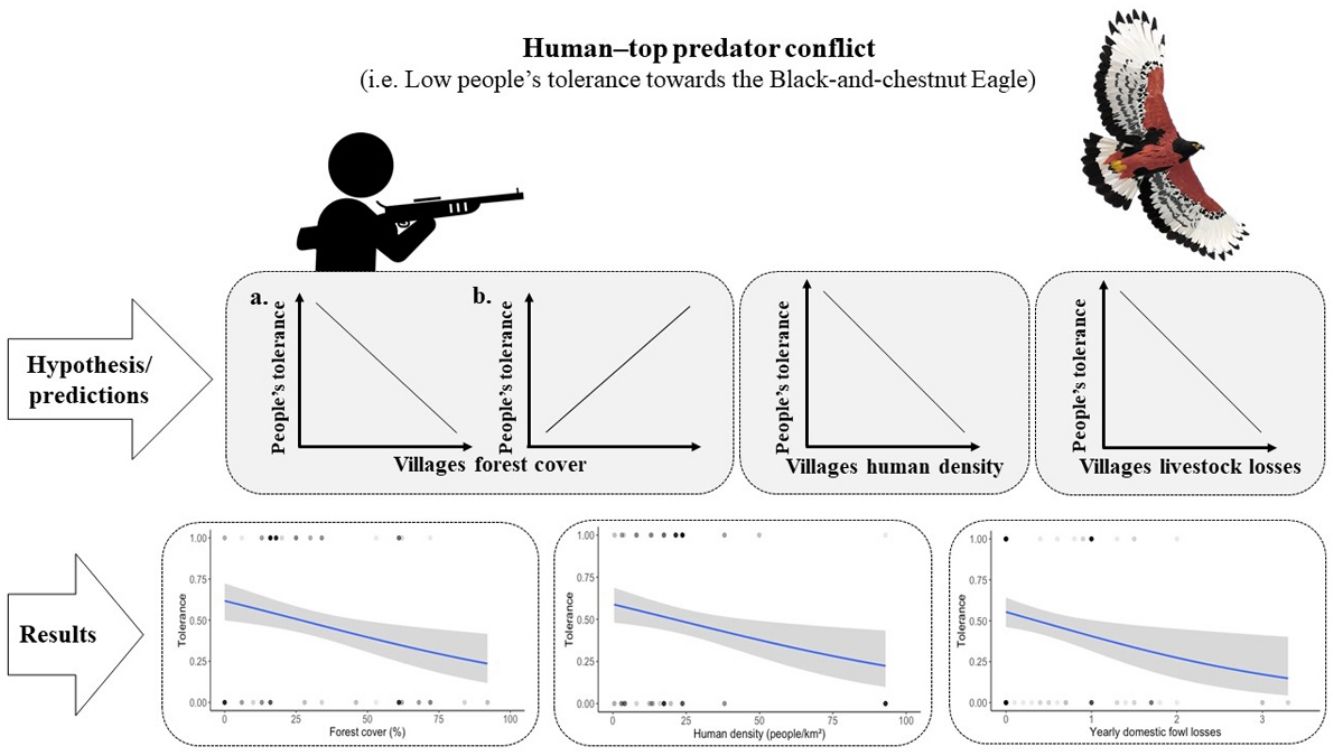
The strategic location of effective mitigation measures to address HWC is as important as the measures themselves (Altringham et al., 2020). In order to make effective decisions on future raptor conservation efforts, and to optimize the use of the limited economic resources, a more comprehensive knowledge of the socio-ecological contexts in which these human–raptor conflicts occur is necessary. As in the case of the BC Eagle populations, other forest raptor species of the Neotropical region are declining primarily due to an increase in HWC (Barbar et al., 2016; Gusmão et al., 2016; Muñiz-López, 2017; Restrepo-Cardona et al., 2020; Sarasola and Maceda, 2006) associated with processes of habitat loss and fragmentation due to agriculture expansion (Grande et al., 2018a; McClure et al., 2018). The mechanisms by which habitat loss and fragmentation, and human persecution in the context of HWC affect a species’ conservation status involves a series of complex social and ecological relationships, which are not completely understood, and thus require socio-ecological studies (Ballejo et al., 2020). The socio-ecological system approach brings together theoretical and analytical techniques from diverse disciplines, including those from social and ecological sciences, to understand complex systems (Binder et al., 2013). Emergent evidence suggests that applying a socio-ecological system approach can inform a better understanding of the socio-ecological contexts in which HWC occur (see Behr et al., 2017; Ceauşu et al., 2019; Dressel et al., 2018; Guerrero and Wilson, 2017; Pooley et al., 2017; Struebig et al., 2018; Teixeira et al., 2020). We anticipate that applying the socio-ecological system approach (Box 1) will improve our current understanding of this specific human–raptor conflict involving the BC Eagle in order to better inform conservation management. The aim of this study is thus to examine the socio-ecological context that exacerbates the human–eagle conflict (i.e. socio-ecological context influencing the people’s tolerance towards BC Eagle domestic fowl predation), in villages of the eastern Andes of Colombia. Our hypothesis is that the people’s tolerance towards this eagle will vary in the different villages influenced by the amount of forest cover (i.e. amount of remaining native forest), the human density, and yearly losses of domestic fowl by BC Eagle in those villages. We predicted that:
- 1.
