Recent bioacoustic studies have shown different responses of insectivorous bats to native habitat loss. We examined the activity and species/sonotypes composition of aerial insectivorous bats present in a human-modified karst landscape in Southeast Brazil, characterized by the presence of semideciduous forest, pastures and Eucalyptus globulus monocultures. Using ultrasonic detectors, we investigated activity and identified bat species and/or sonotypes in the three habitat types. We compared the activity (as a surrogate for abundance) and composition of species/sonotypes present and used Generalized Linear Models to investigated whether canopy density, understory density and food availability influence the response of bats in these habitat types. Our main results demonstrate that the variables general passes and species/sonotype richness did not differ significantly between forest and pasture, however, both variables in these two environments differed significantly from the values found for eucalyptus. We conclude that, in the studied agropastoral landscape, pastures interspersed with forest areas can be used by aerial insectivorous bats during foraging. However, we also found evidence that eucalyptus monocultures, not yet mature and without an understory, have a negative impact on the species/sonotype richness and activity of Neotropical aerial insectivorous bats.
Various anthropic practices related to economic development and subsistence modify natural landscapes, promoting a direct impact on the structure of communities (Oakleaf et al., 2015). Brazilian biomes have historically suffered from the expansion of agropastoral activities (Fonseca, 1985), which currently occupy 255 million hectares or 29.97% of the entire country (MapBiomas, 2021). Considering that legally protected conservation areas on private rural properties, the so-called “legal reserves”, represent about one third of the country's native vegetation (Metzger et al., 2019), understanding the responses of the biodiversity present in these areas is of fundamental portance.
The composition and richness of assemblages of aerial insectivorous bats can vary across an agropastoral landscape with different land uses and levels of native habitat loss (Mendes et al., 2016; Monck-Whipp et al., 2018). Studies suggest that aerial insectivorous bats have different responses to landscape structure. For example, deforestation of native forests can promote the activity of insectivorous aerial bat species with the habit of foraging in open areas and at forest edges (Jung and Kalko, 2011; Falcão et al., 2021). Negative influences on the activity and richness of bat species are observed mainly in places where the proportion deforestation is greater than that of the remnants of native habitat around them (Rodríguez-San Pedro and Simonetti, 2015; Muylaert et al., 2016).
Variation in the structure and composition of insectivorous bat assemblages caused by the loss and/or modification of native habitat is directly linked to the resources that are newly offered by the anthropized landscape (Lentini et al., 2012; Barros et al., 2014; Mendes et al., 2016). The persistence of some species in human-modified landscapes may be determined by the nature of land uses surrounding the natural habitat, improving or limiting landscape connectivity and resource availability (Lentini et al., 2012).
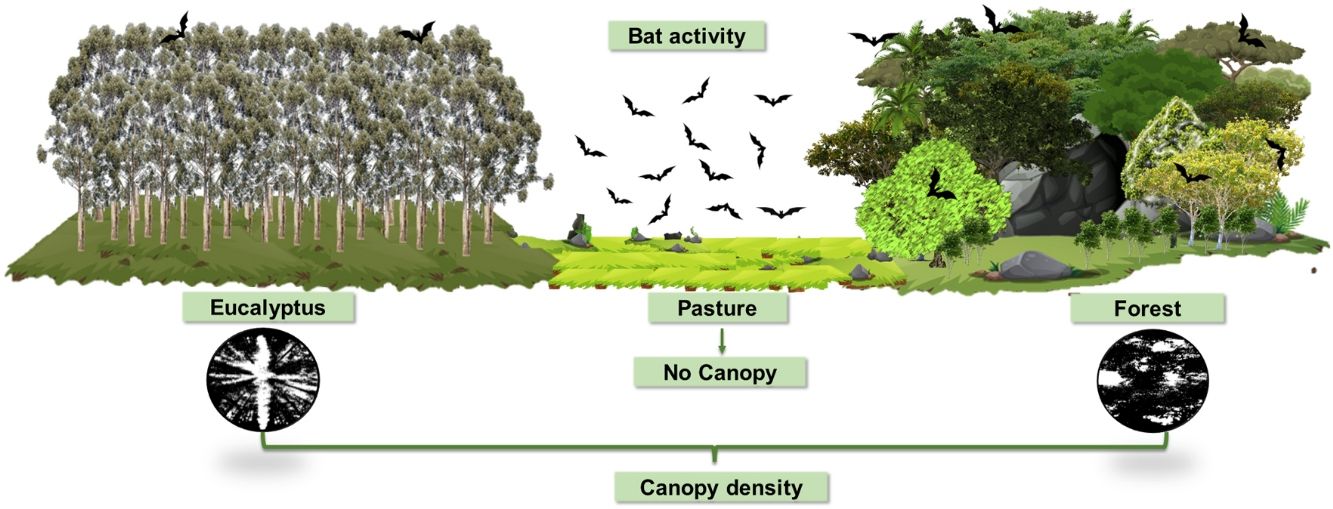
Recent bioacoustic studies have been successful at showing the varied responses of insectivorous bats to changes in agropastoral landscapes (Lentini et al., 2012; Cruz et al., 2015; Falcão et al., 2021). Thus, using this technique, this study aimed to examine the activity and composition of insectivorous bats species present in a human-modified karst landscape in Southeast Brazil, which has part of its native habitat suppressed and replaced by pastures and eucalyptus monoculture.
