Human activities have been increasing pressure on riparian zones, affecting social benefits these ecosystems provide. Invasive species use river corridors as pathways to spread and consequently impact these ecosystems. Willows (Salix spp.) are important tree invaders worldwide. The invasiveness of Salix × rubens and its possible detrimental effects on Brazilian ecosystems have never been addressed. We hereby report the occurrence and invasion of S. × rubens in the highlands of southern Brazil, providing guidelines for the control of established populations and recommendations for safe cultivation to reduce invasions and environmental impacts. Through rapid field surveys and car-conducted roadside surveys performed in different seasons, S. × rubens individuals were located, counted and georeferenced. We registered 1929 individuals growing mainly along watercourses at altitudes between 826 and 1648 m above sea level. Our data provide evidence that S. × rubens has developed populations more than 100 m away from plantations in less than 50 years, likely facilitated by water transport, occasionally forming pure stands along rivers and floodplains. We concluded that S. × rubens is invading riparian ecosystems and thus must be acknowledged as an invasive species in Brazil.
Biological invasions are a major global threat to biodiversity (Diaz et al., 2019; Hulme, 2007; IUCN/SSC, 2000). Invasive non-native species (hereafter “invasive species”) are the second most common cause of species extinctions in the last 520 years (Bellard et al., 2016a). Invasive species have so far contributed to the extinction of 54% of animal species (Clavero and García-Berthou, 2005) and 27% plant species (Bellard et al., 2016b). Currently, a total of 27% of vertebrates (mammals, birds, reptiles and amphibians) are threatened by invasive species worldwide (Bellard et al., 2016a). Aside from that, an uncountable number of communities and ecosystems have been significantly impacted by invasive plants (Vilà et al., 2011) as a result of human-driven introductions (Murphy and Romanuk, 2014).
Invasive species use river corridors as pathways to spread (Pysek and Prack, 1994) and impact these ecosystems by altering the water-table regime, modifying surface hydraulic and geomorphological processes, displacing native species, reducing biodiversity, and increasing erosion (Gallardo et al., 2016; Tickner et al., 2001). Such impacts have direct consequences to the economy and human life (Diaz et al., 2019; Speziale et al., 2012). Furthermore, human activities as a whole have been increasing pressure on riparian zones, affecting social benefits provided by these ecosystems such as flood mitigation, water quality, erosion control, and groundwater recharge (Tickner et al., 2001).
High human pressure, including the introduction of invasive species, is also threatening many of the world biodiversity hotspots in South America (Myers et al., 2000; Pyšek et al., 2020; Speziale et al., 2012). Although research on introduced species is a rather new discipline in the continent (Speziale et al., 2012; Zenni et al., 2016), at least 41 out of the 100 most invasive species in the world (IUCN/SSC, 2000) and more than 840 invasive alien species (data compiled from Ziller et al., 2005 and Dechoum et al., 2020 – in press) are present in South America. In Brazil, the largest country in South America, biological invasions have been recognized as an environmental issue in the past two decades (Zenni et al., 2017, 2016). Still, at least 460 invasive alien species of animals and plants have been registered so far (Instituto Hórus de Desenvolvimento e Conservação Ambiental, 2019), and a large amount of them occur in protected areas (Dechoum et al., 2020 – in press; Sampaio and Schmidt, 2014; Ziller and Dechoum, 2013). Despite the urgency to control and/or eradicate existing invasions in protected areas, concerted management actions are still lacking in Brazil (Dechoum et al., 2019, 2018). Moreover, lack of precaution or proper analyses on the part of government development agencies and the general public on the introduction of non-native species for economic purposes (e.g. Salix spp. - Moura, 2002) results in serious failure in preventing new invasions.
