Vultures are among the most endangered bird species, and changes in their feeding habits due to human activities pose a significant threat to their survival. We studied aspects of the trophic ecology of Andean Condors (Vultur gryphus) in Tierra del Fuego, an archipelago in the southernmost part of its distribution, and compared it with other six sampling sites across Argentina. We estimated the isotopic niche width, the trophic position, and the extent of marine input on condors at a large spatial scale. Andean Condors in Tierra del Fuego exhibit a unique and substantial reliance on marine food webs, which matches the known historical trophic interactions of the species. In contrast to continental Patagonia, the marine trophic input was not homogeneous among individuals, but structured in two groups along the terrestrial – marine gradient. Marine carrion provided by sea lions, seabirds and stranded cetaceans should be monitored since they can be relevant for the long-term persistence of Andean condors in the area. Moreover, the link of condors with the ocean would also include the movement of marine inputs to terrestrial environments. As most condor samples were obtained in protected areas in the terrestrial-marine interphase, this further emphasizes the importance of preserving these areas in the face of environmental change to conserve not only the species and its landscape but also specific trophic interactions.
The accelerating global change has caused significant disruptions to food webs, leading to large-scale degradation of ecosystems and biodiversity (Heleno et al., 2020). Vultures are amongst the most endangered bird species and play irreplaceable ecological roles by significantly enhancing carrion removal (Hill et al., 2018; McClure et al., 2018). As they possess wide home ranges, gathering information at the appropriate spatial scale proves challenging. In that context, non-invasive sampling could be relevant for understanding trophic relationships at a large spatial scale. Shed feathers have been used for studying obligate scavengers through stable isotope analysis (SIA), providing an interesting framework to compare trophic ecology metrics among populations (e.g., Silverthorne et al., 2020). Within the isotopic niche framework, SIA can be used as a proxy for the trophic niche of a species (Bearhop et al., 2004; Newsome et al., 2007), and may provide a powerful tool to assess foraging habitat and diet divergence among populations. Under this approach, dietary breadth, trophic position, and foraging habitats of top consumers are usually estimated through carbon (δ13C) and nitrogen (δ15N) stable isotope values of consumer’s tissues. Even more, since specific tissues reflect consumer diets during synthesis, it provides information at different time scales. For example, feathers are a metabolically inert tissue that reflect birds’ diet when the tissue was growing (Inger and Bearhop, 2008).
The Andean Condor (Vultur gryphus) is a Vulnerable New World vulture, with an extant population of ∼6,700 mature individuals and a decreasing trend (BirdLife International, 2023). It is widely distributed along the Andes, where it is exposed to a myriad of threats such as direct persecution, poisoning, lead contamination, and collision with power lines (Restrepo-Cardona et al., 2022). In Patagonia (Southern South America), introduced herbivores have become a significant component of the food webs, homogenizing the trophic interaction of scavengers (Barbar et al., 2016; Novaro et al., 2000). In that area, condors fed historically on both terrestrial and marine food sources, but they have shifted to a nearly-exclusive terrestrial diet associated to extensive cattle ranching and the harvesting of marine mammals (Duda et al., 2023; Lambertucci et al., 2018). Condors associated with marine prey have been observed or suggested in various coastal locations such as the Pacific coasts of Peru (Gamarra-Toledo et al., 2023) and Tierra del Fuego (Droguett et al., 2023). In contrast, nearby locations such as Southern Chile show only marginal influence of marine food input (Duclos et al., 2020), reinforcing that this marine association may not be widespread across these regions. Therefore, in remote areas with relatively low human impact, ancient trophic interactions may have been retained. In that context, the need to identify and study sites where historical trophic interactions may persist becomes paramount.
The aim of this study was to investigate the relative contribution of marine food webs to the Andean condors that inhabit their Southernmost distribution range. Specifically, we described the trophic iso-scape of Tierra del Fuego samples through clustering analysis; we estimated the relative importance of marine input in Tierra del Fuego and continental Patagonia populations; and we estimated and compared the isotopic niche width and the trophic position corrected by local baselines for seven condor populations. We hypothesized that, as the Southernmost part of South America is an area with low human impact (Mittermeier et al., 2003), the Andean Condors’ feeding habits there are more alike the ancient trophic interactions (i.e., showing a relatively high input from marine food resources, e.g., ∼33%, Lambertucci et al., 2018). If this is the case, we expect condors in Tierra del Fuego to: a) show a significant input from marine trophic web; and b) display relatively high trophic position, since they will be feeding in part on marine predators. Moreover, if marine input is not homogeneous among individuals, we expect Fuegian condors to c) exhibit a wider isotopic niche compared with other populations.