The negative impact of pastures on the bat assemblages is known (Rainho and Palmeirim, 2011), however, it has also been shown that the juxtaposition of linear remnants of intact vegetation, together with less intensive land uses, such as unimproved pastures, benefits insectivorous bat assemblages in agricultural landscapes (Lentini et al., 2012). On the other hand, the impacts of eucalyptus monocultures on bats are poorly studied outside the natural distribution of this type of vegetation (Barros et al., 2014; Cruz et al., 2015).
Thus, in this study, we examined the activity and species/sonotypes composition of bats in the three typical habitats of an agropastoral landscape composed by native forest, pasture and eucalyptus. We predicted that the bat assemblage would differ among habitats, showing less species/sonotype richness and activity in anthropic habitats. Furthermore, we investigated whether forest vegetation structure and insect food availability could explain the responses of these aerial insectivorous bats.
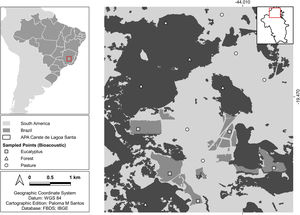
MethodsStudy areaThe study area is in the region known as Lagoa Santa Karst in the municipality of Matozinhos, state of Minas Gerais, Brazil (Fig. 1). The vegetation in the region is transitional between the Atlantic Forest and Cerrado (Brazilian savanna) biomes. The region has more than 500 recorded caves (Hermann et al., 1998), which provide roost for several species of bats (Torquetti et al., 2017). The region has a seasonal climate, with a rainy season from October to March and a dry season from April to September, and an average annual temperature of around 21 °C (Hermann et al., 1998).
Encompassing about 1800 ha, the study area is characterized by an agropastoral landscape matrix with approximately 40% of its area covered by fragmented native forest, mainly on karst rock outcrops, about 40% used for pasture of cattle and horses and 20% covered by monocultures of eucalyptus forests. We collected data on three habitat types, namely semideciduous forest, pasture and Eucalyptus globulus monocultures, hereinafter referred to as forest, pasture and eucalyptus, respectively.
Acoustic monitoring protocolWe chose five sites in each habitat type (15 total) for bat acoustic sampling (Fig. 1). The sites were defined with a minimum distance of 1 km between them and a minimum distance of 250 m to the edge of another habitat type, with the aim of obtaining independence of sample sites. We used two autonomous Song Meter 2 Bat + ultrasonic recorders (Wildlife Acoustics, Concord, USA), which were field mounted at 1.5 m above ground level and with the microphone tilted at 45 ° to the sky. We scheduled the recorders to activate at twilight hours (18:00 h) and to remain active until 06:00 h the following day.
Acoustic data were collected in the months of November 2019, January 2020 and February 2020, with 15 sampling days per month. Each night, we randomly chose two sites belonging to the same habitat type to be sampled and 1 recorder was installed at each. Thus, each site was sampled once a month, totaling 15 samples for each habitat type and a total of 45 samples. We chose the rainy season for sampling because, in the Neotropics, this season presents greater availability of food resources, which is reflected in greater bat activity (Fenton and Zortea, 1999). In addition, collections took place during the dark phase of the moon, as there is evidence of greater bat activity on these occasions (Saldaña-Vázquez and Munguía-Rosas, 2013).