The genus Salix, which includes trees commonly known as willows, is large and taxonomically complex, with approximately 450 species worldwide (Argus, 1997). In Brazil, there are two native species: Salix martiana Leyb. and Salix humboldtiana Willd. (Marquete et al., 2015). Salix martiana occurs in the northern region of Brazil, in the Amazonian phytogeographic domain, while S. humboldtiana occurs in the northern, southeastern and southern regions of Brazil, in the Amazonian, Atlantic Forest and Pampa phytogeographic domains (Marquete et al., 2015). Both species thrive along river margins and floodplains (Oliveira and Piedade, 2002), areas highly disturbed by floods and/ or with high water content in the soil. Among Salix species that have been disseminated worldwide, the most common are S. babylonica L. and S. viminalis L. (Marquete et al., 2015). However, European immigrants and Brazilian government agencies (namely EPAGRI in Santa Catarina and EMBRAPA at the federal level) have recently (since the mid-20th century) introduced several Salix species for economic purposes, mainly for wicker culture (Moura, 2002).
Salix spp. are important tree invaders worldwide (Richardson and Rejmánek, 2011). Salix × rubens Schrank is a hybrid originated from crossing S. alba L. and S. fragilis L. (Holland-Clift and Davies, 2007) (sometimes referred to as Salix cf. viminalis – e.g.: Carpanezzi et al., 2002). Salix × rubens has a single, multibranched trunk that can grow more than 15 m in height (Denardi et al., 2008). Seeds of S. × rubens are dispersed by wind or water (Farrar, 2001), but the species also spreads vegetatively through cuttings (Beismann et al., 2000) or broken parts of branches. This species belongs to willows of the Salix alba–Salix fragilis complex, native to western Eurasia, which represents typical invaders of floodplain ecosystems worldwide (Budde et al., 2011). Salix × rubens was introduced in the highlands of southern Brazil in the mid-20th century by Italian immigrants due to the elasticity and durability of the wood and branches, used to make rope for tying vineyards (Denardi et al., 2008; Wagner et al., 2009). The species adapted well in the region and is currently used for basketry, wicker production (Moura, 2002; Wagner et al., 2009) and live fence posts (Rech et al., 2019). Optimal biomass production occurs in soils with high water retention capacity (Mea et al., 2014). Some studies in Brazil have alleged that the species is not invasive and can be used in ecosystem restoration – especially near watercourses (e.g.: Carpanezzi et al., 2002; Denardi et al., 2008). However, several reports on the invasiveness and impacts of willows worldwide, – including S. × rubens, are clear warnings of potential environmental problems (e.g.Cremer, 2003; Greenwood et al., 2004; Budde et al., 2011; Holland-Clift et al., 2011; Richardson and Rejmánek, 2011).
The invasiveness of Salix × rubens and its possible detrimental effects on Brazilian ecosystems have never been addressed. The species has been tested and studied for economical purposes only. We hereby report the occurrence and invasion of Salix × rubens in the highlands of southern Brazil in protected and non-protected areas. Although the presence of this species has already been reported in other countries and ecosystems, to our knowledge, this is the first report drawing attention to the invasion of S. × rubens in Brazil. Additionally, we provide advice on management methods that can be used to control the invasion and recommendations for safe cultivation to mitigate invasions and environmental impacts.
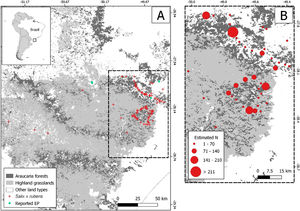
Material and methodsStudy areaThe survey was conducted in private properties and in protected areas (São Joaquim National Park - PNSJ, Leão da Montanha Private Reserve and Grande Floresta das Araucárias Private Reserve) in Rio Grande do Sul and Santa Catarina states, Brazil. These areas are located in the highlands of southern Brazil, mainly represented by subtropical highland grassland (campos de altitude) and mixed rainforest (Araucaria forest) ecosystems. Both ecosystems are part of the Araucaria Moist Forests ecoregion (Olson et al., 2001).
