Among the several noxious characteristics of Persistent Organic Pollutants (POPs) is a low environmental degradation rate, as they remain in the environment for decades. One of the measures adopted to mitigate environmental contamination is the imposition of bans and restrictions to several chemical compounds. But are bans being efficient to reduce the amount of such chemicals in the environment? In this systematic review, we analyzed the efficacy of banning POPs using bats as biomonitors in terrestrial habitats. Although bats provide relevant ecosystem services, these animals are highly exposed to chemical organic pollutants such as POPs due to their feeding and behavioral habits. POP concentrations were observed in biological tissues of bats in the genus Myotis (United States), with levels decreasing over the years since the ban. We also noticed a shortage of studies in neotropical regions, where the information gap on several POPs in tropical systems is still a concern in terms of history and intensive use of these toxic chemicals. Few studies were found on emerging POPs or on POPs recently included in the Stockholm Convention. Besides, the specimens in the analyses in the studies reviewed were not separated by sex or age, which may conceal the potential risk of POPs to the conservation of bat populations. We recommend that future research extends beyond chronic POP contamination in bats to also include risk assessment trials, as wild populations may be affected in the long-term, as well as their role in the ecosystem and the economy, requiring long-term studies.
Studies from all over the world show a decline in bat populations (Frick et al., 2019; Mickleburgh et al., 2002). The main causes are a synergy of factors such as habitat loss, establishment of wind power plants, disease, climate change, and pesticide use (Razgour et al., 2021; Voigt and Kingston, 2016). The order Chiroptera is the second largest mammal order, second only to Rodentia (Simmons and Cirranello, 2018), with more than 1400 species registered globally (Fenton, 2022; Mammal Diversity Database, 2024). Bats play important roles in the ecosystem due to their feeding habits, especially in the tropics. By feeding on fruits and seeds, nectar, invertebrates, and small vertebrates, they contribute to forest regeneration, pollination, and population control (Aguiar et al., 2021; Medellin et al., 2017; Boyles et al., 2011). One other approach on bats has more recently come up regarding their economic importance in parallel to the provision of ecosystem services (Ramírez-Fáncel et al., 2022; Rodríguez-San et al., 2020; Sakoui et al., 2020). In 2011, Kunz and collaborators documented the role of bats as agricultural pest controllers when studying the insectivorous species Tadarida brasiliensis in a cotton plantation, concluding that more than one billion dollars had been saved in pesticide use. However, this important role in the ecosystem and in the economy may be in process of getting silently lost. As bats are found in nearly all continents and coexist with humans in different environments (urban-industrial and agricultural), they are potentially exposed to several threats of human origin, such as chemical pollution (Bayat et al., 2014). Although pesticides are cited as a cause of bat mortality, few studies have investigated this assumption (Torquetti et al., 2021; Oliveira et al., 2020). Among the few that did, cause of death was concluded based on acute, lethal contamination, not covering gradual, chronic poisoning effects of in natura contamination (Bayat et al., 2014). Many species of bats have a migratory habit and hibernate in a certain period of the year (Fleming, 2019; Griflin, 2012). Chronic exposure may lead to sublethal physiological impacts often linked to behavioral cycles of fat deposition and removal that varies between species throughout the world.
The release of chemical contaminants such as Persistent Organic Pollutants (POPs) in the environment is a growing human threat. These chemicals are recalcitrant substances widely dispersed in the environment, thus a matter of concern at the global level (El-Shahawi et al., 2010; Jones and De Voogt, 1999). POPs represent a threat to the environment, animals, plants, and human health. In the current planetary climate crisis, global warming accelerates the process of POP volatilization, potentially increasing the amount of these substances in the environment (Wang et al., 2016). Because POPs have low rates of biological degradation and long half-life, they are detected in the air, in water and in soils in different environments for years, even decades (Zacharia, 2019). POPs are mainly lipophilic compounds associated with the fatty tissues of organisms which usually bioaccumulate in living organisms by ingestion of contaminated food. Moreover, selected POPs potentially biomagnify through trophic chains, remaining in biological systems even after years of exposure (Jones, 2021; Cetin, 2016). Several studies indicate the existence of a relationship between POP concentration and human diseases such as diabetes, obesity, endocrine disorders, cancer, cardiovascular and reproductive problems (e.g., Alharbi et al., 2018; Qing et al., 2006; Adeola, 2004). International recognition of such consequences led to a global effort to control and ban POP emissions through several treaties and conventions. The Stockholm Convention (2001) stands out among these, as several countries have agreed to protect human and environmental health from POPs (Fiedler, 2002; Lallas, 2001). Since the first 12 POP were banned (2001), several halogenated chemicals were listed, including organochlorine pesticides (aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, hexachlorobenzene, mirex, toxaphene); industrial chemicals (polychlorinated biphenyls (PCBs), hexachlorobenzene); by-products (hexachlorobenzene; polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/PCDF), and PCBs); per- and polyfluoroalkyl substances (PFASs) (http://www.pops.int), among other toxic chemicals (Jayaraj et al., 2016; Rae, 2014; Stockholm Convention 2001). Although pesticides were developed with the intent of only affecting target organisms, non-target species are also often impacted (Jayaraj et al., 2016).
