Grassy ecosystems occupy 27% of Brazilian territory and have historically been neglected in conservation efforts and studies about biodiversity. The Campos Sulinos region in Southern Brazil includes open formations in the Pampa and Atlantic Forest domains. This region has the smallest coverage by protected areas in the country and presents high levels of biodiversity and endemism. Besides, this region is under threat due to croplands and exotic tree plantations. Here, we compile a plant population genetics and phylogeography dataset from published studies focusing on species native to the Campos Sulinos to synthesize findings in plant evolution and genetics to better understand the dynamics of genetic diversity in this open ecosystem. We found 58 works on 51 plant species, published from 2005 to 2022. Most studies used only one kind of molecular marker and few loci. The climatic changes during the Pleistocene are likely the main speciation driver either due to the dynamics between grassland and forest or marine transgressions. Overall, high genetic variability and clear structuring of populations were found for the species studied. Regions with high genetic diversity do not coincide with protected areas. We reinforce the necessity of considering high intraspecific genetic diversity and population structure into both conservation and restoration planning, as well as in research within the Campos Sulinos.
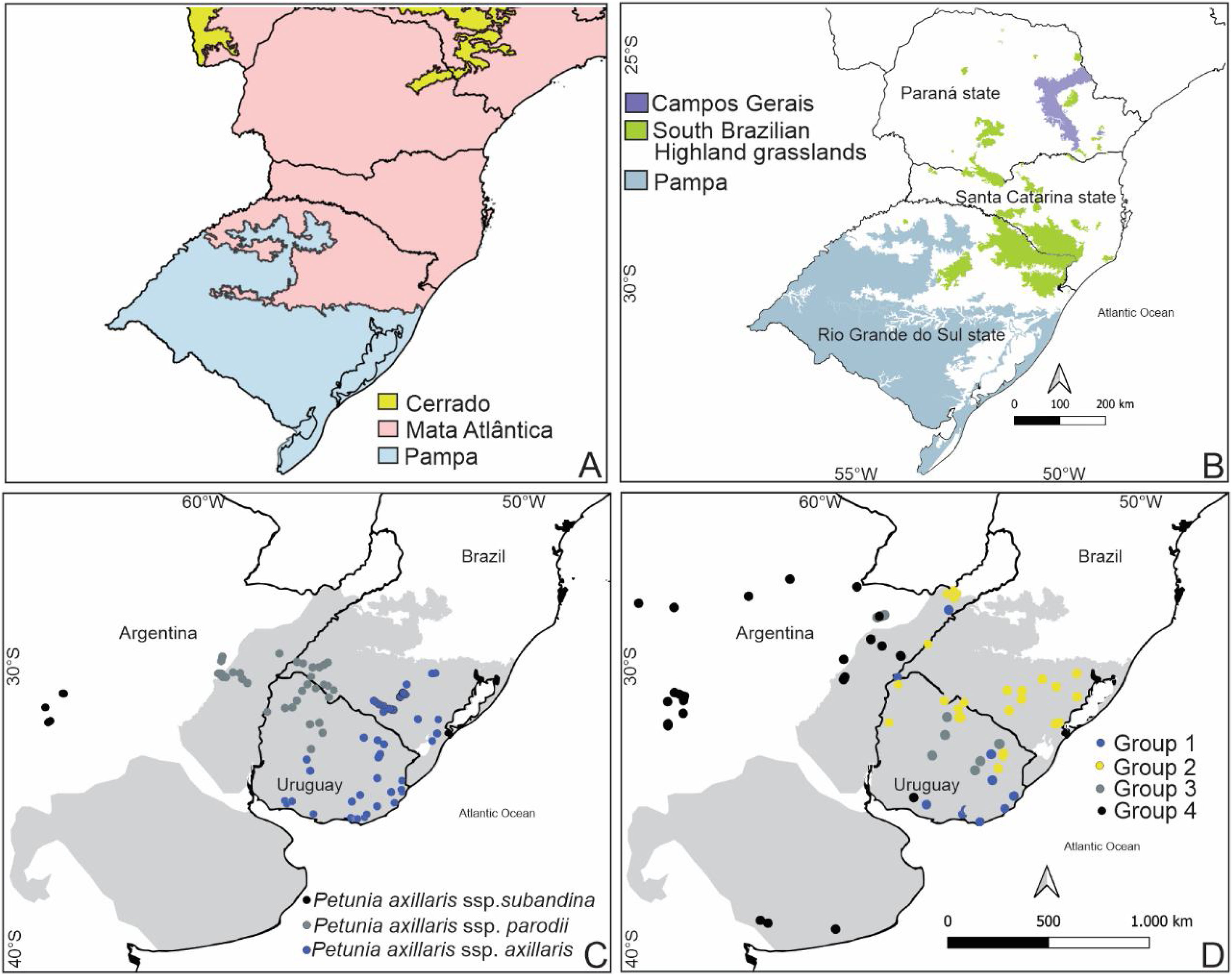
Brazil is known worldwide for its biodiversity, which is often associated with tropical forests. Grassy ecosystems occupy 27% of the country and are highly diverse. Open ecosystems are major components of the Pampa, Cerrado, and Pantanal domains but are also scattered across the Amazon, Atlantic Forest, and Caatinga domains (Overbeck et al., 2022). South Brazilian grasslands (Campos Sulinos) are located in the southernmost part of Brazil (Rio Grande do Sul (RS), Santa Catarina (SC), and Paraná (PR) states), including the Pampa grasslands in the RS, the South Brazilian highland grasslands (SBHG), and Campos Gerais in the Atlantic Forest domain (Fig. 1A, B).
The focal region and the distribution of the two studied species. A) Delimitation of Pampa and Atlantic Forest domains. B) South Brazilian grasslands (Campos Sulinos; Overbeck et al., 2007): Pampa grasslands in Brazil, South Brazilian Highland Grasslands, and Campos Gerais. South Brazilian Highland grasslands and Campos Gerais are grassy ecosystems in the Atlantic Forest biome. C and D) Río de la Plata Grasslands in gray, according to Soriano (1992), this region included the Pampa Grasslands. C) the distribution of Petunia axillaris (Turchetto et al., 2014a), and D) Turnera sidoides haplogroups (Moreno et al., 2018).
The Pampa, a heterogeneous region in terms of topography and soils harboring mosaics of grasslands and low forests in high-elevation regions (Andrade et al., 2019; Overbeck et al., 2022), is part of the Río de la Plata Grasslands that extend to Uruguay and Argentina (Soriano, 1992) (Fig. 1C). The SBHG consists of mosaics of grasslands and Araucaria forests. Campos Sulinos grasslands are grazed, with fire frequently used as a management tool in the SBHG (Dechoum et al., 2018; Overbeck et al., 2022). Grasslands, along with gallery forests, the Araucaria Forest, and savannas, are found in the Campos Gerais region, which is also under fire and grazing. Northward, grasslands are transitional to the Cerrado (Moro and do Carmo, 2007). The floristic patterns of Campos Sulinos support the division in the Pampa and SBHG (Andrade et al., 2019) regions, whereas plant community similarities with Campos Gerais have not yet been evaluated.