Species studiedInvasion by Salix × rubens can cause serious social, economic and environmental impacts (Adair et al., 2006; Holland-Clift and Davies, 2007). Abundant sexual and asexual reproduction, low herbivory and pathogen incidence combined with substantial seasonal vegetative growth likely contribute for invasion success (Adair et al., 2006). Also, riverine environments offer opportunities for dispersal due to connectivity, serving as pathways and increasing the extent of invasion by introduced species (Renöfält et al., 2005), including willows (Budde et al., 2011). In the treeless steppes of northern Patagonia, S. × rubens benefitted from the absence or low abundance of native riparian woody vegetation (Thomas et al., 2015), a similar scenario to the one observed in Brazilian highland grasslands. In Australia, invasive willows are considered invasive species of national significance due to their impacts, with control costs estimated at ca. $A10 million per year (Adair et al., 2006). Some relevant impacts reported for the Salix alba - S. fragilis complex worldwide are: 1) modification of riverine ecosystem properties through obstruction and diversion of streams, leading to erosion (Cremer, 2003); 2) changes in riverbank and hydrological properties such as water quantity and quality (Cremer, 2003); 3) displacement of native species resulting in biodiversity loss (Budde et al., 2011; Cremer, 2003); 4) changes in biotic and abiotic factors of riverine systems, affecting arthropods and higher trophic levels such as bird communities (Greenwood et al., 2004; Holland-Clift et al., 2011); and 5) hybridization with native willows, which poses further risk to native species (Santamour and Mcardle, 1988; Thomas et al., 2012). Salix × rubens was introduced in the highlands of Santa Catarina state in the mid-20th century (Denardi et al., 2008; Wagner et al., 2009). Production and marketing have gradually increased since the year 1960 and the species is currently in cultivation by more than 1200 families on more than 1200 hectares (Brandes and Arruda, 2006).
Data collectionIndividuals were located during field expeditions in the region from 2015 to 2020 in rapid field surveys (Filgueiras et al., 1994) that included a specific search for invasive species in PNSJ and surroundings in 2017. Additionally, from 2017 to 2019, car-conducted roadside surveys were carried out (Henderson, 1991) in the surroundings of invaded areas to increase detection. Car surveys allow covering larger areas in a shorter period of time with less resources compared to other methods, and generate data for species distribution maps (Deus et al., 2016). Surveys were performed in different seasons to increase detection accuracy of Salix × rubens individuals because of the species morphological differences in each season (e.g. deciduousness). The region was chosen because it encompasses one of the largest protected areas in southern Brazil (PNSJ), as well as other private protected areas (Leão da Montanha Private Reserve and Grande Floresta das Araucárias Private Reserve). The car-conducted surveys were carried out along parts of the most important highways in southern Brazil (BR-282, BR-116 and BR-285), including secondary roads. The total area covered was approximately 1070 km. The southwestern search direction was chosen because the majority of watercourses flow toward this direction (Machado, 2013). Search intensity was higher in Santa Catarina state as this is where the protected areas mentioned above are located and where the species is cultivated.
Salix × rubens individuals are easily recognizable due to the format of the crown, leaf drop and orange to reddish color of branches in winter. Once trees were identified, the geographic location of individuals was recorded, the habitat was briefly described (i.e. individuals along watercourses, in yards or along roads) and the number of individuals on each site was visually estimated. When individuals were present in riparian zones, the width of the watercourse as well as elevation data were obtained from freely available satellite imagery (Google Earth). Flowering branches of some individuals were collected and deposited in the Herbarium of the Federal University of Sant Catarina (Herbário FLOR – UFSC).