Finding out how restrictions established in the Stockholm Convention are being applied is an issue of global interest. Although prohibitions have been implemented by most signatory countries, some still use POPs in epidemiological control (Fiedler et al., 2019; Van der Gon et al., 2007), while in others, POPs are illegally trafficked and used (Stechert et al., 2014; Gevao et al., 2010). In this way, reviews are important because they highlight existing information gaps in the literature and can serve as a starting point for the development of future work on POPs in general. Our objective in this study was to systematically review the scientific literature on POP contamination in terrestrial environments, focusing on POP groups that have historically been banned (e.g., organochlorine compounds), using bats as biomonitors at the global level. Our specific objectives were to: (1) test the relationship between years of different POP bans on bat tissues; and (2) identify knowledge gaps in terms of species and regions represented in formal studies. Our hypothesis presumed that the longer POPs are banned, the lower their concentration will be on different bat organs and excreta.
MethodologyOur systematic review was conducted in October 2023, sourced from three data platforms: Web of Science (https://www.isiknowledge.com), Scopus (https://www.scopus.com), and Google Scholar (https://scholar.google.com). No date restrictions were established. The keywords used were: “persistent organic pollutants” OR Aldrin OR Chlordane OR Dicofol OR Dieldrin OR Endrin OR Endosulfan OR Heptachlor OR Lindane OR Mirex OR Toxaphene OR PCB OR DDT OR pesticide OR organochlorine contamin* AND bat OR chiroptera.
The systematic literature review was conducted according to Moher et al. (2015) and Pollock and Berge (2018), considering eligibility criteria; information source; selection of literature; data collection and item selection with synthesis techniques, followed by six refinement stages: 1) As the total number of articles found in the search was very high (more than 1800), they were initially analyzed only if corresponding to the theme proposed for review based on the title. 2) After the first filter, the remaining articles were analyzed based on the abstracts. 3) Theses, dissertations, and abstracts presented at scientific events were not considered. 4) Articles were excluded when covering other chemical compounds, not POP. 5) When the same article was available on two or three data search platforms, it was considered only once. 6) The eligibility criterion was that the article included POP name and concentrations, as well as the scientific name of each bat species assessed. At this point, the text of article was analyzed. 7) Additional searches were carried out in bibliography lists of selected articles to increase the total number of references (Supplementary material 1, 2 and 3).
We extracted important information to highlight the current scenario of studies with POPs in bats worldwide. Bat species were classified according to feeding guild (Reis et al., 2007; Soriano, 2000; Kalko et al., 1996). POPs were classified as pesticides, industrial/unintentional use, or unintended use, according to the classification in the Stockholm Convention (2001).
To obtain the largest amount of data for the quantitative analysis we converted POP concentrations presented in ppm, ppt, mg/kg, or g to μg/g of wet weight, as this unit of measure was used in most publications. Articles containing POP concentrations by lipids, analyses conducted only on dry weight, and those in which the units of measurement were not informed were discarded given the impossibility of conversion. Species in the genus Myotis were selected to minimize phylogenetic variation and because there were not enough data on single species to conduct the analyses. Given the low amount of data and aiming to reduce geographic variation, we decided to use the data of the country with the largest number of records in the quantitative analysis.