Forest cover in villages. Here we postulate two contrary predictions that exclude one another:
- a.
People living in more forested villages will have more contact with wildlife and top predators, and thus will suffer higher losses by predation or at least will perceive a higher predation risk for their livestock, therefore they will be less tolerant of the BC Eagle (Betts et al., 2017; Di Marco et al., 2018; Graham et al., 2005; Michalski et al., 2006; Soto-Shoender and Giuliano, 2011; Teixeira et al., 2020).
- b.
Conversely, more forested villages will have more diverse and abundant populations of wild prey for the BC Eagle and thus, domestic fowl predation rates will be lower there and farmers will be more tolerant to the eagle. While in more deforested areas wild prey will be scarcer and thus, predation rates of eagles on domestic fowl will be higher there and farmers will be less tolerant of the BC Eagle (Acharya et al., 2017; Artelle et al., 2016; Lyamuya et al., 2014; Restrepo-Cardona et al., 2020, 2019).
- a.
- 2.
Human density in villages. Greater human densities in rural villages imply higher use of natural resources and probably a greater perception of competition between humans and wildlife (Artelle et al., 2016; Betts et al., 2017; Di Marco et al., 2018; Kaswamila et al., 2007; Woodroffe, 2000). Additionally, higher human densities may allow more frequent social interaction, allowing livestock predatory events to become more public, giving locals a higher perception of predatory risk and thus a lower tolerance to the BC Eagle (Bruskotter and Wilson, 2014; Carter et al., 2020; Marchini and Macdonald, 2018).
- 3.
Livestock losses. BC Eagle is known to prey on domestic fowl (Aráoz et al., 2017; Echeverry-Galvis et al., 2014; Restrepo-Cardona et al., 2019; Zuluaga and Echeverry-Galvis, 2016). Therefore, the people's tolerance towards the BC Eagle should be lower in villages with higher yearly losses of domestic fowl (Restrepo-Cardona et al., 2020).
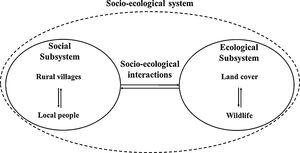
Socio-ecological system approach applied to human–wildlife conflicts, adapted from Carter et al. (2014).
Several research approaches have been developed and applied to different studies in which the interaction between the social system and the ecological system has been explicitly considered (Binder et al., 2013). We used the integrated socio-ecological systems approach proposed by Carter et al. (2014), for guiding our study on the complex relationships between the Black-and-chestnut Eagle and humans in the eastern Andes of Colombia (Guavio Region). Its conceptualization consists of three main components: the social subsystem, the ecological subsystem and the two-way socio-ecological interactions (or feedbacks). The social subsystem comprises local people and rural villages, and the ecological subsystem comprises wildlife and the land cover characterizing their habitat. The dimensions of each of these subsystems (i.e. local people, rural villages, wildlife and land cover) are interrelated and thus influence the characteristics of each other through socio-ecological interactions.
By transcending a single discipline, this approach can account for the patterns and processes that link people and their activities with wildlife and their habitats. Also, it can identify key relationships and feedbacks between people and wildlife. Finally, the approach facilitates understanding of cross-scale (e.g. spatial, temporal, and organizational) interactions between people and wildlife (Carter et al., 2014).