MethodsStudy siteThe Andes in South America primarily run in a North-South direction, but in the Fuegian archipelago, it takes on a Northwest-Southeast orientation. The study area represents the Tierra del Fuego archipelago in Southern Argentina and Chile, an area of ∼74,000 km2. It is comprised of a main island (∼48,000 km2) and thousands of smaller islands and islets. The climate is temperate cold, with an average annual rainfall of 400–500 mm without much seasonality. The average temperature of the coldest month is −4 °C and of the warmest month is 10 °C. The forest canopy is dominated by species of the genus Nothofagus (Austral beeches) and occurs in patches decreasing in size towards the north, from the mountain to the steppe, giving rise to grasslands and meadows. The archipelago is separated from the continent by the Strait of Magellan, which is ∼1.5 km wide in its narrowest point.
There are several carrion sources in the system. Breeding populations and haul-outs of sea lions (Otaria byrona) and fur seals (Arctocephalus australis) are conspicuous throughout the archipelago and show increasing population trends (Milano et al., 2020b, 2020a; Venegas et al., 2002). Additionally, hotspots of cetacean strandings have been described in western Tierra del Fuego (Alvarado-Rybak et al., 2020). Seabird colonies are especially abundant in the outer islands (Kusch and Marín, 2013; Raya Rey et al., 2014). Terrestrial carrion mainly includes guanaco (Lama guanicoe), the only native megaherbivore, and domestic species. Guanacos may exceed 30,000 individuals distributed mainly in lower-altitude and the forest-steppe ecotonal areas of the main island (Flores et al., 2018). Mortality of guanacos may be in the order of 5% annually, with peaks in harsh winter events up to 26% (Montes, 2000). The livestock in the region consists primarily of sheep, and to a lesser extent cattle, raised extensively in large paddocks (Ormaechea et al., 2019).
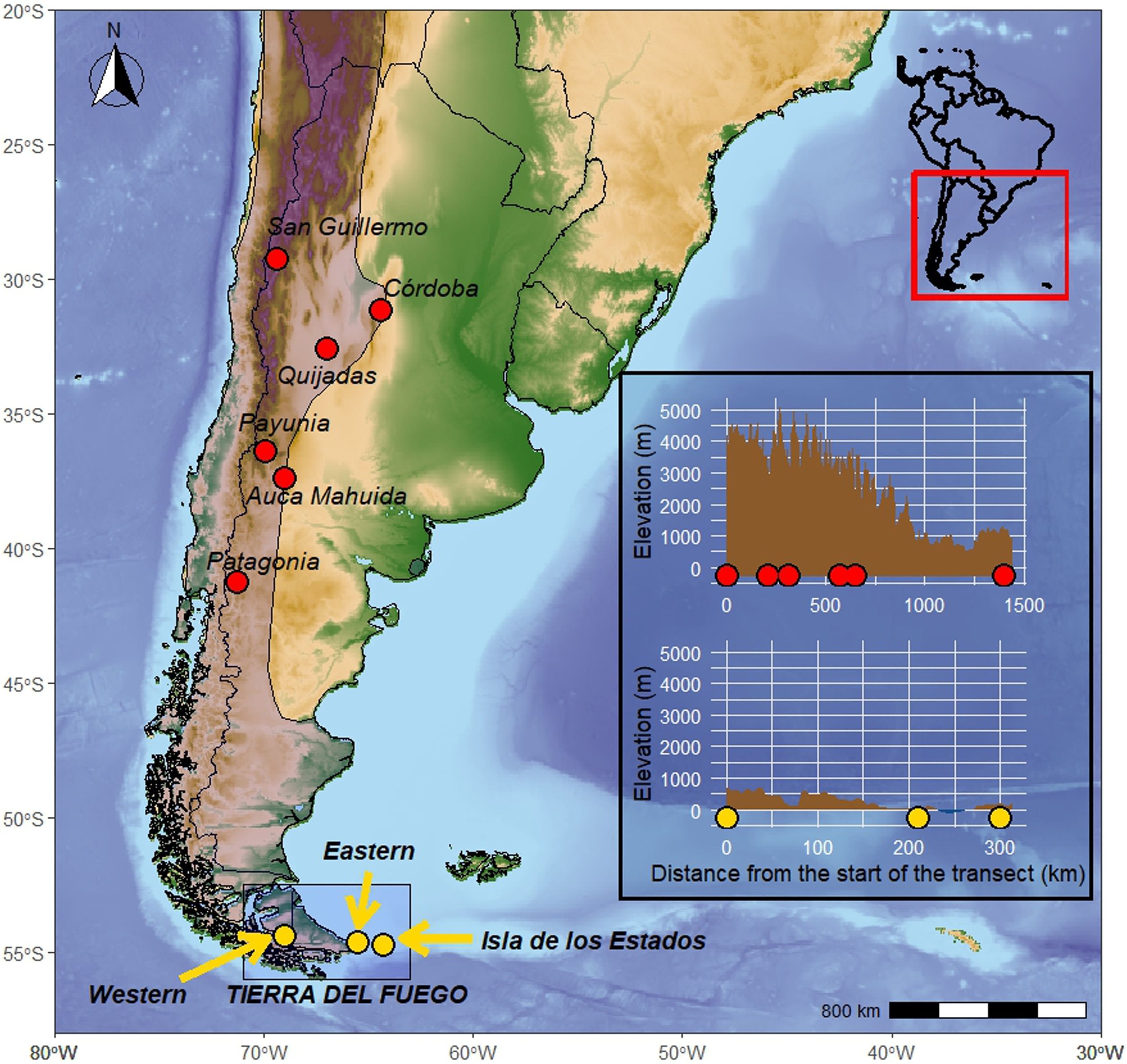
Feathers and baselines samplingShed feathers were collected from known condor roosts or opportunistically throughout the study area (Fig. 1). A total of 43 feather samples were collected in three sites: 1) Western Tierra del Fuego (Admiralty Sound Marine protected area and Beagle Channel; n = 21), embedded in a 2-million ha network of protected areas in both Chile and Argentina; 2) Eastern Tierra del Fuego (Mitre Peninsula, Argentina; n = 14), a 500,000 ha recently established Provincial Reserve, and 3) Isla de los Estados (Staten Island), Argentina (n = 8), a 54,000 ha Provincial Reserve. We also collected muscle from different herbivores in those areas (see Supplementary data).