The POP concentration data were transformed to logarithmic scale (log10) to normalize and homogenize variances. We calculated the time interval between the year of POP ban in the country (EPA, 2020; NOS, 2020; Mattina et al., 2002). We performed an analysis of covariance to evaluate the effect of maximum and average POP concentrations found in bat tissue (carcass, brain and fat), secretory products (breast milk) and excreta (guano) using the time since the POP ban, as a covariant. The same analysis was carried out to check whether there are effects of POP types (DDT, DDE, dieldrin, chlordane and PCBs). All analyses were performed in R environment Team (2009) and Systat 13.
ResultsLiterature reviewInformation extracted from the articles were year of publication, country, POP concentration, tissues and secretions analyzed, bat species, sex, and age class. The articles selected were published between 1971 and 2022 (Fig. 1A). The majority of studies (53%, n = 36) were conducted in the United States (USA), followed by United Kingdom (7%, n = 5) (Fig. 1B), with a total of 68 articles (Supplementary material 4).
A) Number of studies published from 1971 to 2022 grouped every 5 years. On the left Y axis the number of articles published and on the right Y axis the sum of cumulative number of studies. B) Number of publications of Persistent Organic Pollutants (POPs) in bats divided per country between 1971–2022. USA leading the number of publications with 36 articles.
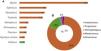
The sex of bats was indicated in 60% of the papers (n = 41), while in 58% (n = 40) bats were divided by age class (young and adults). In all, 64 bat species were evaluated, nearly half in the family Vespertilionidae (almost 59%; n = 38). The insectivorous bat species Tadarida brasiliensis (20%; n = 13), in the family Mollossidae, was more frequently studied. The genus most studied was Myotis (Fig. 2A), while 80% (n = 51) of the bats studied are insectivores (Fig. 2B). About 61% (n = 42) of the 68 articles studied POPs classified as pesticides, 35% (n = 24) as industrial/non-intentional use products, and almost 10% (n = 7) as non-intentional use. The POPs more assessed in the studies were DDT and/or its metabolites (DDE and DDD) (n = 53), followed by dieldrin (n = 29) and PCBs (n = 21) (Fig. 3A). In studies evaluating chronic contamination in bats, the most frequent collection sites were caves (40%), followed by forests (18%) and industrial areas (15%) (Fig. 3B). Studies in which bats were collected and administered doses of POPs for laboratory studies were not taken into consideration (16%) (Fig. 3C; D; E).
Qualitative data extracted from this literature review: A) Persistent Organic Pollutants (POPs) found in bats among articles published between the years 1971–2022. In blue the POPs classified as organochlorines and in orange the only POP studied belonging to the class of organobromines.
Legend Fig. 3A: OB = Organobrominated OC = Organochlorinated DDT = Dichlorodiphenyltrichloroethane; DDE = Dichlorodiphenyldichlorethylene; DDD = Dichlorodiphenyltrichloroethane; PCBs = Polychlorinated biphenyls; HCHs=Hexachlorocyclohexanes; CHLs = Chlordane compounds; PFHxS = Perfluorohexane sulfonate; Heptachlor=Heptachlor (epoxide); PBBs = Polybrominatedbiphenyls; PBDEs = Polybrominated diphenyl ethers; HCBs=Hexachlorobenzene; PCP = Pentachorophenol.
B) Type of environment studied classified according to the authors of the articles; Types of environments that were detailed by the authors of the article: C) Detail of the surroundings of cave sample areas D) Detail of the surroundings of forest areas E) Detail of the surroundings of urban areas.
The bat species used in our analyses were Myotis velifer, Myotis lucifugus, Myotis leibii, Myotis septentrionalis, Myotis sodalis, Myotis grisescens, Myotis californicus, Myotis volans, and Myotis evoti. All studies in the articles selected for our review were conducted in the United States (USA). We obtained 65 values of concentrations for the following organochlorine POPs: DDT and its metabolite DDE, dieldrin, chlordane and PCBs. This result is due to the larger number of studies on these pollutants (Supplementary material 5).