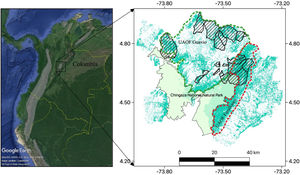
The study area is located in the Guavio Region, in the northeast of Cundinamarca department, eastern Andes of Colombia. The area is located within the buffer zone of the Chingaza National Natural Park, a national protected area which encompasses high Andes, tropical rain forest, sub-Andean forest and Andean forest (766 km2; Vargas and Pedraza, 2004). The governmental authority of this region is Corporación Autónoma Regional del Guavio (CORPOGUAVIO; www.corpoguavio.gov.co) which has a jurisdiction of approximately 3660 km2 including eight municipalities of the northeast of Cundinamarca department.
About 40% of the original vegetation cover of Andean forests in the Guavio Region has been lost mainly to agriculture and mining (CORPOGUAVIO, 2009). The original vegetation cover is managed by CORPOGUAVIO which has five forest management units (or Unidades Administrativas de Ordenación Forestal – UAOF). This study was conducted in two of these forest management units: UAOF Guavio and UAOF Farallones. The UAOF Guavio has 274 km2 (55%) of dense forest and 119 km2 (24%) of fragmented forest, while the UAOF Farallones has 293 km2 (79%) of dense forest and 42 km2 (11%) of fragmented forest. The UAOF Farallones is very important because it has the largest contiguous forest in the whole Guavio Region (Fig. 1).
Black-and-chestnut Eagle distribution range (left; http://www.birdlife.org). Forest cover in the study area of the Guavio Region is shown in green (right), Cundinamarca department of Colombia. Villages (n=20, hatched areas) were selected in two forest management units (or Unidades Administrativas de Ordenación Forestal – UAOF): the UAOF Guavio and UAOF Farallones (dashed line polygons).
The study area covered six municipalities, located at altitudes between 1000 and 3000m, in an area of approximately 2000 km2 (5.13804, −73.78346; 4.48784, −73.31499) (Fig. 1). Overall, 25% of the people live in urban areas (approximately 10,000 people) and 75% in rural areas (approximately 30,000 people). Rural areas in municipalities are divided into a lower administrative level called rural villages, or veredas in Spanish, with populations usually ranging from 25 to 1500 people (Tables S1, S2). In these rural villages, properties greater than five hectares are used mainly for rearing extensive livestock and for commercial agriculture (i.e. mainly in greenhouses), while properties less than five hectares are used mainly for low scale intensive livestock rearing and self-consumption agriculture. Overall, pasture lands have a few scattered trees and native forest cover in the villages is mainly in the most inaccessible places (i.e. places with highest slopes and far away from main roads). These conditions set an abrupt wilderness-agriculture interface, mainly in the most forested villages, where there are large areas of native contiguous forest in contrast to pasture lands with very few scattered trees.
Data collectionWe sampled 24 rural villages with confirmed presence of BC Eagle and/or human–eagle conflict from two sites within the selected study area: (1) 13 rural villages were randomly selected from a set of 20 with evidence of human–eagle conflict (Table S1), based on information collected between 2006 and 2012 by CORPOGUAVIO, and (2) 11 villages were selected based on field observations of juvenile BC Eagle and evidence of human–eagle conflict collected between 2014 and 2016 by the first author (unpublished data). We estimated the interview sample size based on Bernard's probabilistic sampling procedure with a 95% confidence level and confidence interval of seven percentage points (Bernard, 2006). Considering that the studied area represent an unequally distributed human population, we used a proportionate stratified sample (i.e. Probability Proportionate to Size – PPS; Bernard, 2006) by number of households in each village (see SM 1, Table S2). We obtained a complete list of the people resident in the villages. Interviewees were contacted in their homes and only one person older than 18 years-old was interviewed from each household. First author and three trained field assistants conducted 172 usable interviews in 20 rural villages: 94 people between April and May 2014 (in 12 villages), and 78 people between February and March 2017 (in 8 villages). Four villages were excluded because we could not interview at least two persons. In all cases, ethical standards of social surveys were met by informing respondents that their participation was voluntary and that we would ensure their anonymity.
We built a Geographic Information System for our study area using QGIS 3.4 (www.qgis.org). A Land Use Land Cover (LULC) map (scale 1:10.000) of the study area was provided by CORPOGUAVIO (CORPOGUAVIO, 2009) and the geographical villages’ boundaries were downloaded from DANE (https://www.dane.gov.co). Using these two layers, we estimated the forest cover in each one of the 20 rural villages. The proportion of forest cover in the villages – hereafter forest cover (i.e. amount of remaining native forest; see Teixeira et al., 2020) – varied from 0% to 92%. Finally, using the geographical villages’ boundaries and the list of the people resident in the villages, we estimated the human density in each rural village.