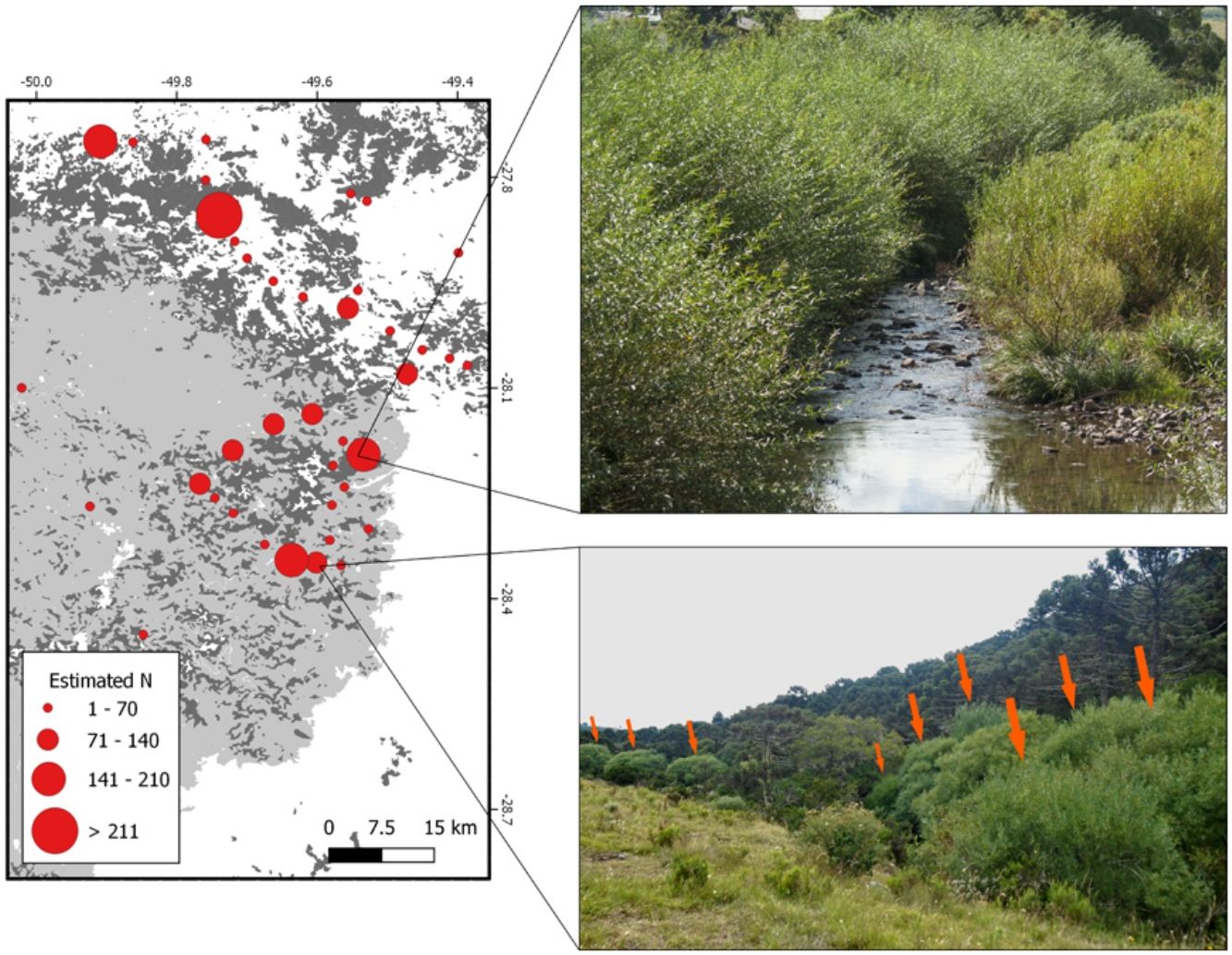
The location of the individuals registered in field expeditions was used to build a detailed map of the estimated number of individuals in the region of interest. In addition, a 1 km radius buffer was created for every occurrence, and buffers that overlapped were combined (sum of estimated number of individuals for each occurrence) in order to visually improve the spatial distribution of the individuals. Then, the centroid of the aggregated occurrences was plotted following a classification based on the final number of individuals. The location of willow plantations (for experimental and/or commercial purposes) reported in literature was also plotted (Rech et al., 2011, 2006). Calculations and maps were produced using the QGis platform (QGIS, 2017).