In our analysis, we were unable to include all data on concentrations found in the review due to lack of data standardization. POP concentrations (LogMax and LogMean) have decreased significantly in bats of the genus Myotis over the years since the ban in the USA (p = 0,003). There was no significant difference in POP concentrations considering POP type (LogMax p = 0.467 and LogMean, p = 0.404; Table 1). But 47% of the variation in the concentration of POPs was explained considering time since the ban and biological matrix (brain, fat, carcass, breast milk and guano) (Table 1). Higher initial concentrations were observed in fats and carcasses (Fig. 4).
Statistical results of analysis of covariance (ANCOVA) of POPs concentrations (maximum and mean values) and biological matrix, with banning time as a covariate. The POPs that entered the analyzes were DDT, DDE, dieldrin, chlordane and PCBs. The biological matrices considered were carcass, brain, fat, guano and breast milk.
| POPs Log - Maximum Values | POPs Log – Mean Values | |||||||
|---|---|---|---|---|---|---|---|---|
| R2 | F | p | N | R2 | F | p | N | |
| Banning time | 0.1257 | 9.0581 | 0.0038 | 65 | 0.1306 | 9.4625 | 0.0031 | 65 |
| Banning time | 0.4696 | 15.6019 | 0.0002 | 65 | 0.5121 | 19.0889 | 0.0001 | 65 |
| Biological tissues and products | 9.5641 | <0.0001 | 11.5331 | <0.0001 | ||||
| Banning time | 0.1763 | 10.5029 | 0.0020 | 65 | 0.1868 | 11.0167 | 0.0016 | 65 |
| POPs type | 0.9050 | 0.4670 | 1.0202 | 0.4044 | ||||
All the bold values are statistically significant (P < 0.05)
Logarithmized concentrations (ng/g wet weight) of organochlorine Persistent Organic Pollutants (POPs)-DDT and its metabolite DDE, Dieldrin, Chlordane and PCBs- in relation to the year of ban in the USA. A) Biological matrix brain. B) Biological matrix carcass. C) Biological matrix fat. D) Biological matrix guano. E) Biological matrix breast milk and F) Maximum POP concentration (Log) in all biological matrices.
POP bioaccumulation and biomagnification mechanisms and their transport are better established in aquatic environments compared with terrestrial environments. Several species of marine organisms, including top-of-the-chain mammals, are commonly studied (Sanganyado et al., 2018; O’Hara and Becker, 2002). In the terrestrial environment, there are few studies using mammals as biomonitors (Capella et al., 2023; Armitage and Gobas, 2007). In aquatic environments, many associate risks to human health with the ingestion of contaminated fish (Ko and Pan, 2018; Pérez-Parada et al., 2018), thus increasing the demand for studies. This review covers POP contamination in a unique land mammal, bats, around the globe. Existing POP contamination studies only cover 64 bat species among more than 1400 species (Fenton, 2022). Additionally, although more than 30 POPs are listed in the Stockholm Convention, only 13 have been studied using bats as biomonitors. The data compiled in our study provide an overview of current knowledge on the association of POPs and bats, showing a decrease of POP concentrations over time since these products were banned, as well as differences in concentration in different bat tissues. Environmental contamination studies in bats go beyond impact assessments on populations, being highly relevant for the understanding of possible impacts on ecosystem services provided by this animal group, which in turn contributes to the balance of ecosystems and human populations. Although the relationship between the use of chemical products and bats is recognized (Benvindo-Souza et al., 2022), studies that evaluate this negative impact along with bat ecosystem roles are scarce (Torquetti et al., 2021).