Questionnaire. Variables defined a priori from HWC literature were included in a questionnaire (Kansky and Knight, 2014). To ensure that the interviewees were really familiar with the BC Eagle they were asked to identify the eagle from a picture (i.e. we showed pictures of a BC Eagle adult, another of a BC Eagle juvenile, and a third of both ages). After this, we conducted a closed-ended question survey asking about personal experiences with BC Eagle (i.e. observation frequency of BC Eagle by people, yearly losses of domestic fowl by BC Eagle, and historical or current records of killed eagles) and tolerance. The tolerance was selected as the response variable and was defined as “the ability and willingness of an individual to absorb the potential or actual costs of eagle predation on domestic fowl” since anyone living in an area with eagles has to bear the risk of added costs which would not be present in the absence of the bird (i.e. livestock losses, Kansky et al., 2016). Therefore, we measured tolerance as the capacity for people to accept BC Eagle. We used scenario-style questions concerning hypothetical livestock predation by asking respondents about how many individual domestic fowl would they tolerate losing before killing BC Eagle. Possible answers were: none, between one and five, up to 10, and more than 10. Finally, we asked about demography and socio-economy (i.e. sex, age, education, economic activity and domestic fowl number). We codified: age in six classes (one decade per class: 18–27, 28–37, 38–47, 48–57, 58–67, >67; see White et al., 2018), education in four classes (university professionals, high school education, elementary school education, and no formal education), and economic activity in three classes (farming production, mining, and others).
Statistical analysesDescriptive statistics were used for presenting results on socio-economic and demographic variables, while a GLM framework was used to test our hypothesis (Zuur et al., 2009). Villages were categorized according to the amount of forest cover, as: a) “low proportion” proportion up to 29%; b) “medium proportion” between 30 and 60%; and c) “high proportion” with more than 60%, based on the minimum and maximum proportion obtained. A Kruskal–Wallis test was run to determine differences between the mean yearly domestic fowl losses of people in villages with low, medium and high proportion of forest cover. χ2 test of independence was run for estimating the influence of the category of forest cover on: the proportion of people observing BC Eagle at least annually (i.e. from weekly to yearly) and the proportion of killed eagles (i.e. historical or current records of killed eagles reported in the questionnaires) in the 20 villages.
A socio-ecological model needs at least three components: the social subsystem, the ecological subsystem and an interaction among them (i.e. interaction component). Our hypothesis was then translated into a hypothetical mathematical model (HM) as follows:
where tolerance represents a feedback of the social subsystem to the ecological subsystem, forest cover and human density represents the ecological and the social subsystems, respectively, and yearly domestic fowl losses by BC Eagle represent the interaction component between both subsystems (Box 1).In order to determine if our socio-ecological HM was the one that better explained the HWC, we compared it with two sets of alternative models. First, we compared it with simpler alternative models (AM) which included all the combinations of two of three variables in the HM (e.g. a model including amount of forest cover+human density; another model including amount of forest cover+the yearly losses of domestic fowl by BC Eagle, and so on) plus simple models including only one of the three variables (Table 1). Second, we compared our HM with a set of other demographic and socio-economic alternative models defined a priori from the literature on HWC (LAM; Table 2) with raptors in the Neotropical Region (see Ballejo et al., 2020, 2019; Cailly-Arnulphi et al., 2017; Restrepo-Cardona et al., 2020).
Alternative models (AM) with different combinations of the variables included in our hypothetical model (HM). The tolerance was selected as the response variable to all models (see Material and methods).
| Model | Variables include |
|---|---|
| HM | Tolerance ∼ Forest cover+human density+yearly domestic fowl losses |
| AM1 | Tolerance ∼ Forest cover+human density |
| AM2 | Tolerance ∼ Forest cover+yearly domestic fowl losses |
| AM3 | Tolerance ∼ Forest cover |
| AM4 | Tolerance ∼ Human density+yearly domestic fowl losses |
| AM5 | Tolerance ∼ Human density |
| AM6 | Tolerance ∼ Yearly domestic fowl losses |
Alternative models from published literature (i.e. literature's alternative models; LAM), on assessments of human–raptor conflicts in the Neotropical Region (see Ballejo et al., 2020, 2019; Cailly-Arnulphi et al., 2017; Restrepo-Cardona et al., 2020), used for validation. Original response variable refers to those presented in the original manuscript (see References). Equivalent alternative model refers to our interpretation of the original model to our data. The tolerance was selected as the response variable to all alternative models (see Material and methods).