The Campos Sulinos region has historically been neglected in conservation efforts, as most grassy ecosystems worldwide (Stevens et al., 2022). No systematic compilation of the genetic diversity distribution of this plant species exists; thus, our understanding of genetic patterns across regions is scarce. Major threats to Campos Sulinos conservation include the expansion of croplands and exotic tree plantations (Andrade et al., 2023). For example, the Pampas domain has lost 34% of its natural vegetation since 1985 (Baeza et al., 2022). Recently, efforts have been made to catalog species richness in the region (e.g., Iganci et al., 2011; de Magalhaes et al., 2016; Andrade et al., 2018; Külkamp et al., 2018; Andrade et al., 2019, 2023); however, no attention has been given to genetic diversity. Genetic diversity within species and populations is fundamental for adaptation to new environmental conditions for short- and long-term survival, especially in the face of climate change. Evolutionary processes and genetic diversity within species should be considered in biodiversity conservation efforts to avoid inbreeding depression and preserve the potential for adaptation to ongoing and future environmental changes (Hoban et al., 2021; Nielsen et al., 2023).
In this study, we investigated the patterns and processes involved in plant microevolution in Campos Sulinos by synthesizing existing knowledge from published data. We systematically reviewed the plant evolution in Campos Sulinos and obtained information on seed dispersal and pollination modes. The resulting dataset is a tool for conservation efforts and helps understand the dynamics of genetic diversity in these open ecosystems. We summarized the research through meta-analyses and narrative reviews, investigated the patterns of genetic diversity and structure associated with environmental variables, and discussed biodiversity conservation in Campos Sulinos.
Material and methodsLiterature searchWe searched for articles that presented intraspecific genetic data in plants from the Campos Sulinos region, including the Río de la Plata grasslands, when the population distribution included this region. We built our bibliographic database (1980 until July 2022) using two search strategies (Fig. S1). First, we searched for published articles on the Web of Science (Institute of Scientific Information, Thomson Scientific— https://apps.webofknowledge.com/) and Google Scholar (https://scholar.google.com.br/) containing specific keywords. Second, we searched the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) through the R (R Core Team, 2022) package rentrez v.1.2.3 (Winter, 2017) to further identify studies presenting molecular genetic data. A nucleotide database was searched based on the species list from Andrade et al. (2018). We arbitrarily limited the search to species with more than 10 sequences per genetic marker to direct the results to phylogeographic studies. During the search, the titles, abstracts, and keywords of each study were screened, and we used geographic distribution and experimental studies applying molecular markers as inclusion criteria. Additionally, we reviewed some studies that presented integrative analyses, such as ecological niche modeling, and discussed the results using molecular data from previously published studies. We proceeded with the full-text screening of all selected studies.
Finally, we extracted the following information from 58 studies: (i) sample taxa, (ii) biological aspects of studied taxa, (iii) molecular markers, (iv) geographic distribution if extensive (sampling covered most of the geographic distribution of species) or limited (sampling did not cover the species distribution), (v) population structure and genetic diversity patterns, (vi) contact zones, (vii) refuges, (viii) demographic patterns and lineages divergence time estimation (Table S1).
Mapping genetic diversity and structureWe extracted the population genetic parameters from the articles (Table S2) and mapped them across the study area. It was impossible to recover data from all studies because some did not present the indices used. We obtained different statistics to measure genetic diversity parameters according to the molecular markers analyzed in each study. We used haplotype diversity for cpDNA and nuclear markers; for AFLP, the proportion of polymorphic data; for SSRs, the allele richness; for SNPs, the proportion of polymorphic sites; and for ISSRs, the percentage of polymorphic bands. After tabulation, we used the data to construct diversity maps using different grid resolutions (0.25°, 0.5°, 1°, and 1.5°) for populations from Pampa, SBHG/Campos Gerais, and all regions of Campos Sulinos (widespread species). The results across different grid resolutions were generally similar; thus, we only report the results based on 0.25°. We generated diversity maps for each marker type (AFLP, cpDNA, ISSRs, nrITS, SNPs, and SSRs) for each of the three groups (Pampa, SBHG/Campos Gerais, widespread). All indices from the different markers were scaled to 0–1 prior to mapping. Additionally, a summary map, including all types of markers, was generated by calculating the mean-scaled value across the markers. All maps were generated using the R package raster v.3.6-11 (Hijmans et al., 2023).