Results and discussionOur data provide evidence that Salix × rubens has developed populations more than 100 m away from plantations in less than 50 years (likely facilitated by water transport), sometimes with a high number of individuals forming pure stands along rivers and floodplains. In other words, our data show that S. × rubens is invading riparian ecosystems. Willow trees occur in the surroundings (Essl et al., 2018) and far away from plantations (Richardson et al., 2000), probably as a result of multiple establishment or introduction events (Blackburn et al., 2011). The main conclusion of our study is that Salix × rubens must be acknowledged as an invasive species in Brazil. We registered 1929 individuals of S. × rubens in altitudes between 826 and 1648 m above sea level in nine different municipalities (Fig. 1, Table 1). Individuals were mainly observed along watercourses and/or in yards (cultivated). Although some of the S. × rubens individuals were present in yards or plantations, i.e., cultivated (26.6% of reported occurrences), in wetlands or along roads (1.5% of reported occurrences), most individuals occurred exclusively along watercourses and/or on floodplains (71.9% of reported occurrences) (Table 1). The occurrence of this species along watercourses in a considerable geographic extension is unlikely due to planting. The most probable source of invasion is cultivation, as several occurrences were found close to plantations. The management technique employed on planted individuals in order to collect branches for basketry consists in removing yearly grown branches once a year. These are younger and thinner, thus flexible and easier to work with. Therefore, planters and collectors manage willows to remain as a low tree that is constantly pruned and does not grow tall. Several individuals registered in natural areas in this study consisted of trees with one main trunk and thick branches, taller than managed individuals, which provided additional evidence of uncontrolled dispersal. Moreover, the willow plantations observed were not located on river margins, as this placement complicates branch collection.
Individuals were observed along several watercourses in three drainage basins of the Canoas and Pelotas rivers (Santa Catarina state) and Apuaê-Inhandava river (Rio Grande do Sul state). These basins belong to the Upper Uruguay Freshwater Ecoregion (Abell et al., 2008) and are among the most important in terms of water supply for both human and agricultural uses in both states. The width of watercourses in which individuals were observed ranged from 0.5 to 62 meters, which demonstrates that the species is able to occupy different types of watercourses, from streams to rivers and probably withstand different levels of flooding. Salix × rubens branches are brittle at the base, therefore breaking off easily. The branches develop roots when in contact with water (Fig. 2A), increasing opportunities for medium to long-distance dispersal and establishment of new individuals (Beismann et al., 2000), especially during floods or strong water flows. Once established, the species can develop dense stands with a large number of individuals, and even form pure stands (e.g.Fig. 2B and 2C). Although many trees were large, younger individuals (juveniles) that probably established vegetatively from broken twigs were also present, as well as one seedling (Fig. 2D). Dissemination and invasion by this species can also occur during the transportation of branches, as they are carried in uncovered trucks. Branches can eventually fall from trucks and reach wetlands or watercourses. The species is benefited by river corridors (Pysek and Prack, 1994) as an invader of riparian zones.
A: Salix × rubens branches develop roots when in contact with water. Combined with the brittleness of branches, this characteristic can increase dispersal, establishment and invasion. B: High abundance of S. × rubens along the margins of the Pericó river. C: Pure stand of S. × rubens on the Bonito river floodplain. D: A seedling of S. × rubens growing in a watercourse. Images by the authors.
Salix × rubens has already been reported as an invasive species in other regions of the world, such as Patagonia in Argentina (Budde et al., 2011), Colorado state in the USA (Shafroth et al., 1994), and southeastern Australia (Greenwood et al., 2004; Holland-Clift et al., 2011). In Australia, S. × rubens as well as S. fragilis and S. alba are considered weeds of national significance due to their widespread distribution and ecological impacts (Adair et al., 2006). In Brazil, especially in the region covered in this paper, S. × rubens has already established (Moura, 2002; Wagner et al., 2009). Considering the methodology used in our study (rapid surveys and car surveys), it is likely that the abundance of S. × rubens in the region might be underestimated and that the area invaded is larger than so far reported (Catry et al., 2015). Despite the already known problems caused by species introductions, especially concerning biodiversity loss and ecosystem changes (Bellard et al., 2016a, b; Diaz et al., 2019; Vilà et al., 2011), the Brazilian federal government has not so far adopted risk assessment protocols to evaluate requests for the introduction of species. Besides, researchers in rural development agencies, who are mainly focused on the agribusiness sector, often introduce species without requesting permission from the federal environmental agency, increasing the risk of species invasions for the lack of proper assessments. As invasion by S. × rubens has been reported in other regions in the world, invasiveness in Brazil has been disregarded by rural development agencies such as the Agribusiness Research and Rural Extension Company of Santa Catarina (EPAGRI SC), which has been promoting the use and commerce of this willow. Such conduct goes against the recommendations proposed in the Convention on Biological Diversity, which state that “A good predictor of invasiveness is whether a species has successfully or unsuccessfully invaded elsewhere” (https://www.cbd.int/invasive/).