Map of the study area in southern South America. Yellow dots indicate sites where feathers samples of Andean Condors were collected for this study. Red dots represent sites sampled in previous studies. The shaded area indicates the distribution of the Andean Condor in the study area (BirdLife International, 2023). The inset shows the elevation (m.a.s.l.) of each sampled transect, with dots representing sampling sites along each elevation profile (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
We removed surface contaminants by washing feathers with distilled water. Then, we cut pieces within three sections from each individual feather (from the top, the middle and the base) and we pooled them. Lipids and surface oils were removed from the feathers by repeatedly rinsing them with a 2:1 chloroform:methanol solution for 12 h. Feathers were then rinsed with distilled water and left to dry during 12 h. The muscle of the different herbivores collected were lyophilized and homogenized using a mortar and pestle prior to analysis. Feather pieces and muscles of mussels and herbivores were weighed (mean ± SE: 0.600 ± 0.025 mg) in tin capsules, then flash-combusted (Costech ECS 4010 elemental analyzers) and analyzed for carbon (δ13C) and nitrogen (δ15N) stable isotope values via an interfaced Delta XP continuous-flow stable isotope ratio mass spectrometer at Louisiana State University (USA). USGS 40 and USGS 41 glutamic acid reference materials were used to normalize sample values. Sample precision based on repeated samples and reference materials was 0.1 ‰ for both δ13C and δ15N. Stable isotope values are expressed in δ notation in per mil units (‰), according to the following equation:
where X represents either δ13C or δ15N, and R is the ratio between 15N/13N or 13C/12C. Rstandard was based on atmospheric N2 for δ15N and Pee Dee Belemnite for δ13C.
Clustering analysisWe estimated the optimal number of isotopic clusters by calculating the goodness of clustering measure through the gap statistic (Tibshirani et al., 2001) using the cluster package in R software (Maechler et al., 2022; R Core Team, 2023). We used the non-hierarchical k-means clustering function setting a maximum number of clusters of three (i.e., the number of sampling locations in Tierra del Fuego). This partitioning method is adequate for continuous data and performs a single partition of objects (i.e., each data point is unequivocally assigned to one cluster) (Borcard et al., 2011). We specified and visualized the optimal number of clusters using the firstmax method with the fviz_gap_stat function (Maechler et al., 2022). We then assigned the individual samples to each one of the optimal clusters, and computed the proportion of individuals of each sampling site belonging to each cluster. Finally, further analyses were performed using these clusters as independent groups to assess if they exhibited trophic differences (see below).
Regional isotopic niche and trophic position analysisWe compiled isotopic data from different studies throughout the Andean Condor distribution for which there was available information on both condor feathers and prey items (Fig. 1). Terrestrial baseline was composed of herbivores, including domestic (e.g., cattle and sheep), exotic (e.g., deer, feral goats), and native species (e.g., camelids), assigned to target populations in past works or sampled for this work (Lambertucci et al., 2018; Perrig et al., 2021, 2017). Marine baseline included intertidal mussels (Mytilus sp.) found in the literature and sampled for this work. Data used specifically for each case can be found in the Supplementary data. We converted hair values into putative muscle values using a metanalysis of trophic discrimination factors for C3 plants-depending herbivores as this is the largely dominant carbon pathway in South America (Stephens et al., 2022; Still et al., 2003).
We used the nicheROVER package in R software to estimate the isotopic niche width of each population (Swanson et al., 2015); nicheROVER utilizes Bayesian computation to estimate isotopic niche width, incorporating a probabilistic Bayesian modeling to account for uncertainties in niche measurements, producing sample size-corrected metrics. Isotopic niche width is a complementary metric to estimate the diversity of resources consumed: While it cannot be directly interpreted as trophic niche width (Hette-Tronquart, 2019), it roughly integrates the variability in prey types and feeding grounds over the sampled period. For each sampling site, we produced 2,000 estimations of Bayesian isotopic niche width and used boxplots and violin plots to show the variability among different runs.
