Understanding how biological communities respond to human-caused landscape disturbances is urgently needed to identify optimal spatial scenarios for preserving biodiversity in anthropogenic landscapes. Forest loss is increasingly cited as a major disturbance in these landscapes, but its impact on biodiversity in mountain regions with high endemism is not well understood. Here we evaluated how bird species diversity responds to forest loss in ‘La Montaña’ mountain region of Guerrero State, Mexico. We separately assessed the complete bird assemblage, and the diversity and spatial distribution of three different ecological groups (forest-specialists, habitat-generalists, and disturbance-adapted species) in the whole landscape mosaic. We found that the diversity of the complete assemblage decreased linearly with forest loss. However, species responses to forest loss differed among ecological groups, with the diversity of forest-specialist and habitat-generalist species increasing in more forested landscapes, and the diversity of disturbance-adapted species following the opposite pattern. Similarly, the proportion of sites occupied by forest-specialist birds decreased with forest loss, but site occupancy by habitat-generalist and disturbance-adapted birds was independent from forest cover. Our findings highlight that the optimal landscape scenarios for preserving bird biodiversity in general and forest species in particular, are those that maintain as much forest cover as possible.
With natural forests increasingly threatened by human-caused disturbances (Laurance et al., 2012) and deforestation rates advancing quickly worldwide (World Resources Institute, 2021), biodiversity conservation cannot solely rely on protected areas. It also requires the implementation of biodiversity-friendly strategies in human-modified landscapes (Arroyo-Rodríguez et al., 2020; Melo et al., 2013). To this end, assessing species’ responses to landscape changes is critically needed to identify optimal landscape scenarios for biodiversity conservation (Arroyo-Rodríguez et al., 2020). Of particular importance is assessing the effect of forest loss on the abundance and diversity of different species, as our understanding on this topic is far from complete. Whereas several studies indicate that forest loss can be negatively related to the diversity of several vertebrate groups (Watling et al., 2020), in many other cases, forest loss can have weak or even positive effects on some species (e.g., Gestich et al., 2021; Pardini et al., 2009; Vallejos et al., 2020). The positive responses to forest loss can be related to the fact that populations of some species can survive the initial phase of deforestation and crowd in the remaining forest patches (the so-called ‘crowding effect’; Gestich et al., 2021; Vallejos et al., 2020). Therefore, additional studies on the effect of forest cover on biodiversity are needed to assist the implementation of adequate conservation strategies in human-modified landscapes.
Forest loss not only limits the availability of suitable habitat for forest species; it also causes an exponential increase in the isolation distance between forest patches (Fahrig, 2013, 2003). This can explain the decline in taxonomic diversity (Moreno-Opo, 2020), and the alterations in the structure of remaining communities in more deforested landscapes (Best et al., 2001). However, the effect of forest loss on species largely depends on their habitat requirements, and their ability to use resources from different land cover types (see “cross-habitat spillover hypothesis” Tscharntke et al., 2012). For instance, forest-specialist species are usually specialized in resources that can only be found within the forest (Miranda et al., 2021), and many of them have low vagility (Linnell and Lesmeister, 2019). Thus, forest loss can strongly impact forest-specialist species (e.g., Morante-Filho et al., 2015). In contrast, the so-called ‘disturbance-adapted’ species usually have high vagility in human-modified areas, where they can find food resources and space to reproduce (Fehlmann et al., 2021). Finally, many species are habitat-generalists; i.e., they can use resources from different land covers and adjust their diets to the resources found in various vegetation types, including both native and anthropogenic vegetation (Fehlmann et al., 2021). In addition, habitat-generalist species can change their vagility depending on the degree of landscape deforestation (Salinas-Melgoza et al., 2013). Therefore, separately assessing the effect of forest cover on different ecological groups is paramount for a deeper and more accurate understanding of species’ responses to deforestation.