The independence among sampling sites was investigated using the Mantel Test, to determine whether the sites can be treated as true replicas. This test has been performed using species/sonotypes passes to calculate dissimilarity matrices with the Bray-Curtis Index, with 9999 randomizations.
Sound analysis and species identificationSpectrograms and acoustic identification of bat sonotypes were performed using RAVEN software (Cornell Lab of Ornithology Bioacoustics Research Program). A bat pass was considered as the sequence of two or more echolocation calls (or pulses) recorded the moment after the recording was triggered (Schnitzler and Kalko, 2001). To avoid recording multiple passes of the same individual, we considered an interval of two minutes before recording another pass of the same sonotypes. Feeding buzzes and social calls were counted with no break between passes. We visually inspected the spectrograms of all recordings to identify each recording to species level based on the following acoustic parameters: structure of the echolocation pulse, highest emitted frequency, lowest emitted frequency, peak frequency, pulse duration and interval between pulses. We classified sound recordings as sonotypes when identification to species level was not possible.
Characterization of each habitat typeWe characterized forest vegetation structure and food availability in each habitat type to see if these characteristics could influence the responses of aerial insectivorous bats.
Vegetation structureVegetation density of the understory and canopy of the studied habitats were used to illustrate the vegetation structure of each habitat type and were estimated following the methodologies proposed by Nobis and Hunziker (2005) and Bianchi et al. (2017), with modifications. Except in pasture, which does not contain understory and canopy formation, a 100 m transect was drawn at each sample site covering the distance between the recording station and the light trap. Photographs of the understory and canopy vegetation were taken every 20 m along the transect.
Local understory vegetation was photographed with a Nikon Coolpix P510® camera in four distinct positions using a 1 m² (1 m × 1 m) white screen as a background. A team member carrying the white screen was positioned 2 m away from another team member carrying the camera. The member holding the screen alternated among four positions, east, west, north and south of the camera.
The canopy was photographed along the same transect as traced for the understory photographs, however, only one photographic record was taken every 20 m. Photographic records were obtained using a Xiaomi Redmi 7® smartphone coupled to a fisheye lens, as described by Bianchi et al. (2017). The smartphone was fixed and aligned on a 1.5 m high tripod, focusing only on the canopy, with its top always facing geographic north. To standardize the incidence of sunlight, canopy photographs were taken only between 06:00 and 08:00 h and between 16:00 and 18:00 h.
Each digital photographic record of vegetation structure was analyzed according to Nobis and Hunziker (2005). Image resolution was initially adjusted using PHOTOSHOP CC2015.5 software (Evening, 2016), followed by contrast adjustment, with black representing vegetation and white representing open area (Fig. S1 in Supporting information), using IMAGEJ software (Rasband, 2014). The average number of black and white pixels was calculated for the understory and for the canopy of each sample site. Finally, we used the averages of pixels to calculate vegetation density (VD) for the understory and canopy for each sample site using the following formula:
Food availabilityFood availability for bats in each habitat type was estimated based on the dry weight of insect biomass obtained at each sampling site. Insects were collected with HP light traps during the recordings, as described by Pugedo et al. (2005). The places where the traps were installed followed the same assumptions of independence between sampling sites as described previously for the recorders. The traps were installed 100 m away from the recorders and emitted a low-voltage yellow incandescent light (1.5 V) to ensure they did not interfere with bat activity and did not attract insects from other habitats. The traps were installed at least 1.5 m above ground level at twilight hours (18:00 h) and kept in operation for 12 h. Each site was sampled once a month, for a total of 45 samples. The collected insects were stored in 70% ethanol. Only winged insects with body sizes between 0.5 and 3.0 cm were considered in obtaining biomass dry weight. The selected insects were kept in an oven at a temperature of 70 °C for 72 h, or until stable dry weight was reached, and then weighed on a precision scale.
Data analysisClassifying the bat passesThe ratio between the total number of bat passes recorded at a sample site divided by the number of sampling hours at that site was calculated, resulting in bat passes/hour (Jung and Kalko, 2011). This variable was later used at the levels: i) bat species/sonotype passes: passes identified to the level of species or sonotype group; ii) general bat passes: all registered passes, without taxonomic distinction; iii) feeding buzzes; and iv) social calls.