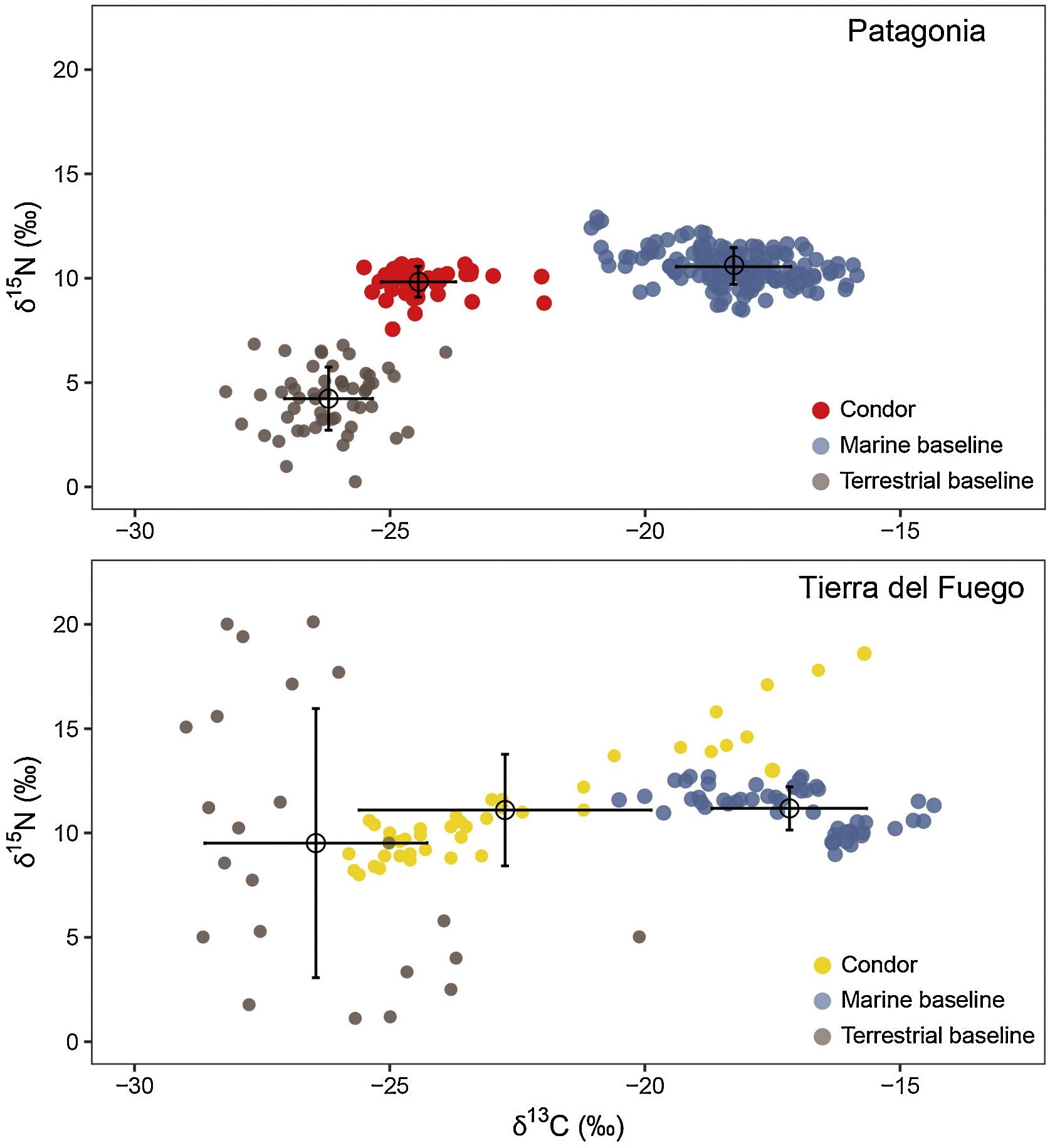
For estimating marine input and trophic position among populations, we used the tRophicPosition package in R software (Quezada-Romegialli et al., 2018; R Core Team, 2023). For both Tierra del Fuego and Patagonia (the only other site in which previous evidence showed that they were potentially linked to marine resources), we used the twoBaselinesFull model, including ungulates and marine mussels as terrestrial and marine baselines respectively (Fig. 2). For those populations for which there was no previous evidence or suspect that a marine trophic association was possible, we only used ungulates to estimate the trophic position, using the oneBaseline model (Quezada-Romegialli et al., 2018). We generally retained the baselines as assigned in previous works for those sites not sampled here (Perrig et al., 2021, 2017). For the Patagonia site, we added mussels (Mytilus sp.) from the nearby marine environment (Del Rio-Lavín et al., 2022; Mayr et al., 2011). For Tierra del Fuego, we choose own and published baselines (Balza et al., 2020; Dodino et al., 2022, 2020; Riccialdelli et al., 2017). In total, we used 279 samples of condors and 475 baseline samples for the analyses. Since the tRophicPosition package requires raw data and some published data was only available as mean ± SD data, we generated a simulated dataset assuming a normal distribution while maintaining the original sample using the rnorm function in R software. We assumed that terrestrial herbivores and mussels occupied a trophic position = 2 and we selected a trophic discrimination factor of 3.1 ± 0.2 ‰ for δ15N and 0.4 ± 0.4 ‰ for δ13C, estimated for feathers samples of California Condors (Kurle et al., 2013). Since two Tierra del Fuego clusters unrelated with sampling site were supported (see Results), we further test whether or not they were differentiated by their marine-terrestrial input and trophic position.