Among vertebrates, birds comprise one of the leading groups affected by forest loss (Sekercioglu, 2012). However, as research on birds frequently focuses on forest-specialist birds (Boesing et al., 2018), or on the complete bird assemblage (Leyequien et al., 2010), the response of different ecological groups to forest loss remains to be relatively misunderstood (but see Matuoka et al., 2020; Morante-Filho et al., 2015). Furthermore, most landscape studies of birds use a patch-landscape design to assess how the bird community in focal sites is affected by the surrounding landscape (Bennett et al., 2006) and, usually the birds are sampled in a single land cover type (e.g., forest cover, Boesing et al., 2018; Carrara et al., 2015; Morante-Filho et al., 2015). Thus, we know relatively little about the effect of landscape changes on bird assemblages in different land cover types of the landscape mosaic.
As suggested by Bennett et al. (2006), mosaic-level sampling can be done by evaluating multiple sample points in a single type of land cover, or by evaluating several sample points in multiple land covers (the design adopted in our study). The first approach is particularly adequate when the research question involves a single but spatially heterogeneous land cover type, as it allows the researcher to capture the habitat heterogeneity (e.g. caused by different soil types) within the focal land cover. However, when species can use different land covers and habitat heterogeneity is smaller within land covers than among them, the second approach is more adequate, as it allows the researcher to better understand the impact of landscape forest cover on birds across the whole landscape mosaic (Bennett et al., 2006). Yet, as bird research is more frequently based on the first approach (Boesing et al., 2018; Matuoka et al., 2020; Morante-Filho et al., 2015), additional studies on the effect of the forest cover on bird communities in the whole landscape mosaic are needed, particularly in mountain regions, in which different land covers can provide habitats for different species (Mendenhall et al., 2016).
The “La Montaña” mountain region of Guerrero State, harbors a high percentage of endemic birds in Mexico (Navarro-Sigüenza et al., 2014). However, it has been subjected to a wide variety of land-use changes, resulting in heterogeneous landscapes with different-sized forest patches being surrounded by an anthropogenic matrix composed of small villages, low-input crops, and grasslands (Borda-Niño et al., 2017). Despite this, to our knowledge, only two studies have evaluated the response of birds to land-use changes in this region (Almazán-Núñez et al., 2018; Vázquez-Reyes et al., 2017). These studies demonstrate that anthropogenic disturbances negatively affect bird diversity. However, these studies use a patch-landscape design and do not evaluate the effect of landscape composition on bird diversity in other land cover types than forest.
Here, we assessed changes in species composition along a forest cover gradient and investigated how changes in forest cover affect the diversity and distribution of birds sampled in different land covers, including second-growth vegetation, annual crops, pastures, and villages. We conducted this analysis not only with the complete assemblage, but separately for species with different habitat requirements (i.e., forest-specialist, disturbance-adapted and habitat-generalist species). Specifically, we predicted that more forested landscapes would exhibit higher diversity of the bird complete assemblage, mainly by maintaining forest-specialist birds that depend more on forest resources (Morante-Filho et al., 2015). In contrast, we predicted that landscapes composed of low forest cover would present high diversity of disturbance-adapted birds. We also predicted a higher proportion of occupied sites by forest-specialist birds in more forested landscapes, whereas disturbance-adapted birds likely follow the opposite pattern. Finally, we predicted that the diversity of habitat-generalist birds and the proportion of sites occupied by these species are probably weakly affected by landscape changes.
MethodsStudy areaWe carried out this study in the "La Montaña" region of Guerrero State, Mexico — a mountain region with rough topography (Salgado Terrones et al., 2017). The localities studied are inhabited by Me'phaa, Nahualt and Mixteco indigenous people. The management by local people was similar in all landscapes, so in all of them, we can find the same cover types but in different proportions. The villages are small rural human settlements that do not exceed 1000 inhabitants, with rustic adobe houses and dirt roads or small paths between the houses. The native vegetation of the region is composed of pine-oak forests that are widely used by local people for the collection of fuelwood, construction materials and forage for goats (Salgado Terrones et al., 2017). The predominant crop is the traditional “Milpa” (intercropping of corn, beans, and squash; Moreno-Calles et al., 2016), and a lesser extent of sugar cane, intended primary for self-consumption. Crop rotation is usually practiced, so is common to find open areas in a fallow period (Borda-Niño et al., 2017). Total annual rainfall averages ∼1800 mm, with a rainy season between April and November. The mean annual temperature is 25.7 °C (SMN, 2013).