Characterization of the structure of the bat assemblageTo analyze the bat assemblage structure, we used species/sonotype activity as a proxy for abundance (Falcão et al., 2021); in this case, activity can reflect the abundance of bat sonotypes present during the sampling. Through this analysis, we hope to verify if the activity of each of the species/sonotype recorded differs in each habitat. For this, we use the Non-Metric Multidimensional Scaling (NMDS) analysis and Multivariate Permutational Analysis of Variance (PERMANOVA) on the activity data, both calculated using the Bray-Curtis Index. For the dissimilarity analysis of the composition of the insectivorous bat assemblage, NMDS and PERMANOVA analyses were performed using the Jaccard Index with presence/absence data of species/sonotype in each sampled habitat type.
The data for species/sonotypes of the family Molossidae were excluded from the analysis. The ultrasonic pulses emitted by bats of this family have a narrow band, long duration and relatively high power (dB) when compared to those of other bat families (Denzinger and Schnitzler, 2013). These characteristics allow these pulses to travel distances greater than 10 m (Denzinger and Schnitzler, 2013). Thus, due to the low canopy height of the studied forest, as the vegetation in question is of recent secondary growth, due to selective logging and coffee cultivation in the area in the last 50 years (Brina, 1998), molossid bats could produce a sampling bias, since their pulses could be detected inside the forests, even when foraging in the open space above this canopy (E. Bernard, personal information). The recorded molossid sonotypes are provided in the Supporting Information (Table S4; Fig. S4). All analyses described above were performed using PAST software version 4.10 (Hammer et al., 2001).
Effects of habitat metrics on bat responsesA Generalized Linear Model (GLM) analysis was performed to test the effects of habitat type, food availability and habitat vegetation density on overall bat activity and species/sonotype richness. Bat passes obtained in the form of general passes, feeding buzzes and social calls, in addition to species/sonotype richness, were used as response variables. The habitat metrics of habitat type, canopy density, understory density and insect dry mass were used as explanatory variables. The variables and covariates were tested using the Pearson correlation test before performing the GLM to see if they could be considered true. Seeking a better fit of the model, after analyzing the residuals, the response variables general passes, feeding buzzes and social calls were transformed into log10. Since species/sonotype richness is a count, the model was adjusted for the Poisson distribution. All models were tested using R software (R Core Team, 2022), and the distribution of the residuals of each model were observed using the “rdiagnostic” function of the RT4bio package (Reis et al., 2013). Here, species/sonotype of the family Molossidae were also excluded from the analysis.
ResultsAcoustic monitoring and species identificationWe extracted 2569 general bat passes, 502 feeding buzzes and 43 social calls of insectivorous bats for analysis. We registered 1477 passes in pasture, 955 in forest and 137 in eucalyptus (Table 1). We recorded 326 feeding buzzes in pasture, 170 in forest and only six in eucalyptus. On the other hand, we recorded 34 social calls in forest, followed by eight in pasture and only one in eucalyptus. It was possible to identify seven species and four sonotype groups (Table 2) belonging to the families Vespertilionidae (Fig. S2 in Supporting Information) and Emballonuridae (Fig. S3 in Supporting Information). The most frequent taxa were Myotis sp.1 (571 passes), Histiotus velatus (443 passes), Peropteryx sp. (423 passes) and Eptesicus furinalis (335 passes) (Table 2); Myotis ruber had the lowest number of passes (8) (see Table 2). The Mantel Test showed that the distance between sample sites did not affect bat composition, thus indicating independence among sites (R = −0.065, P = 0.745).
Acoustic records and results {p-Values (P) and F-Fisher (F)} of generalized linear models (GMLs). The number of acoustic passages, sonotypes richness and social calls registered are given for each sampled habitat type. The table also describes the relationships between response variables (general bat passes, bat species/sonotype passes and social calls) and explanatory variables (habitat type, canopy density, understory density and food availability). Contrast indicates similarities or dissimilarities only to the variable Habitat Type, where: P = pasture; M = forest; E = eucalyptus.