Isotopic (δ15N and δ13C) data from Patagonia (above) and Tierra del Fuego (below) used to estimate marine input into condor feathers and trophic positions. Red and yellow dots represent data from each condor site; brown and blue dots represent data from terrestrial and marine baselines, respectively. Black circle and bars denote the mean and standard deviation of each group. Each site is shown separately to highlight that the isotopic context (i.e., the range and variation of terrestrial and marine baselines) is site-specific (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
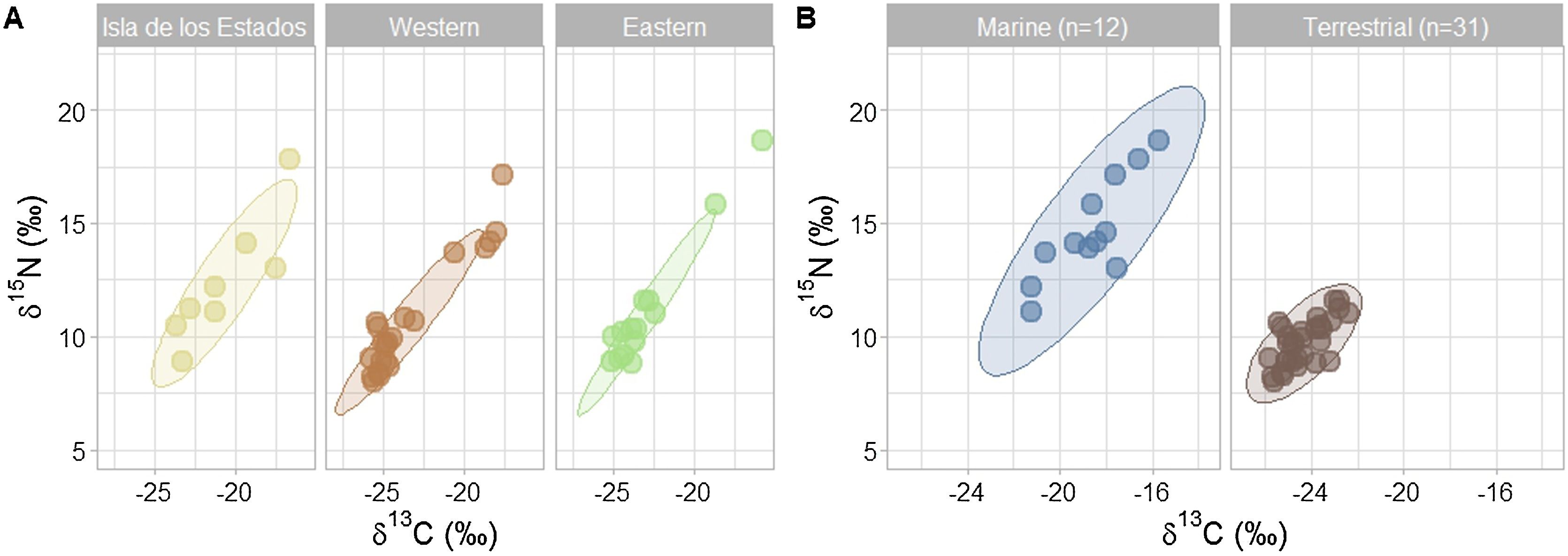
For Tierra del Fuego, the optimal number of clusters was two. The samples did not cluster by geographical origin (Fig. 3A), but rather differed along the carbon-nitrogen (putatively marine-terrestrial) axis (Fig. 3B). Overall, 28% of the samples were assigned to the ‘marine’ cluster. Isla de los Estados had the largest proportion of their samples assigned to the ‘marine’ cluster (63%, n = 8), followed by Western (24%, n = 21) and Eastern (14%, n = 14) Tierra del Fuego.
Andean condors in Tierra del Fuego showed an overall estimated marine input (% input, [C.I.: 2.5%–97.5%]) of 38% [C.I: 25–50]. This value was higher than the estimated marine input for continental Patagonia (15%, [C.I: 10–20]). For Tierra del Fuego, the assigned marine and terrestrial cluster differed in their estimated food web baselines input. While the ‘terrestrial’ cluster showed a median marine input of 21% [C.I: 10–30], the ‘marine’ cluster showed a median marine input of 79% [65–93].