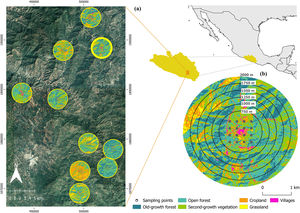
Sampling designWe selected 10 landscapes along a wide forest cover gradient (i.e., from 15% to 81% forest cover; Lambert Conformal Conic ITRF92 coordinates 16°52′32″, 17°15′56″N and 99°08′40″, 98°55′11″W). We focused the study on the landscapes surrounding ten different villages, considering that human settlements largely determine the configuration and composition of human modified landscapes (Tolessa et al., 2016). The villages were located ≥4 km apart between each other, and at 900–1600 m a.s.l., thus limiting our study to the distribution range of pine-oak forest in the region (Borda-Niño et al., 2017). In the center of each landscape we established a 1000-m × 1000-m grid centered on each village, divided every 250-m, thus creating 16 squares. The sampling sites were systematically established in the center of each square (i.e., 16 sampling sites per landscape), thus sampling different cover types present in the landscape (Fig. 1). Villages’ sizes ranged between 1 and 20 ha, so just a few sampling sites were within the villages (between one and seven sampled sites per landscape).
As we do not know a priory which the best scale to measure forest cover is, we followed the multi-scale analysis protocol proposed by Fahrig (2013) to identify the so-called 'scale of effect' (i.e., the spatial scale that yields the strongest response to landscape forest cover). This protocol is explained in more detail in Appendix S1 (Supplementary material), but a brief overview is given here. We established six circular landscapes around each village, ranging from 750 to 2000-m radius, every 250 m (Fig. 1b). The smallest landscape represents the minimum size to cover all sampling sites surrounding each village. The largest landscape was established to prevent spatial overlap between landscapes (Fig. 1a). We used QGis 3.4.12 software to estimate forest cover in each landscape. We performed an unsupervised classification to define the land cover types using high-resolution satellite images (Sentinel February 2, 2020). This classification included old-growth forest, open-forest (i.e., low-density forests resulting from selective logging), second-growth vegetation (i.e., agriculture areas with different fallow ages—usually <10 years—which after this period, are cultivated again and therefore, fall far short of natural forest habitats, both structurally and functionally), annual crops, pastures, and villages. Considering that the extent of habitat has been recognized as one of the properties of the landscape structure with greatest influence on bird diversity (Bennett et al., 2006; Watling et al., 2020), we decided to use the percentage of forest cover as the descriptive variable of the landscape. While forest cover has particularly explained the diversity patterns of forest specialist birds (Bennett et al., 2006), the amount of forest in the landscape also influences the bird community of non-forest habitats (Cabral et al., 2021; Leyequien et al., 2010). We included both old-growth forest and open forest to quantify the percentage of forest cover within each landscape.
Bird surveysWe surveyed bird assemblages using limited radius (50 m) point counts (Ralph et al., 1995). We sampled 160 points, 16 per landscape, limiting the survey to resident birds because it is reasonable to expect that these species rely more strongly on the landscape characteristics than migratory species (Şekercioglu et al., 2019). All surveys were carried out from 7 to 11 am by one observer (FV-C) using 10 × 42 binoculars and, a digital recorder for capture unidentified vocalizations at the time of sampling, so that they could be identified in the laboratory. Each point was surveyed once during the rainy season, from July to September 2019. We recorded all birds seen or heard at each point during a 7-min period. We used a field guide for visual identification (Howell and Webb, 1995) and a bird vocalization database (Myska, 2019) to identify recorded vocalizations. We classified each bird species according to its habitat requirements following Billerman et al. (2020) as forest-specialist species (i.e., those that use principally forest habitats), disturbance-adapted species (i.e., those that use principally open areas, including forest edges, isolated trees, agricultural lands, and human settlements), and habitat-generalist species (i.e., species that can use both forest and non-forest habitats, whether native or anthropic) (Table S1).