| Variables | Acoustic records by habitat type | General linear models | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forest | Pasture | Eucalyptus | Total | Habitat type | Canopy | Understory | Food availability | ||||||
| P | F | Contrast | P | F | P | F | P | F | |||||
| General passes | 955 | 1477 | 137 | 2569 | 0.0032 | 9.618 | E ≠ M = P | 0.0090 | 6.818 | 0.3997 | 0.709 | 0.5555 | 0.366 |
| Species/sonotype richness | 11 | 11 | 9 | 11 | 0.0006 | 7.266 | E ≠ M = P | 0.0065 | 7.402 | 0.2184 | 1.514 | 0.7289 | 0.125 |
| Social calls | 34 | 8 | 1 | 43 | 0.1056 | 2.248 | E = M = P | 0.1005 | 0.756 | 0.1299 | 2.293 | – | – |
Species and sonotypes recorded during samplings for each habitat type.
| Family/species/sonotypes | Acoustic records by habitat type | |||
|---|---|---|---|---|
| Forest | Pasture | Eucalyptus | Total | |
| Emballonuridae | ||||
| Peropteryx macrotis (Wagner, 1843) | 6 | 72 | 0 | 78 |
| Peropteryx sp. | 136 | 285 | 2 | 423 |
| Vespertilionidae | ||||
| Eptesicus brasiliensis (Desmarest, 1819) | 43 | 230 | 8 | 281 |
| Eptesicus furinalis (d’Orbigny, 1847) | 94 | 230 | 11 | 335 |
| Histiotus velatus (I. Geoffroy, 1824) | 287 | 111 | 45 | 443 |
| Lasiurus blossevillii (Lesson and Garnot, 1826) | 26 | 16 | 7 | 49 |
| Lasiurus sp. | 57 | 18 | 2 | 77 |
| Myotis riparius (Handley, 1960) | 20 | 30 | 0 | 50 |
| Myotis ruber (E. Geoffroy, 1806) | 2 | 4 | 2 | 8 |
| Myotis sp.1 | 147 | 408 | 16 | 571 |
| Myotis sp.2 | 73 | 49 | 3 | 125 |
| Unidentified | 64 | 24 | 41 | 129 |
The activity of the insectivorous bat assemblages differed among habitat types. The PERMANOVA analysis (P = 0.006; Table S1 in Supporting Information) and the distribution of points in the NMDS plot, suggests that the bat species/sonotypes activity is different in relation to the habitats (stress = 0.100; Fig. S5 and Table S2 in Supporting Information). Species/sonotypes composition did not differ between forest and pasture, however, the composition of these two habitat types differed significantly from that of eucalyptus (PERMANOVA, P = 0.237, Table S1 in Supporting Information). This result is also observed through the NMDS plot (NMDS stress = 0.091; Fig. S6 and Table S3 in Supporting information).
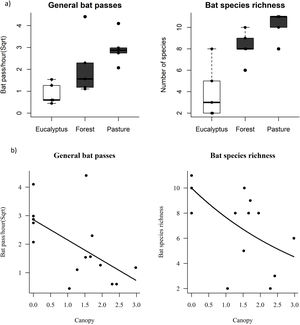
Effects of habitat metrics on bat responsesPearson correlations showed collinearity between feeding buzzes and the covariate general passes (R = 0.943, P = 0.007), and so we excluded feeding buzzes as a response variable in subsequent GLM analyses. The variables general passes and species/sonotype richness did not differ significantly between forest and pasture, however, both variables in these two environments differed significantly from the values found in eucalyptus (Table 1; Fig. 2a). No significant differences were detected for social calls (GLM: F = 2.248, P = 0.100; Table 1).
a) Boxplot comparing bat activity and species/sonotypes richness in each habitat type. Black dots represent sampling sites. Points outside the whiskers are outliers. Dark boxes indicate significant differences by contrast test. b) Linear regression of bat activity and species/sonotypes richness as a function of canopy density. All responses were canopy density dependent.
The explanatory variable canopy density had a negative effect on the general activity and species/sonotype richness of bats (GLM: F = 6.818, P = 0.009; Table 1; Fig. 2b). Finally, the variables understory density and food availability had no effect on bat activity (GLM: P > 0.05, Table 1).
DiscussionIn this study we observed variation in the composition and activity of aerial insectivorous bats in different habitats of a karst landscape modified by agropastoral activities, confirming our initial predictions. The variables general passes and species/sonotype richness did not differ significantly between forest and pasture, however, both variables in these two environments differed from the values found in eucalyptus, which presented significantly lower values. This result shows that eucalyptus plantations probably exert negative pressure on insectivorous bats in the landscape. Our results also demonstrate that the lower activity and species/sonotype richness of these bats is associated with denser canopy structure in the studied habitats, while insect availability and understory density did not result in any effect.