| Model | Equivalent alternative model(response variable ∼ explanatory variables) | Original response variable | Reference |
|---|---|---|---|
| LAM1 | Tolerance ∼ Education+gender | Lethal control vs. Non-lethal strategy | Ballejo et al., 2020 |
| LAM2 | Tolerance ∼ Economic activity+gender | Harmful vs. Beneficial | Restrepo-Cardona et al., 2020 |
| LAM3 | Tolerance ∼ Domestic fowl owner+gender | ||
| LAM4 | Tolerance ∼ Domestic fowl owner | Harmful vs. Non-harmful | Ballejo et al., 2019 |
| LAM5 | Tolerance ∼ Education | ||
| LAM6 | Tolerance ∼ Economic activity+education+gender | Injurious vs. Beneficial | Cailly-Arnulphi et al., 2017 |
Through an information-theoretic approach using Akaike's information criterion (AIC) and Akaike weights (ωi), we determined the parsimony of our HM describing the data, respect to the six AM and the six LAM, respectively (see Richards et al., 2011). Before analysis, collinearity of continuous variables was assessed using Pearson's correlation coefficients, with all predictors used having r <0.7. Multicollinearity was assessed to all models by calculating the variance inflation factors (VIF) using the package car. The VIFs for all predictors used were <2, well below the common used threshold value and thus we are confident of the absence of multicollinearity among variables (see O’Brien, 2007). Models were ranked according to the Akaike Information Criterion corrected for small sample sizes (AICc). Akaike weights (ωi) estimate the probability to be the best model. The model with lower AICc value, and higher Akaike weights, was the model that best fitted our data. We considered models in which the difference in AIC relative to the best model is<2 as alternatively well supported models (Burnham and Anderson, 2004, 2002). The area under the ROC curve (AUC) was also estimated to compare the model's performance (i.e. AUC measures the overall performance of a model; a model that does not perform better than chance has an AUC of 0.5). This was made using the ModelMetrics package. We used a Binomial Regression Model using a logit link function.
Tolerance was our response variable. Interviewees were regarded as tolerant if they accepted to lose more than ten domestic fowl before killing BC Eagles (i.e. they have no intention to kill BC Eagle), and non-tolerant if they would kill eagles even if eagles killed up to or less than ten domestic fowls (i.e. they have any level of intention to kill BC Eagle) (see Marchini and Macdonald, 2012). Based on these two conditions, we considered tolerance as a binomial variable (1=tolerant, 0=non-tolerant). People were also classified as highly tolerant and lowly tolerant based on these values, respectively. We used R language to the estimated tests and through the package lme4, we fitted the models and compared them to each other (Bates et al., 2015). In all cases we used R 3.6.3 (R Development Core Team, 2014).
ResultsSocio-ecological characteristics of samplePeople. From all interviewed respondents, 55% were men and 45% were women. There were 4.7% university professionals, 15.7% had a high school education, 71% had an elementary school education, and 8.7% had no formal education. Their ages ranged between 18–27 (12%), 28–37 (15%), 38–47 (16%), 48–57 (22%), 58–67 (19%) and more than 67 years (16%). Most were engaged in farming production (77%) as their main economic activity, while others were pensioners, housewives or employees (21%). The remaining were involved in mining (2%). The majority (57%) had more than twelve domestic fowl. People who currently do not have domestic fowl (16%) declared they would be interested in having some in the near future. Over sixty percent of the people reported between weekly to yearly observations of BC Eagle (among the rest of the people 9% hardly ever saw it and 29% never saw it in the wild). Twenty-nine people had never seen it in the wild, but they properly identified as an BC eagle. Over a half of the people had low tolerance to losing domestic fowl by BC Eagle (52%).
Villages. Thirty percent of the villages had a high proportion of forest cover (between 61 and 92%), and twenty percent of the villages had medium proportion of forest cover (between 30 and 53%). The rest of them (50%) had low percentages of forest cover (between 0 and 28%; Table S3). The yearly domestic fowl losses by BC Eagle were higher in villages with high proportion of forest cover than in those with medium and low proportions (Kruskal–Wallis=4.5616, p=0.033). The mean human density in the 20 villages was 17.4±21.9 (±SD) people/km2. The human density had a negative correlation with the forest cover proportion in each village (Pearson=−0.62, t=−10.374, p<0.001). We did not find an influence of the proportion of forest cover on the observation frequency of BC Eagle by people (χ2=0.099, p=0.951).