Data analysesTo avoid pseudoreplications problems, we pooled the information from the 16 sampling sites per landscape and used the landscapes as independent replicates. First, we constructed rank-abundance curves for each landscape to assess changes in bird species composition along the forest cover gradient. We then estimated the sampling completeness in each landscape to assess the accuracy of bird inventories (Chao et al., 2014). To this end, we used the sample coverage index (Cn) suggested by Chao et al. (2014), which is available in the “entropart” package (Marcon and Hérault, 2015). As we found a high variation in sample coverage among landscapes (Cn = 0.68–0.9), we used an extrapolation protocol to estimate the accumulated number of species (γ-diversity) per landscape for samples with similar coverage (Cn = 1). We estimated the extrapolated γ-diversity with Hill numbers of order 0 (°Dγ, species richness), 1 (1Dγ, exponential Shannon entropy), and 2 (2Dγ, inverse Simpson concentration) (Jost, 2006) with the “entropart” package. °Dγ is independent of species' abundance variations, giving a disproportionate weight to rare species. 1Dγ weights each species according to their relative abundance, without giving more or less importance to rare or abundant species. Thus, it is interpreted as the effective number of typical species in the community (Jost, 2006). Finally, 2Dγ gives greater importance to dominant species, being interpreted as the effective number of dominant species in the community (Tuomisto, 2010). Then, we analyzed the relationship between γ-diversity and forest cover considering the complete assemblage and each ecological group separately, using linear models. To test whether the responses to forest loss differed among ecological groups, we carried out analyses of covariance (ANCOVA), one per response variable. For this, we used the “stats” package (R Core Team, 2021), and then the function “emtrends” of the “emmeans” package (Lenth, 2021) to post-hoc tests. In the models, we included the effect of a single continuous variable (forest cover in 2000-m radius landscapes, which was identified as the 'scale of effect'; Appendix S1), a categorical factor (i.e. the ecological group), and the interaction between these two explanatory variables. Significant interactions indicate that the effect of forest cover differs among ecological groups. We also assessed changes in the proportion of occupied sites by each ecological group along the forest cover gradient to understand how these bird groups were spatially distributed across landscapes. For this, we calculated the number of sites occupied by each group of species within each landscape (range = 0–16) divided by the total number of sampling sites (n = 16). Then, we fitted a linear model to the complete assemblage and again carried out an ANCOVA to test for differences among ecological groups using “stats” package (R Core Team, 2021). Since our explanatory variable data is a proportion, we transformed proportions to logit to meet the normality assumption (Warton and Hui, 2011). All the analyses were performed in R software (R Core Team, 2021).
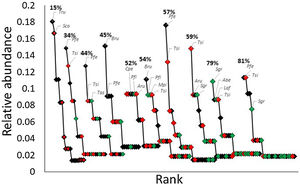
ResultsWe recorded a total of 497 individuals belonging to 56 bird species. Thryophilus sinaloa, Pheugopedius felix, Basileuterus rufifrons, and Turdus rufopalliatus – all habitat-generalist species – were the most abundant species in most landscapes (Fig. 2). However, forest-specialist species such as Setophaga graciae, Lepidocolaptes affinis, Myioborus pictus, and Piranga flava dominated the landscapes with higher forest cover (Fig. 2).
Rank-abundance curves showing the relative abundance of bird species recorded in 10 landscapes with different percentage of forest cover (indicated on each curve). We classified the species as forest-specialist (green diamonds), disturbance-adapted (red diamonds), and habitat-generalist (black diamonds). Dominant species: Aru = Aimophila rufescens; Abe = Amazilia berillina; Bru = Basileuterus rufifrons; Cpe = Contopus pertinax; Laf = Lepidocolaptes affinis; Mpi = Myioborus pictus; Pfe = Pheugopedius felix; Pfl = Piranga flava; Sco = Saltator coerulescens; Sgr = Setophaga graciae; Tas = Turdus assimilis; Tsi = Thryophilus sinaloa; Tru = Turdus rufopalliatus.