Management actions and restoration of invaded areasOur study suggests that S. × rubens started to spread and establish new populations, invading Brazilian subtropical highlands. Since the species has already been reported as invasive elsewhere with well-known impacts, it is likely that Brazilian ecosystems invaded by this species are also facing negative consequences. Early detection and control are important components of invasive species management, as eradication and control actions should ideally be undertaken in stages of initial invasion (Hobbs and Humphries, 1995). Moreover, research based on adaptive management must be undertaken so that efficient techniques can be defined and to provide a better understanding of the impacts of invasive willows in the region studied and in South America in general. The control techniques developed by institutions and researchers for Salix spp. invasions in Australia can provide an important basis for application in Brazilian ecosystems. We therefore recommend application of the methods suggested by Holland Clift & Davies (2007) until a more specific method can be defined for local conditions. A brief summary of management actions is presented below.
Despite the large impacts of willows in riparian ecosystems, removing them without further action can have unpredictable consequences (Holland-Clift et al., 2011; Rutherfurd et al., 2000). Although each situation must be evaluated independently, the effective control of S. × rubens invasions requires planning, resources, skills and appropriate equipment (Holland-Clift and Davies, 2007). From a conservation management perspective, the restoration of invaded areas must begin with the elimination of willows (including stock exclusion) followed by revegetation with native species (Holland-Clift et al., 2011) when S. × rubens is dominant and native species tend not to regenerate well. The control of S. × rubens can be carried out mechanically i.e. cutting and removing willows, piling and burning, combined with a chemical phase of herbicide application on cut stumps to avoid resprouting (Holland-Clift and Davies, 2007). When trunks are too large to be cut off at ground level, ringbarking combined with herbicide application should be used (Holland-Clift and Davies, 2007). The use of herbicides must be evaluated for each situation to avoid contamination of aquatic ecosystems. Application on cut stumps tends to be fairly safe (Dechoum and Ziller, 2013) and should comply with applicable laws and management policies. Trees can be stem-injected around the entire trunk with herbicides followed by removal after 2–3 months or leave the tree standing, although trees may take a long time, over a year, to dry up (Holland-Clift and Davies, 2007). Considering the local conditions of our study, the best herbicide available is Triclopyr for cut stump applications and ring-barking, as it is most often effective on woody plants, is not expelled through the root system, and will therefore not contaminate soil or water as long as carefully applied. Glyphosate may be tested for foliar application, stem injection and cut stump application (Holland-Clift and Davies, 2007). It is important to make sure all plants are removed from the invaded area, including branches, especially to prevent broken twigs to be carried by water and establish downstream (Holland-Clift and Davies, 2007). Chemical control must be conducted on dry days in the absence of rainfall at safe distance from water courses as needed (e.g.Machado et al., 2020). Moreover, chemical application should be topical and targeted, only using active ingredients that are not exudated by plant roots (Dechoum and Ziller, 2013).