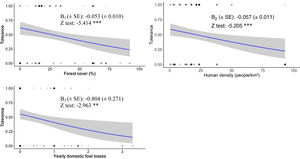
Socio-ecological model of the human–eagle conflictWe found a negative relationship between the people's tolerance towards BC Eagle and the amount of forest cover, human density, and the yearly losses of domestic fowl by BC Eagle (GLM; β=−0.053, p=6.17e−08, β=−0.057, p=1.94e−07, and β=−0.804, p=0.00304, respectively, R2=0.224; Fig. 2). Our socio-ecological model correctly classified 80% of people's tolerance towards BC Eagle (AUC=0.801; Table 3). This model also performed better than all alternative models tested (see Table 3).
Predicted effects of people's tolerance towards the Black-and-chestnut Eagle in the Eastern Andes of Colombia, according to each of the predictor variables included in the best-fitted model (i.e. Tolerance∼forest cover + human density + yearly domestic fowl losses by BC Eagle). Coefficients and statistical significance (codes: ***=0, **=0.001) are included. Light grey delimits 95% confidence intervals.
Comparison of our hypothetical socio-ecological model (HM) with other non-socio-ecological models. i. with a set of simpler models (AM) and ii. with a set of alternative models derived from the literature (LAM). Models are ranked according to the Akaike Information Criterion corrected for small sample sizes (AICc). Besides AICc, ΔAICc, Akaike weights (ωi), AUC and the number of parameters (k) are provided.
| Model | Variables include | k | AICc | ΔAICc | AUC | ωi |
|---|---|---|---|---|---|---|
| HM | Forest cover+human density+yearly domestic fowl losses | 4 | 193 | 0.00 | 0.803 | 0.982 |
| AM1 | Forest cover+human density | 3 | 201.1 | 8.04 | 0.786 | 0.018 |
| AM4 | Human density+yearly domestic fowl losses | 3 | 226.4 | 33.3 | 0.664 | 0 |
| AM2 | Forest cover+yearly domestic fowl losses | 3 | 230.9 | 37.85 | 0.609 | 0 |
| AM3 | Forest cover | 3 | 234 | 41.01 | 0.628 | 0 |
| AM5 | Human density | 2 | 235 | 41.94 | 0.485 | 0 |
| AM6 | Yearly domestic fowl losses | 2 | 235.1 | 42.09 | 0.589 | 0 |
| HM | Forest cover+human density+yearly domestic fowl losses | 4 | 193 | 0.00 | 0.803 | 1 |
| LAM2 | Economic activity+gender | 3 | 233 | 39.96 | 0.628 | 0 |
| LAM6 | Economic activity+education+gender | 6 | 237.4 | 44.39 | 0.640 | 0 |
| LAM3 | Domestic fowl owner+gender | 3 | 238.2 | 45.17 | 0.583 | 0 |
| LAM4 | Domestic fowl owner | 2 | 238.8 | 45.80 | 0.552 | 0 |
| LAM1 | Education+gender | 5 | 243.3 | 50.30 | 0.592 | 0 |
| LAM5 | Education | 4 | 244.1 | 51.08 | 0.552 | 0 |
The assessment of historical or current records of killed BC Eagles by the interviewed respondents indicates that the species was disproportionately hunted in the villages with high (83%; 4 of 6 killed eagles) and medium proportion (17%; 2 of 6 killed eagles) of forest cover (χ2=75.102, p<0.001). We did not find evidence of killed eagles in those villages with low proportion of forest cover (Table S3). Percentage of respondents declaring that they had killed at least one BC Eagle was low (3%; 5 of 172), nevertheless, among people living in villages with high proportion of forest cover the percentage increased to 8% (4 of 50). Only one person reported having killed two eagles and four admitted to each having killed one BC Eagle. Four BC Eagles were killed between 30 to 40 years ago, one another was killed 15 years ago, and one respondent was not willing to report a date. Among the people who had killed at least one BC Eagle in the previous years, all had declared low tolerance (i.e. intentions to kill the BC Eagle in the future if it fed on their domestic fowl).
DiscussionOur socio-ecological model used to analyze the socio-ecological factors that affect the local people's tolerance towards the BC Eagle, in a region of the Eastern Andes of Colombia, showed the best performance to explain tolerance among several alternative ecological, demographic and socio-economic models. The people's tolerance to the BC Eagle was lower in those villages with higher forest cover, higher human density and higher yearly domestic fowl losses. Forest cover was also positively associated with BC Eagle yearly attacks on domestic fowl. Increasing human density in areas that still hold important forest cover is likely exacerbating the severity of the human–eagle conflict in areas with an abrupt wilderness interface.