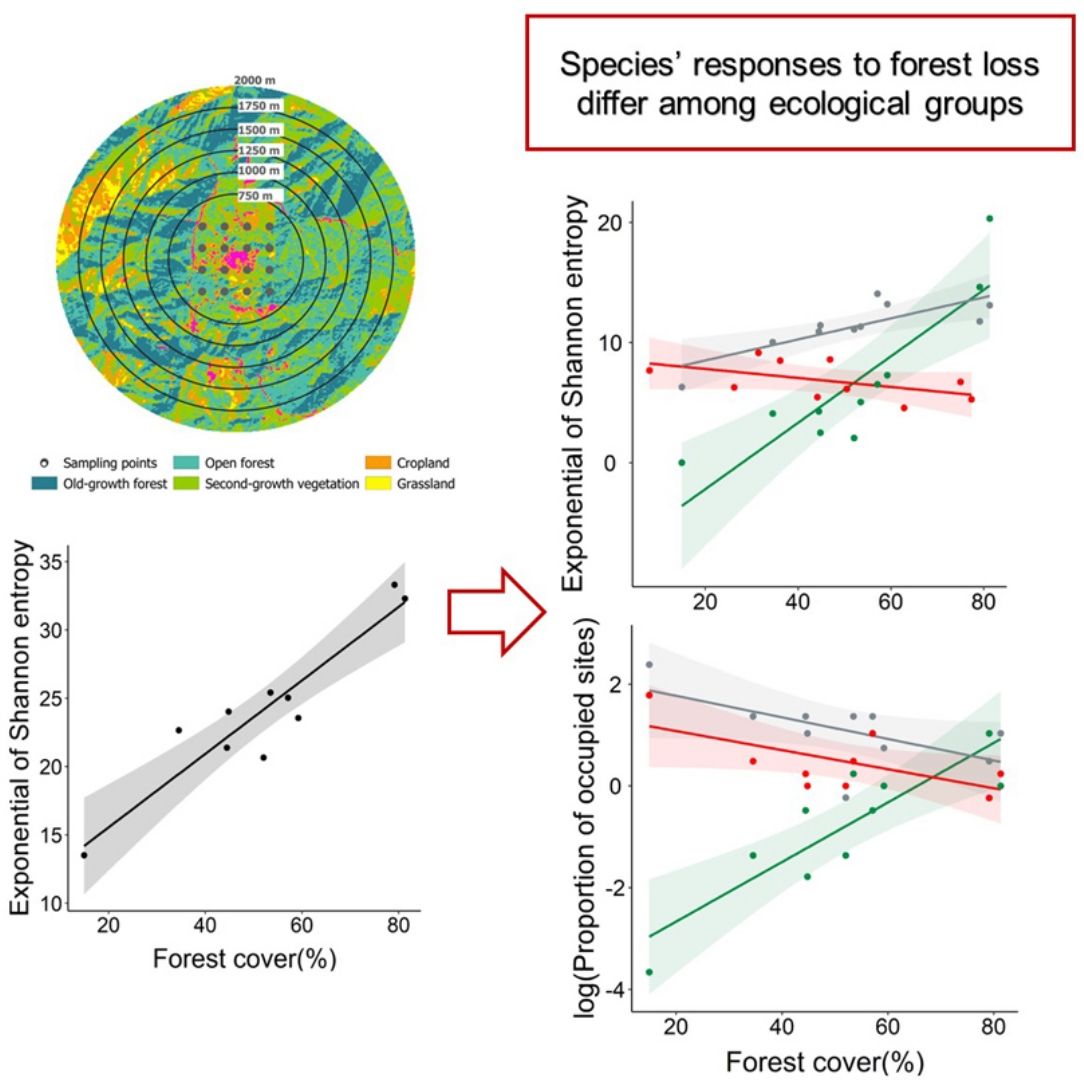
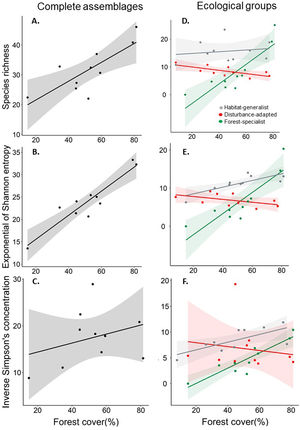
The species richness (°Dγ) and the diversity of common species (1Dγ) of the complete bird assemblage were positively related to forest cover (R2 = 0.62; P = 0.007, and R2 = 0.87; P < 0.001, respectively; Fig. 3a,b), but the diversity of dominant species (2Dγ) was not associated to forest cover (R2 = 0.10; P = 0.37; Fig. 3c). The relationship between species richness and forest cover differed among ecological groups (interacting term within the ANCOVA: F1;24 = 14.90, P < 0.001, Fig. 3d). In particular, the species richness of forest-specialist birds was positively related to forest cover, but the opposite pattern was observed when assessing disturbed-adapted species (Fig. 3d, Table S2). The species richness of habitat-generalist birds remained constant along the forest cover gradient (Fig. 3d, Table S2). The effect of forest cover on the diversity of common species also differed among groups (F1;24 = 19.76, P < 0.001, Fig. 3e), with the diversity of forest-specialist and habitat-generalist birds being positively related to forest cover, whereas the diversity of disturbance-adapted species being weakly related to forest cover (Fig. 3e, Table S2). Conversely, the effect of forest cover on the diversity of dominant species did not differ among ecological groups (F1;24 = 3.17, P = 0.06; Fig. 3f, Table S2).
Response of species richness (0Dγ, A and D), diversity of common species (1Dγ, Shannon's entropy exponential; B and E) and diversity of dominant species (2Dγ, Simpson's inverse concentration; C and F) to landscape forest cover, separately assessing bird complete assemblages and different ecological groups (forest-specialist birds, disturbance-adapted birds, and habitat-generalist birds). In all cases, we showed the accumulated alpha diversity in 16-point counts (i.e., gamma diversity per landscape). The shaded area is the 95% confidence interval of the linear models.