Most of the studies evaluated here are more than 30 years old, and more than half were carried out in one country. According to our evaluation, of the 68 articles assessed, 36 were carried out in the United States, where the number of bat species is approximately 60, most of which are insectivores (O’Shea et al., 2003). Although these publications were important for pioneering the study of POPs on bats, they were also restricted to practically one feeding guild and region of the globe. Punctual studies were carried out in other regions of the planet, with little research in tropical areas. Mansour et al. (2017) used insectivore bats of the species Taphozous perforatus as biomonitors of DDT and PCB concentrations in Egypt, one of the signatory countries to the Stockholm Convention. African, as well as, South American and Asian countries, have a history of prolonged use of DDT and other POPs, and late control measures (Sadasivaiah et al., 2007). In the year 2000, DDT was reintroduced for use in the malaria control program in Africa (e.g., in Cameroon, Kenya, Liberia, Nigeria, Senegal, and Tanzania), then pulverized in traditional mud houses in low-income communities (Hargreaves et al., 2003). The highest diversity of bats occurs in tropical regions, with more than 1000 species of diversified feeding habits with fundamental roles in the ecosystem (Mickleburgh et al., 2002; Alberico et al., 2005; Mickleburgh et al., 2002). Tropical regions are also represented by most developing nations with sociopolitical and environmental conflicts, where the use of chemical pollutants and POP bans occurred late in history (Schreinemachers and Tipraqsa, 2012; Schiesari and Grillitsch, 2011). The articles reviewed from tropical areas accounted for only 22.3% (n = 15), which corresponds to less than half of the articles published in the United States. Most North American bat species live in caves, hibernate in the winter, or migrate between seasons of the year, habits that differ in tropical bat species. Behavioral habits may influence the bioaccumulation of persistent polluters (Valdespino and Sosa, 2017; Frouin et al., 2012), and differ between bat species in temperate and tropical areas.
DDT and other POPs were intensively used between the 1940s and 1960s, then banned in the early 1970s. Although most pollutants assessed in our review were banned in the 1970s, contamination was still reported in the years 2000. Despite the known fact that certain POPs bioaccumulate in bats, being toxic, few studies investigated POPs as possible threats to their health and behavioral changes. In our review, 55 of the 68 publications only determined POP residues and concentrations without reporting on their effects on individuals. Although it is essential to verify the potential concentration of POPs that may bioaccumulate in bats, it is also important to indicate possible agents leading to population decline.
POP bioaccumulation occurs mainly through ingestion of contaminated water and/or food (Armitage and Gobas, 2007; Jones and De Voogt, 1999). Bats have a diverse diet, especially in tropical regions, being insectivore, carnivore, piscivore, frugivore, hematophagous or nectarivore (Clare et al., 2011; Mickleburgh et al., 2002). Some insectivore bat species can feed on up to 1000 insects in one night (Wetzler and Boyles, 2017). Eighty percent of the species cited in the articles we reviewed were insectivore (Fig. 2B), present in different landscape types, and play a role in pest control in nature and in agricultural areas (Maslo et al., 2022; Rodríguez-San et al., 2020; Kunz et al., 2011). These results were expected, as most studies were carried out in the USA, where insectivorous bats are more abundant (see Fig. 1B). According to Cisneros et al. (2015), frugivorous bats are more capable of exploring resources in secondary vegetation compared with insectivores. Differences in bat abundance within a landscape may thus imply different levels of intoxication risk between feeding guilds (Valdespino and Sosa, 2017), and indicate which guilds are more vulnerable to POP effects. Brinati et al. (2016) have already demonstrated noxious effects of small endosulfan doses on the frugivore bat Artibeus lituratus in tropical areas. In our review, however, only one study covered other feeding guilds (Racero-Casarrubia et al., 2021), of which none was piscivore. Longevity is another characteristic of bats (Brunet-Rossinni and Austad, 2004), with a record established for a free-living Myotis brandtii bat at 41 years of age (Podlutsky et al., 2005). This may result in longer exposure time and, when at the top of the food chain, greater biomagnification (Jones et al., 2009; Wilkinson and South, 2002). Therefore, it is important to analyze species with other feeding habits, such as omnivores and/or carnivores.
As expected, most of the species studied were collected in caves. However, it is important for this type of work to detail the surroundings of the collection sites, considering that the bioaccumulation of chemical pollutants is directly affected by the type of environmental matrix in the surroundings. Some articles described the entire sample area, mainly pointing out the existence of agriculture, pastures, houses, and industries (see Fig. 3B and Supplementary material 4). It was not possible to conclude on any pattern regarding the type of POP and the type of environment in the collection areas due to the lack of description of surrounding areas in most articles. Shore et al. (1996) report that many species of bats roost on roofs and may come into contact with pesticides used to treat roof wood against rotting. This work was important to identify dermal exposure, taking into account the type of environment bats are using as homes and roosting sites. Still, few studies have investigated bioaccumulation in bats exposed to direct contamination.