Our results suggest that the risk of persecution of BC Eagles in the context of HWC is relatively high in the most suitable habitats of the eastern Andes of Colombia. We found that the people's tolerance was negatively affected by the proportion of forest cover and by the domestic fowl losses by BC Eagle, both higher in the most forested landscapes. Based on knowledge of habitat requirements of the species, it is likely that BC Eagle selects villages with highest proportions of forest cover (i.e. those with minimal deforestation; Thiollay, 1991), where they had a higher impact on domestic fowl. Overall, the proportion of forest cover in the 20 villages was 44%. Around half of the people interviewed showed low tolerance to losing domestic fowl by BC Eagle (52%), but this proportion increased up to 76% in the six villages with the highest proportion of forest cover.. Human persecution was also most frequent in these villages where 8% of people acknowledged to have killed the species. Therefore, our results suggest that it is in those areas with higher forest cover and high domestic fowl losses where conservation action should be prioritized in the eastern Andes of Colombia.
Our outcomes supported the overall evidence that forest cover is positively associated with wildlife attacks on livestock and thus forest cover is indirectly and negatively associated with people's attitudes and tolerance towards predators (Graham et al., 2005; Michalski et al., 2006; Soto-Shoender and Giuliano, 2011; Teixeira et al., 2020). However, a study made in the vicinity of four BC Eagle nests suggested the contrary (Restrepo-Cardona et al., 2019, 2020). This difference could be due to the geographic scale of both studies as well as to its designs. While Restrepo-Cardona et al. (2019, 2020) worked in the vicinity of nests, in both the Central and the Eastern Andes mountains, we worked in several villages only in the Eastern Andes but in a wider area not restricted to the vicinity of nests. This divergence clearly shows the need to consider the approaches and the different scales of analysis. Future studies considering the interplay among forest cover, hunting of native prey by farmers, and prey availability for the eagle (i.e. free-range domestic fowl and wild prey) are necessary to have a better ecological understanding of the system and how it interplays with social factors to increase or reduce local HWC, and thus to improve our socio-ecological evidence to drive decision-making and implementation of conservation measures (Lyamuya et al., 2014; Restrepo-Cardona et al., 2019; Teixeira et al., 2020; Woodroffe et al., 2005).
Low tolerance in villages with high human density where the remaining habitat for the BC Eagle can be scarce could be related to some underlying issues related to a human–human conflict which are also present (Fraser-Celin et al., 2018). HWC are frequently complex and it is well known that mistrust between management agencies and other stakeholders, or the mere communication of predation events among stakeholders can create or aggravate conflicts (Bruskotter and Wilson, 2014; Marchini and Macdonald, 2018). The presence of the BC Eagle in these villages may be less frequent because there is a lower proportion of forest cover (Thiollay, 1991), and thus domestic fowl losses should be lower here. Although we did not find an influence of the proportion of forest cover on the observation frequency of BC Eagle by people, low proportion of forest cover was associated with low BC Eagle attacks on domestic fowl. However, the relationship between high human density and low forest cover may cause a high demand of natural resources which are scarce in these villages, but necessary to small scale subsistence farming production, (i.e. trees for firewood and construction materials for homes and fences, or wooded pastures with forage for livestock). Access to these natural resources may be limited by authorities through regulations and laws which foster discontent and environmental conflicts among stakeholders. These two factors have been shown to influence human–jaguar (Panthera onca) and human–cougar (Puma concolor) conflicts in Sao Paulo, Brazil (Engel et al., 2016), human–black bear (Ursus americanus) conflict in Colorado, USA (Lischka et al., 2018), and human–African wild dog (Lycaon pictus) conflict in Botswana (Fraser-Celin et al., 2018). Further socio-ecological research to deepen the this aspect of the conflict is certainly needed.