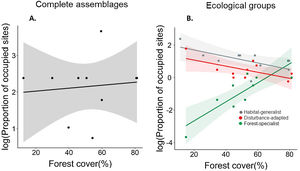
Regarding the effect of forest loss on birds’ distribution, we found that the proportion of occupied sites was not related to forest cover in the complete assemblage (R2 = 0.011; P = 0.77; Fig. 4a), but we found a strong relationship when analyzing ecological groups separately (Fig. 4b). This relationship differed among ecological groups (F1;24 = 20.99, P < 0.001), forest-specialist birds responded positively to forest cover (Fig. 4b, Table S2), but disturbance-adapted and habitat-generalist birds presented a negative trend (Fig. 4b, Table S2).
Association between the logarithm of proportion of sites occupied by birds and landscape forest cover separately assessing the complete assemblage (A) and different ecological groups (forest-specialist, disturbance-adapted, and habitat-generalist birds) (B). The shaded area is the 95% confidence interval of the linear model.
Our study indicates that the diversity of the complete bird assemblage, and particularly forest-specialist and habitat-generalist species, decreased in more deforested landscapes. In contrast, the richness of disturbance-adapted species increased in more deforested landscapes. Forest-specialists were also distributed in a lower proportion of sites in more deforested landscapes, while habitat-generalists and disturbance-adapted species tended to show the opposite pattern. Taken together, these findings support previous studies conducted with a patch-landscape design (Boesing et al., 2018; Morante-Filho et al., 2020). Therefore, forest loss not only affects the bird community within forest patches, but the complete community across different land cover types in the studied landscapes.