Bats may therefore be affected in different ways once POP exposure and effects vary with feeding habits, foraging, behavior, age, sex, and metabolism (Schneeberger et al., 2014; Wickramasinghe et al., 2003; Stansley et al., 2001). In our review, we observed that some studies report lower POP concentrations in females than in males (e.g., Land et al., 2019). However, most did not find significant differences between sexes in the same age class (e.g., Schanzer et al., 2022; Kuzukiran et al., 2021; Allinson et al., 2006). Although no significant differences were found, this may be due to the fact that statistical analyses in this type of work are usually performed with small sets of sampling data (n), which leads to type II errors. Qualitatively, the literature generally reports lower values for females than for males (Bayat et al., 2014). In addition, other factors act in synergy, generating differences in POP concentrations between sexes. This variation has been attributed to foraging activities by both sexes in relation to reproductive cycles and seasonal behavior such as hibernation (Valdespino and Sosa, 2017; Kunz and McCracken, 1996). Although more than half of the papers reviewed identified the sex of bats (n = 41), most of them did not separate or indicate sex during POPs analyses or in the results. Even fewer informed the reproductive condition of females (pregnant, lactating, post-lactating, or inactive). The lack of such references may mislead the interpretation of data on young and adults, affecting data on bat life expectancy. Additionally, the lack of distinction between males and females may mask potential risks to populations as they behave distinctly and play different roles in the population.
Quantitative analysisOur analyses showed that POP concentrations differed between tissues and elimination products. This can be explained by a synergy of factors, such as compound composition, tissue turnover, differences in lipid content, and bioaccumulation and exposure time in organisms. In our analyses, concentrations in fat seemed to have been metabolized faster compared with concentrations elsewhere. In humans, it has already been demonstrated that although adipose tissue is linked to endocrine activities (insulin sensitivity, blood pressure and other physiological functions) (Yu et al., 2011; Müllerová and Kopecký, 2007), it is not responsible for enzymatic activities performed by organs such as the liver. Furthermore, for highly lipid-soluble POPs, up to 99% of the body content of these compounds can be found in adipose tissue (Lind and Lind, 2020). POPs that accumulate in the liver can have effects on lipid metabolism and their excretion rates. Bratberg et al. (2013) suggest that POPs induce the CYP1a detoxification system in the liver of fish, and an increase in the elimination rate of PAHs and POPs. Thus, this difference may reflect different rates of metabolism and/or different durations of exposure.
Shore et al. (1991) compared POPs in the carcass, liver and kidney of insectivorous bats and found similar relationships, although with slower metabolism and higher concentrations in the liver when compared to kidneys. In kidneys, POPs were quickly eliminated as an excretion product, resulting in smaller amounts. The liver is known to be the body's main organ of storage, regulation, and detoxification in mammals (Alvarado-Rico and Castro, 2010), which may indicate more recent exposure to contaminants (Noschese et al., 2022) compared with other organs such as the brain. Clark and Prouty (1977) measured DDT and PCB concentrations in the brain and carcass of Eptesicus fuscus bats, noting that substantial quantities of residue may be deposited in the brain when fat reserves are metabolized, indicating different exposure and tissue metabolization times. High concentrations of chemical pollutants in the brain may pose higher risk and signal chronic, long, gradual exposure (Robinson et al., 2018; Clark and Krynitsky, 1983). The fact that these pollutants are found in the brain indicates that they can overcome the blood-brain barrier (BBB), responsible for protecting the passage of various extraphysiological substances and toxins. Thus, the presence of POPs in the brain can have a negative impact on individuals, promoting neuroinflammation and dysregulation of homeostatic control (Cresto et al., 2023; Akash and Rehman, 2021). Clark (Clark and Krynitsky, 1978; Clark and Kroll, 1977) found lethal doses of DDT and DDE in the brains of Myotis lucifugus and Tadarida brasiliensis bats. The mean brain concentration of DDE upon the diagnosis of death was 519 ppm (Clark and Kroll, 1977). Lethal concentrations in the brain of adult females reached a mean value of 24.52 ppm for DDT, while juveniles were 1.5 times more sensitive to DDT than adult bats (Clark et al., 1978).