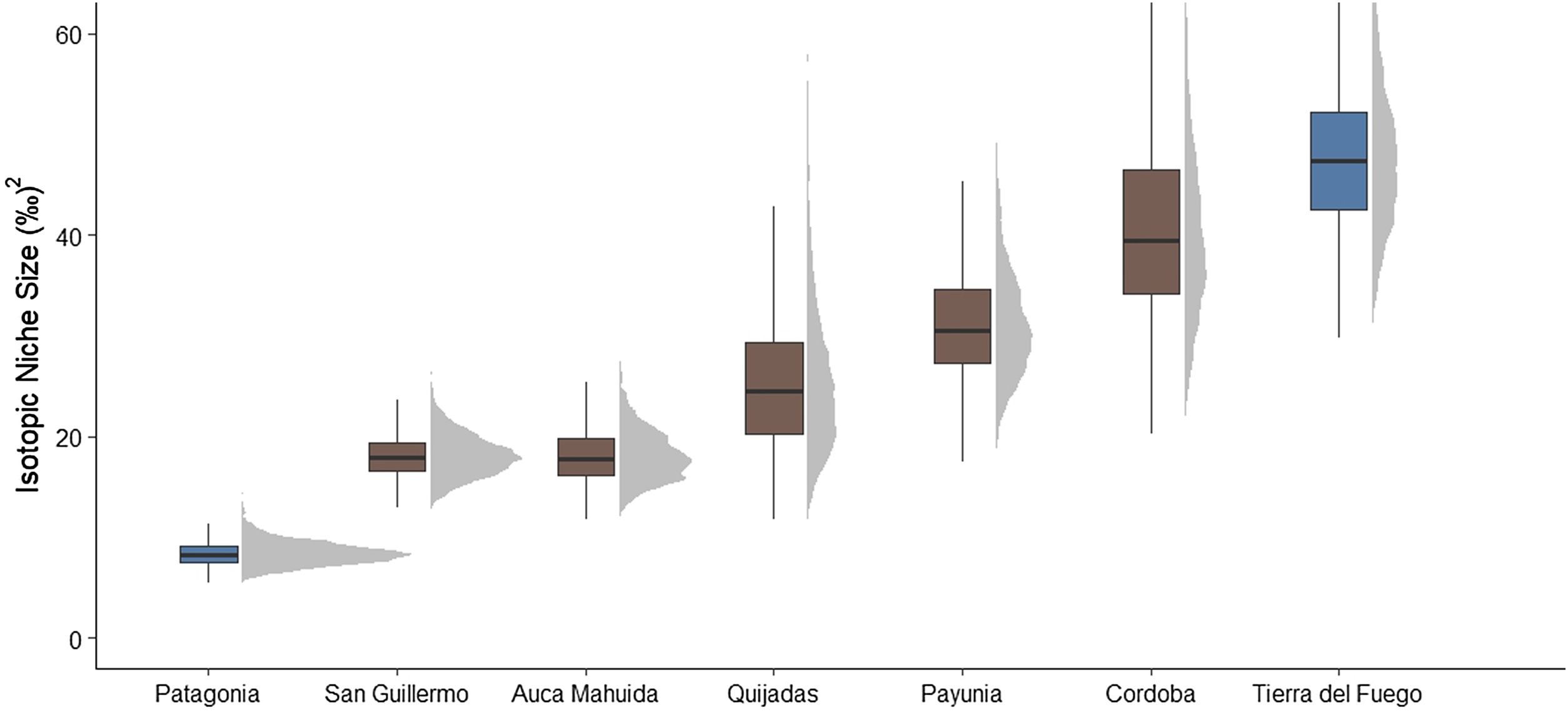
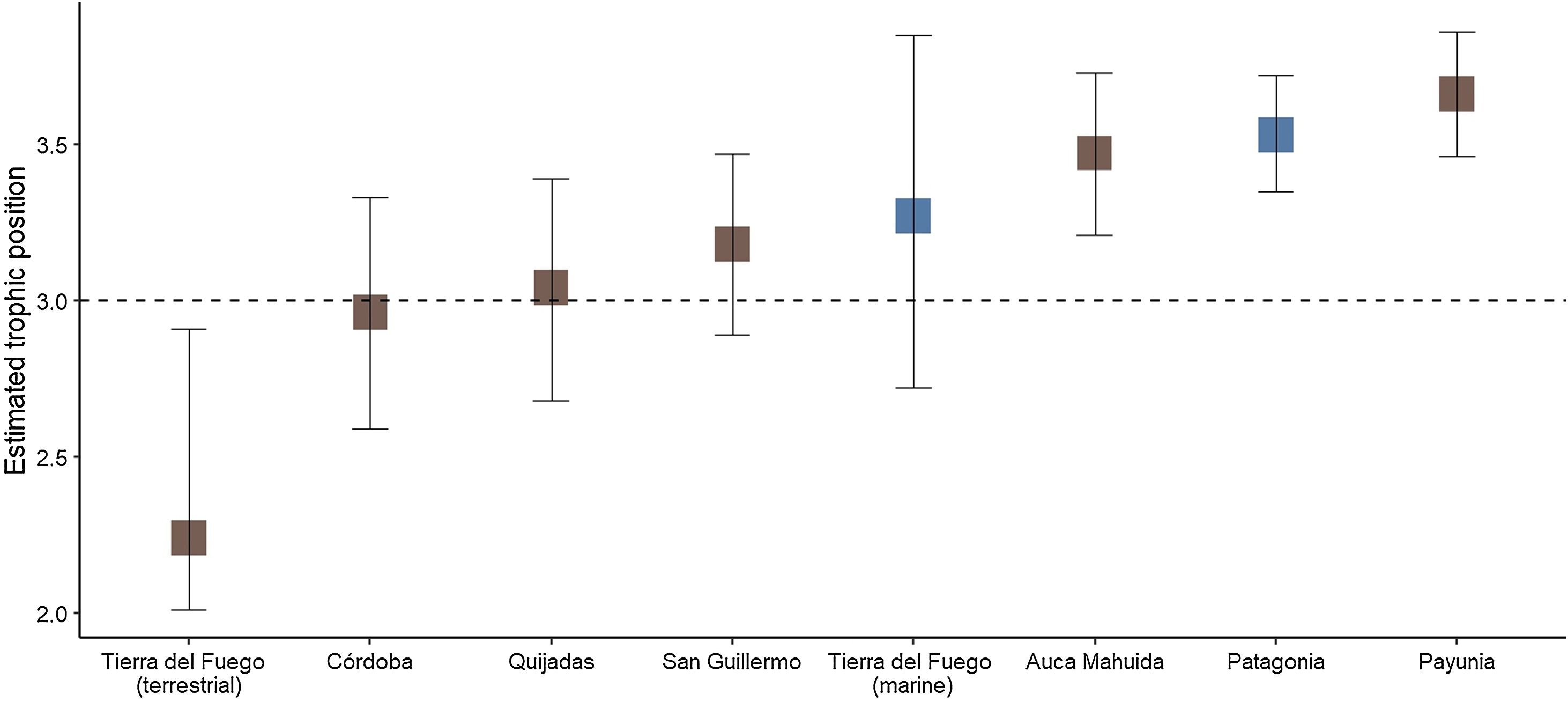
Among populations, isotopic niche width was highly variable: Patagonia and Tierra del Fuego showed the smaller and the largest isotopic niche, respectively (Fig. 4). Estimated median trophic position among populations varied about one trophic level. Populations with marine input were amongst the ones with the highest trophic position values, while the terrestrial cluster of Tierra del Fuego showed the lowest trophic position (Fig. 5).
Isotopic niche width among Andean Condor sites ordered by increasing estimated median value. Populations with estimations based on both marine and terrestrial trophic baselines are shown in blue, while those based solely on a terrestrial baseline are shown in brown. See Fig. 1 for the geographical location of each sampling/study site. In blue, populations with marine trophic input (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Estimated trophic position among Andean condors ordered by increasing median trophic position. Dashed line shows trophic level = 3, a value suggestive of an exclusive herbivore-carrion diet. Populations with estimations based on both marine and terrestrial trophic baselines are shown in blue, while those based solely on a terrestrial baseline are shown in brown. See Fig. 1 for the geographical location of each sampling site (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Our study provides the first insight on the Andean Condor’ trophic ecology at Tierra del Fuego, an area with low anthropogenic impact. The realized isotopic niche of Tierra del Fuego, in comparison with continental Patagonia, supports our hypothesis that the southernmost condors are retaining a historical trophic link with the marine environment. The estimated marine trophic-web contribution in the Fuegian population was similar to the historical (∼100–2,000 years before present) contribution of the marine environment to condor diet in continental Patagonia (Duda et al., 2023; Lambertucci et al., 2018). In addition, a wider isotopic niche was consistent with the highest estimated marine input among all condors studied. Previous work with SIA on feathers samples of Andean condors did not find isotopic metrics to differ with sex (Perrig et al., 2021) or with other co-occurring scavengers (Silverthorne et al., 2020). Our results suggest the occurrence of two consumer types among condors in Tierra del Fuego, with one third of the individuals showing a strong association with the marine trophic web. Since we pooled three fragments of the feathers for the analyses, this would imply some level of temporal consistency in feeding habits that should be further investigated. In Patagonia, in contrast, the marine trophic input was not correlated with a wide trophic niche, suggesting that individuals there may be less specialized.