As expected, our results indicate that forest loss causes a strong impoverishment of the bird assemblage in a human-modified mountain region. The diversity of forest-specialist species decreased in more deforested landscapes. This finding is consistent with the habitat amount hypothesis (Fahrig, 2013), which predict that species richness in equal-sized sample sites should increase with the total habitat amount (forest in this case) in the local landscape surrounding the sample sites. Interestingly, and contrary to what it has been documented in previous studies (Carrara et al., 2015; Morante-Filho et al., 2015), forest loss also decreased the diversity of habitat-generalist species, likely because these previous studies only surveyed forest patches and did not assess the changes in the bird community across different land covers. Thus, a novel contribution of our research is that forest loss not only impact forest species in forest lands, but also habitat-generalist species in the whole landscape mosaic. This implies that forest cover is not only important for forest species, but for a larger number of generalist species that also depend on the resources offered by the forest (e.g., food, refuge, nesting sites, Cornelius et al., 2008; Neuschulz et al., 2011). In other words, forest-specialist and habitat-generalist species may be more reliant on forest cover than disturbance-adapted species.
In fact, the diversity of disturbance-adapted species slight increased in more deforested landscapes. Some of the disturbance-adapted birds that dominated these deforested landscapes were Saltator coerulescens and Thryophilus Sinaloa, species that are known to do relatively well in human-modified lands (Billerman et al., 2020). Although for many native species, some elements of the landscape such as villages, crops, living fences, among others, can be ecological traps (i.e., habitats that animals prefer in which their fitness is lower than in other available options, Hale and Swearer, 2016), disturbance-adapted birds can proliferate in deforested landscapes because of their high capacities to obtain resources from different human-modified lands (Escobar-Ibáñez and MacGregor-Fors, 2015; Fehlmann et al., 2021).
Regarding the distribution of species, our findings indicate that, as expected, the proportion of sites occupied by forest-specialist species decreased with increasing forest loss. However, habitat-generalist species were widespread across the landscape. In fact, many these species can move among different land cover types (natural and anthropogenic) in search for supplementary or complementary resources (Dunning et al., 1992). Following the cross-habitat spillover hypothesis (Tscharntke et al., 2012), this movement capacity drives the whole-landscape community structure and associated processes. In birds, the movement capacity greatly differs among ecological groups. In our study, the relatively narrow distribution of forest-specialist birds in deforested landscapes is consistent with previous studies (Miranda et al., 2021), and could be related to the low dispersal capacities of species within this group (Robertson and Radford, 2009), and its specialization on forest resources. In contrast, habitat-generalist birds have a relatively high vagility, which allow them to use resources from different land cover types (Cadavid-Florez et al., 2020), occupying a widespread distribution across the landscape. Finally, the less noticeable effect of forest cover on the spatial distribution of disturbance-adapted birds may be due to the high vagility of this group, which allows these species to occur in different environments (Fehlmann et al., 2021), regardless of the degree of landscape deforestation.
We conclude that the amount of forest cover in the landscape is of paramount importance for predicting the diversity of birds in a human-modified mountain region. As forest loss has negative impacts on both forest-specialist and habitat generalist species, our findings support the idea that preventing forest loss and increasing forest cover is a top priority for preventing biodiversity loss (Arroyo-Rodríguez et al., 2020). Therefore, increasing forest cover in the landscape surrounding villages is good for preserving birds and their ecological function in the ecosystem, and for improving human well-being.
FundingThis work was supported by project PAPIIT-UNAM IN300119.
Declaration of interestThe authors declare that there is no conflict of interest regarding the publication of this article.
We thank the NGO Xuajin Me’phaa, especially Margarita Munciño for helping us approaching the community and facilitating field work, Paulo Dionicio and Daniel Dionicio for their assistance in field data collection. Also, we thank the authorities and interpreters in each village, who made possible for us to communicate with the local people. We are grateful to Francisco Mora for statistical advice. FVC thanks the Posgrado en Ciencias Biológicas of the Universidad Nacional Autónoma de México-UNAM and the Consejo Nacional de Ciencia y Tecnología-CONACyT (scholarship number 825713). This paper is part of the requirements of the Posgrado en Ciencias Biológicas of the UNAM for the PhD studies of FVC.