Despite being pretty simple, our socio-ecological model performed much better in explaining tolerance than all alternative models. These alternative models included a sample of simple models including only the ecological component, only the social component or the output of the interaction of both subsystems in the number of domestic fowl preyed upon by the eagle, as well as several other models including demographic and socio-economic drivers previously reported in the literature as relevant in human–raptor conflicts (Tables 1–3; see Ballejo et al., 2020, 2019; Cailly-Arnulphi et al., 2017; Restrepo-Cardona et al., 2020). Our results are thus in consonance with the emerging evidence suggesting that applying socio-ecological models to HWC can be informative and beneficial (Behr et al., 2017; Carter et al., 2019, 2014; Ceauşu et al., 2019; Dressel et al., 2018; Guerrero and Wilson, 2017; Pooley et al., 2017; Teixeira et al., 2020). Similarly, a recent study combining people's tolerance for critically endangered Sumatran tigers (Panthera tigris sumatrae) in socio-ecological models, with underlying attitudes, emotions, norms, spiritual beliefs and geographic profiles yielded predictions of tolerance that were 32 times better than models based on social predictors alone (Struebig et al., 2018). Those outcomes are clear examples of how a socio-ecological approach can improve our understanding of HWC with several species, including raptor species of conservation concern. These human–raptor conflicts have been widely studied from the biological sciences perspectives (e.g. Madden et al., 2019; Restrepo-Cardona et al., 2019; Sarasola et al., 2010; Valkama et al., 2005) but have usually ignored the socio-ecological evidence to inform decision-making and implementation of conservation measures (e.g. Ballejo et al., 2020, 2019; Cailly-Arnulphi et al., 2017; Grande et al., 2018b; Restrepo-Cardona et al., 2020), thus hampering the success of the proposed conservation measures.
The Conservation Plan for the BC Eagle in the Guavio Region considers that threats related to farming encroachment into intact native habitat of the species, and the increase of severity of human–eagle conflicts, should be mitigated (see Zuluaga, 2018). Based on the new socio-ecological evidence generated in this work, we suggest that the implementation of conservation measures related to these threats should focus mainly on specific areas where there is a larger risk of human–eagle conflict (i.e. villages with most forest cover, and among them, those with higher human density) to be more cost effective. This strategy would optimize the use of limited economic resources and would help to proportionally decrease BC Eagle mortality by poaching where this threat is higher. In the most forested villages where people have lower tolerance towards BC Eagle, more economic and human resources are needed in order to increase people's tolerance towards BC Eagle. In the same way, environmental education programs for saving the BC Eagle should be targeted to a broad audience (Zuluaga, 2018), and focused on specific areas with historical human–BC Eagle conflict (that could be measured by historical or current records of BC Eagles killed by people; see Nilsson et al., 2020). Approaches based on the science of behavioural change, such as ‘Theory of planned behaviour’ and ‘Theory of change’ have proven to make a more tangible difference for human behavioural changes (Altringham et al., 2020; Center for Theory of Change, 2019; Nilsson et al., 2020) and should be applied. A way to assess the success of the Conservation Plan for the Black-and-chestnut Eagle in the Guavio Region might be to measure whether this program's actions truly reduced the number of BC Eagles killed in those villages with the most intact native forest in the Guavio Region (Nilsson et al., 2020).
ConclusionOur study represents a good example of how an abrupt ‘wilderness’ interface can exacerbate a human–eagle conflict. The socio-ecological approach allowed us to better understand the complex interplay between people's tolerance, forest cover, human density and livestock losses. In this way, we captured the multiple ecological as well as social dimensions of this human–eagle conflict, identifying the combinations of attributes that should be considered for decision-making and implementing of conservation measures for the BC Eagle. Our findings supported the overall evidence that forest cover is positively associated with wildlife attacks on livestock and were in consonance with the most recent evidence indicating that population declines of top predators as well as of other vertebrate biodiversity can be severely affected by the exacerbation of HWC in areas of wilderness-agriculture interface.
Conflict of interestsNone declared.
We want to specially thank CORPOGUAVIO for funding (Agreements 422/2013 and 504/2016) and interviewees by their kind participation that made possible this study. L.F. Gómez, M. Schulze, and C. Lee supported us in the field work. Y.M. Salazar, M. Schulze and D.F. Aristizábal supported the desk work. S.A. Lambertucci, S. Marchini and J.T. Ibarra made comments that deeply improved the manuscript. The Black-and-chestnut Eagle Project – South America team, especially S. Kohn and T. Rivas-Fuenzalida, provided valuable discussions about the validity of our hypothesis and its application, based on their field observations on the species in Ecuador and Peru. Marta Curti kindly reviewed the English grammar. We also to thank the directors of Fundación NEOTROPICAL, F. Sáenz Jiménez and F. Cirí León. Santiago Zuluaga wrote this paper while funded by a doctoral grant from CONICET, The Peregrine Fund and Fundación Proyecto Águila Crestada-Colombia. Three reviewers provided constructive suggestions that allowed us to significantly improve this manuscript.