We did not find a consistent pattern correlating marine trophic input with trophic level. While both marine condors of Tierra del Fuego and those from Patagonia had a relatively high estimated trophic level (Fig. 5), the absolute values for the terrestrial cluster in Tierra del Fuego (less than 3), suggests that certain limitations prevent a direct interpretation of these absolute values. We consider that this may be related to the unique isotopic processes that occur near marine mammals and seabird breeding areas in insular systems. Both seabirds and marine mammals subsidize terrestrial ecosystems with allochthonous nitrogen, enriching the surrounded environment in δ15N (Caut et al., 2012; Erskine et al., 1998). As a result, higher values and a higher variability of δ15N in prey types is expected, and the trophic position of both clusters from the Fuegian condors would be underestimated. Consistently, both the maximum and the range of δ15N values for the Tierra del Fuego terrestrial baseline was nearly three times that of Patagonia (Fig. 2).
The high marine input found for the Fuegian condors is likely favored by the geographical characteristics of this area. Tierra del Fuego is an insular system that also represents the lowest altitude limit of the Andes. The maximum distance from any point in the archipelago to the coast is less than 50 km. This distance can be easily cover daily by condors, as they perform low-cost soaring and can cover distances of up to 350 km/day (Lambertucci et al., 2014). The lesser extent of terrestrial prey items on the diet of the Fuegian condors could be also related to biogeographical factors. Tierra del Fuego lacks pumas (Puma concolor), a carnivore that frequently produces terrestrial carrion further exploited by condors in the continent (Perrig et al., 2017). In Southern Patagonia and Tierra del Fuego, moreover, cattle and sheep abundance has decreased in the last century (Aagesen, 2000), and pinniped carrion (including placenta), may have increased due to the recent recovery of populations in the area (Milano et al., 2020b, 2020a).
In Andean condors, the association with nearly exclusive terrestrial carrion is related to risk of exposure to pesticides used in cattle ranching to control predators (Estrada Pacheco et al., 2020) or lead poisoning associated to hunting (Lambertucci et al., 2011). In that context, the association with a broader array of food sources may enhance condor resilience in a changing world. However, this could also be associated with different risks: on the one hand, most marine food sources have higher level of heavy metals, and this has been already identified as a potential threat for California Condors (Kurle et al., 2016). In fact, some areas of the Fuegian archipelago have been identified as a hotspot of mercury that is magnified to scavengers (Balza et al., 2021; Dodino et al., 2022; Lois et al., 2022). On the other hand, colonies of marine mammals and seabirds can serve as sources of pathogens such as influenza viruses. During the 2022/2023 avian influenza outbreak, many birds and marine mammals in South America died, providing carrion for scavengers such as condors (Gamarra-Toledo et al., 2023; Plaza et al., 2024). In fact, the virus affected the critically endangered California Condor (Kozlov, 2023).
Some limitations of this study that need to be considered are that isotopic signatures depend on the variability in trophic discrimination factors and isotopic baselines, which we assumed to be fixed (Barnes et al., 2008). While this approach likely reduces our ability to do finer-scale discrimination among groups, it does introduce any particular bias that we know of. Also, assuming that all individuals within certain groups (in this case, the clusters and sampling sites) represent the same trophic role overlooks individual variability, a dimension that, if included, have several advantages (Clutton-Brock and Sheldon, 2010). To improve resolution power, future studies could estimate the trophic position of Andean condors using compound-specific SIA of amino acids. A prior work using this approach on Andean Condor feathers samples showed that quantifying the ratio of 15N between an amino acid that reflects the source (e.g., phenylalanine), and one that reflects the fractionation by the consumer (e.g., glutamic acid), can robustly predict the trophic position without the need of baseline data (Barceló et al., 2022).
ConclusionUnderstanding the dynamics of trophic interactions and conserving their structure has emerged as a novel, but rarely assessed approach to safeguarding the integrity of ecosystems (Heleno et al., 2020). Our results highlight that studying the trophic ecology in insular, remote regions, can help to better understand historical food webs which have been disrupted by human impacts in more anthropic, continental areas. Our study further enhances the importance of areas in which trophic links can be conserved and further shows the potential of non-invasive sampling to monitoring trophic links in a cost-effective way. Most samples from this work were obtained in protected areas that intend to preserve the marine-terrestrial interphase in a challenging, rapidly changing world. Thus, there is the potential for these areas to preserve ancient trophic links, and the long-term persistence of the relationship of the condor with the ocean will likely depend on these areas and their future management.
We thank Rodrigo Munzenmayer, Adolfo Imbert, Pablo Torres Carbonell, Laura Muiño, Darío Urruty, the Asociación Civil Conservación de Península Mitre, Wildlife Conservation Society Chile, Ignacio Domato, and Amira Salom for their invaluable field assistance. Special thanks to Mónica Torres and Fernando Encinas for their help in processing samples, and to Michael J. Polito for his support with the stable isotope analyses. We also extend our gratitude to the associate editor, an anonymous reviewer, and Eneko Arrondo for their insightful comments. Lastly, we are grateful to PECON for providing a free open-access platform that promotes quality, inclusive science for conservation in Latin America, especially during a period when political challenges and denialism present significant obstacles for the scientific community in Argentina. This research was supported by CONICET and the Wildlife Conservation Society.