Analyzing excretion products (guano) and secretion (breast milk) resulted in the lowest N sample and concentrations, which made it difficult to infer any decline in the compounds over the years. However, organochlorine compounds have been detected in dead bats, even in cases when the concentrations of these compounds in guano were below the fatality rate (Clawson and Clark, 1989). Therefore, even low concentrations of POPs may generate harmful, long-term effects on bat health.
In mammals, the transfer and elimination of POP through breastfeeding has already been confirmed (Hu et al., 2021; Berghe et al., 2012). In bats, the highest concentrations of POP were eliminated during the first pregnancy and the first few days of lactation (Clark and Lamont, 1976). Although Clark et al. (Clark and Krynitsky, 1978; Clark and Lamont, 1976, 1975) were not able to corroborate damage to the offspring due to POP exposure, they verified that the residues excreted in milk may be accumulated by newborns through milk feeding. In 1989, Clawson and Clark demonstrated that a diet based on milk can be more critical for young bats than feeding on contaminated insects, as young bats are more vulnerable. Thies and McBee (1994) proved that DDT was transferred through the placenta from female bats to their offspring. These data suggest a quick loss of DDT residues associated with gestation and lactation, as well as the danger of exposure of fetuses and newborns during pregnancy and breastfeeding.
Another issue is the need for data standardization. Observing more recent publications, not only on bats (e.g., Brown et al., 2018), we found that authors tend to use two measuring units (ng/g ww-wet weight; ng/g lw-lipid weight). Thus, we emphasize the importance of standardization to enable comparisons between different regions and species. This is especially important because POPs can be transported in different ways, do not respect country borders, and are a problem of global concern. We suggest that the measurement of concentrations is standardized in wet weight, and that organochlorine pollutants include data on lipid concentrations because they are lipophilic (ng/g ww; ng/g lw).
ConclusionAlthough bats may be directly or indirectly exposed to pesticides, scarce data are available to allow for an evaluation of impacts or to assess whether their roles in the ecosystem are being lost. For these reasons, new studies should be directed not only to assess POP concentrations, but also behavioral changes and possible impacts on bat health. In this review, we highlight the relevance of bans on chemical pollutants and the application of control measures using bats as terrestrial biomonitors. Yet, more studies are necessary to detect sentinel species and cover more feeding guilds. More than 50% of the publications reviewed studied insectivorous species in the United States. Only 13 of the 67 papers included not only data on POP concentrations, but also assessed changes in bat health or behavior. There is a gap in the scientific literature on the contamination and effects of POPs recently listed in the Stockholm Convention (PFAS, bromides, and Short Chain Chlorinated Paraffins), as well as on emerging contaminants that have not been listed by the Convention (chlorpyrifos and pyrethroids). The successful conservation of bat species must integrate research and conservation practices to identify stressors and solutions. The articles reviewed did not provide any conclusive difference on the variation between concentrations found in bats of different sex and age, which may be due to the low number of individuals analyzed. The lack of data undermines efforts to evaluate POP impact and bioaccumulation in bat populations. Despite the scarce data on POP contamination that may be useful to define conservation measures for bat species, our results show that bats can be used as terrestrial biomonitors. We highlight the need to develop assessments on POP contamination on bats in tropical regions, as well as techniques that do not require euthanasia for data collection. In the long term, the longevity of bats in the wild may be affected by POP exposure due to gradual, chronic contamination.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
PSMA thanks FAPERJ for the scholarship (E-26/204.129/2022), Bat Conservation International for the Student Scholars 2021 scholarship (SS2102) and the environmental compensation resources of Vale S.A. administered by the National Center for Research and Conservation of Caves (Cecav/ICMBio), and intended for the Brazilian Society for the Study of Chiropterans - SBEQ, as part of the Small Scholarships Program in Biology, Ecology and Conservation of Bats. ECL thanks UERJ Research and Teaching Support Program (PAPD). HGB thanks FAPERJ for the CNE grant (E-26/202.757/2017), and Prociência/UERJ and the National Council for Scientific and Technological Development/CNPq (307781/2014-3) for research and productivity grants. This study is part of the “Atlantic Forest Biodiversity Research Program (PPBio Mata Atlântica Network)” of the Ministry of Science, Technology, Innovation and Communications (MCTIC) and was supported by the National Council for Scientific and Technological Development (CNPq) (Process no 457458/2012-7).