After willow removal, site rehabilitation and monitoring are required to prevent erosion, regrowth or reinvasion (Holland-Clift and Davies, 2007). The re-establishment of native vegetation can be left to natural regeneration (when a seedbank still exists), planting/seeding when the site has low potential for natural regeneration (Holland-Clift and Davies, 2007), or a combination of these methods. The native willow species Salix humboldtiana, which occurs naturally along riparian zones in the study area and was observed in several sites, is the best option for restoration in this ecosystem, as well as to improve ecological recovery and long-term stability. Salix humboldtiana can be easily propagated through cuttings (Santos et al., 2011), grows well under stressful conditions and has proven to be successful for the restoration of embankment areas (Rauch et al., 2014). Along with S. humboldtiana, other common riparian native tree species in the region such as Araucaria angustifolia (Bertol.) Kuntze, Eugenia uniflora L., E. involucrata DC., Gymnanthes klotzschiana Müll.Arg., Luehea divaricata Mart. & Zucc., and Parapiptadenia rigida (Benth.) Brenan can be used along with other native species to increase taxonomic and functional diversity of affected sites, accelerating restoration. Restoration interventions must differ depending on the ecosystems originally present, since the region in our study covers a mosaic of forests and grasslands. Tree species must be planted/sown only in original forest habitats, whereas a different approach is required for native subtropical grasslands. Grasslands, along with other non-forest ecosystems such as savannas, are frequently undervalued by environmental policies at high risk of being mistakenly afforested, a practice that would negatively affect biodiversity and ecosystem functioning (Buisson et al., 2018; Veldman et al., 2015). Grassland restoration requires special attention as current knowledge on the restoration of tropical and subtropical grasslands is limited and restoration research in Brazilian grasslands is still scarce (Buisson et al., 2018; Overbeck et al., 2013). Although some alternatives can involve sowing of native forbs and grasses (e.g.Thomas et al., 2019), transfer and propagation of grassland species, and topsoil transposition (see Buisson et al., 2018 for a detailed list), it is likely that restoration attempts must include continuous active management to maintain disturbance regimes (Buisson et al., 2018) and improve forage productivity (Overbeck et al., 2013).
Our study is the first report on invasion by Salix × rubens affecting subtropical highland ecosystems in Brazil. This first assessment shows that riverine ecosystems are the habitat types mostly affected, and possibly provide a pathway for its spread. Despite its economic importance, we recommend that cultivation includes measures to prevent biological invasion as it can lead to economic, social and environmental impacts. People working in the willow industry and on cultivation must be informed of the potential impacts on ecosystems, on native willow species, and on water resources. Such information for producers must be provided by environmental and rural development agencies, such as EPAGRI SC and IMA SC. In addition, we recommend the development of specific legislation to regulate the species usage, such as others focused on invasive species and specific pathways published by the State Environmental Agencies of Santa Catarina and Rio Grande do Sul (for more information, see: https://institutohorus.org.br/marcos-legais/). This legislation must be discussed in the framework of state programs implemented in the southern states of Brazil (Rio Grande do Sul State Program for the Control of Invasive Alien Species - https://www.sema.rs.gov.br/programa-invasoras-rs; and Santa Catarina State Program for Invasive Alien Species - http://www.ima.sc.gov.br/index.php/biodiversidade/biodiversidade/especies-exoticas-invasoras). We also suggest the addition of Salix × rubens to the official lists of invasive species of both states. Research institutions such as universities and practitioners in NGOs should also be involved in order to select alternative species which can substitute the invasive species in its current use. Plantations must not be established along watercourses to avoid propagation downstream, while existing S. × rubens trees that are not in use or growing in places from where they can easily spread must be eliminated. Once they understand the negative consequences of invasions, the people who live in areas of cultivation and participate in the willow industry may be able to suggest other management alternatives if given the chance. Caution should also apply to other non-native willow species introduced for experimentation, of which plantations should be eliminated once experiments are concluded. This is the first study outlining the invasion of riparian ecosystems by Salix × rubens, an invasive species that poses a risk to subtropical highland grasslands and araucaria alluvial forests in southern Brazil.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Márcia P. Hoeltgebaum and Johnny Silveira for field assistance; the Brazilian Program for Biodiversity Research (PPBio) Atlantic Forest Network CNPq (457451/ 2012-9) for logistic support and ICMBio for permissions and support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.