Activity and composition of the insectivorous bats assemblages among habitat typesThe composition of insectivorous bat assemblages found in forest and grassland habitats is characterized by the presence of species/sonotypes belonging to the guilds of open space foragers (emballonurid species) and edge space foragers (vespertilionid species) (Denzinger and Schnitzler, 2013), which possess morphological, sensory and motor characteristics that favor foraging at the forest edge, close to the canopy line, between treetops or in open spaces, avoiding the interior of forests (Denzinger et al., 2016).
The similarity among assemblages indicates that different resources available in these habitats, such as food and roosts (Mendes et al., 2016; Monck-Whipp et al., 2018), may be influencing their use by bats, as many roosts are available on rocky outcrops within these forests and are used by several of these species, as verified by previous studies (Talamoni et al., 2013; Torquetti et al., 2017). Considering that insect availability does not have a significantly different effect among habitats, these aerial insectivorous bats probably move between forest and pasture in search of satisfying their foraging and roosting needs. The bat activity observed in pasture, and considering its correlation with feeding buzzes, may indicate that species benefit from these open areas by using them as foraging sites. Thus, apparently the resources present in forest and in pasture allow these insectivorous bats to use these areas in a complementary way (Dunning et al., 1992).
The bat activity observed in pasture demonstrates that, apparently, in the studied landscape, the conformation of pastures interspersed with areas of native habitat influences insectivorous bat activity, which may reduce the distance between roosting and foraging sites. A similar result was found in Chile, where bat activity levels can be significantly associated with the amount of forest in relation to their fragmented surroundings (Rodríguez-San Pedro and Simonetti, 2015). Furthermore, it has been demonstrated that bat activity in areas of extensive grazing is greater at the edges than in the interior (Rainho and Palmeirim, 2011), since within vast pasture areas bats become more exposed to adverse weather conditions and predators.
The results regarding eucalyptus plantations demonstrate that they have a negative effect on bat activity and species/sonotypes richness, as also observed for phyllostomid bats in an area of Brazilian Cerrado (Pina et al., 2013), although the cause is not clear. Some studies carried out in subtemperate regions in the Neotropics, where native forests have been replaced by exotic forest plantations, show that some bats species are able to use these areas to move, forage and roost (Barros et al., 2014; Rodríguez-San Pedro and Simonetti, 2015).
A hypothesis that can be raised to explain our results is related to the canopy structure of the studied stands. The plots are recent and were planted where there were other pastures. The trees were planted relatively close together and their growing branches are intertwined, which can make it difficult for bats to navigate inside these plantations, since the wing morphology of the recorded species is not suitable for highly maneuverable flights, as they are adapted for flying in open areas (Schnitzler and Kalko, 2001). Furthermore, the absence of understory may have contributed to this result. The occurrence of understory with a complex vegetation structure and with a height above 3 m, common in mature eucalyptus plantations, is associated with higher levels of bat activity and species richness (Simonetti et al., 2013; Cruz et al., 2015; Burgar et al., 2017).
Factors influencing bat activity and composition across habitatsThe finding that activity and species/sonotype richness levels decrease as canopy density increases can be expected, since the characteristics of the studied landscape apparently favor the permanence of these open and edge space foragers. The lack of a relationship between bat activity and food availability must be related to the fact that we did not find significant differences in insect abundance among habitats. However, a study carried out in Panama (Estrada-Villegas et al., 2012) also did not observe an effect of insect availability on insectivorous bat activity.
The experimental design of this study has some limitations, such as the small number of sampling sites, due to the small size of the study area. Although this, we consider our results important because our data reinforce the importance of maintaining preserved areas of native vegetation in agropastoral landscapes and demonstrates that exotic forest plantations can exert a negative effect on insectivorous bats assemblage.
FundingThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, and by Fundo de Incentivo à Pesquisa (FIP PUC Minas).
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. SAT thanks Fundo de Incentivo à Pesquisa (FIP PUC Minas) for research grants. We thank José Hein and the staff of the Cauaia farm for allowing us to collect the bat passes, and to Erik Wild for English revision. We also thank the team from the mastozoology laboratory at Pontifical Catholic University of Minas Gerais for helping with fieldworks.