Environmental correlates of genetic diversityWe generated correlative models for each of the three groups using the scaled mean local genetic diversity value across all markers as the response to investigate the extent to which the spatial patterns of genetic diversity were explained by the environmental descriptors. The models were built using a machine-learning algorithm (randomForest, Liaw and Wiener, 2002). We used five groups of environmental predictors: variables describing the current temperature, current precipitation, past temperature, past precipitation, and soil features. Current precipitation and temperature variables included all 19 bioclimatic descriptors (Fick and Hijmans, 2017). Historical climate layers were built to reflect climatic stability over the last 120,000 years (120 kyr), considering 19 bioclimatic descriptors available every 4000 years during this period (Karger et al., 2017). For each bioclimatic descriptor, the coefficient of variation over the past 120 kyr was estimated in R and summarized in the new raster layers. The coefficient of variation of each bioclim was used as the input for principal component analysis (PCA). Soil descriptors (11 variables) were obtained from soilgrids.org (Poggio et al., 2021) and used as the input for the soil PCA. The standard deviations of the bioclimate descriptors and soil data are presented in Table S3. The first three components of the PCA were obtained for each group, totaling 15 predictors at a 0.05° resolution. PCAs were conducted in R using the prcomp function (R Core Team, 2022) and summarized using the functions of the raster package. Each dataset was split into model training and testing datasets (containing 70% and 30% pixels, respectively) to build the models. A 10-fold cross-validation was repeated 10 times and was used for each model. All models were built using the R package caret (Kuhn et al., 2016), and variable importance (VI) was computed using the varImp function of the same package. The predicted model was projected and binarized (the threshold applied was the 0.95 quantile) to highlight areas with high genetic diversity.
Pollination and dispersal modeThe dispersal mode and pollination syndrome for each species were recovered from selected studies or additional references, as indicated in Tables S1 and S2.
ResultsWe retrieved 58 works that were published from 2005 to 2022 (Table S1). The number of published works per year ranged from 5 to 10 from 2014 to 2020; in 2021, only two were published. We compiled information on the intraspecific genetic variation for 28 genera and 19 families. Solanaceae Juss. was the most studied family, with 26 studies, and the genus Petunia Juss. appeared in 23 articles. Other families and genera had one, two, or three appearances. Fifty-one species were studied, most of which were herbs (∼64%); however, seven trees, seven climbing species, three shrubs, two epiphytes, and one cactus were included. All the studied species were native (except Senecio madagascariensis Poir.), 21 occurring exclusively in Pampa, 18 occurring exclusively in SBHG and Campos Gerais, and 13 occurring in all regions (widespread species). Most studies (33) evaluated the entire species distribution, whereas 25 were limited to a part of the species distribution (Table S1).
Most studies (42) used only one kind of molecular marker. The most commonly used marker was cpDNA (12 alone and 13 combined with another marker), followed by SSRs (18 alone and 6 combined with another marker). Many studies have combined molecular markers with other data, such as climatic variables (16), morphology (8), cytogenetics (6), crossing experiments (4), archaeological/palynological data (1), bioregion analysis (1), conservation status (1), pollen viability (1), and seed viability (2).
Species demographyRecent and ancient contact zones were reported in 16 studies for the genera Petunia (10), Calibrachoa Cerv. (1), Turnera L. (2), Passiflora L. (1), Ipheion Raf. (1), and Podocarpus L'Hér. ex Pers. (1) (Table S1). Ancient contact was described as having occurred in the last glacial maximum (LGM) for Petunia axillaris (Lam.) Britton, Sterns & Poggenb. subspecies in Bagé, RS, Brazil, and the Rio Negro region of Uruguay. The connection between the southern and northern populations of Podocarpus lambertii Klotzsch ex Endl. was observed during the LGM. Recent contact for Petunia inflata R.E.Fr. and Petunia interior T.Ando & Hashim. was reported in the northwestern RS, with the possibility of recent hybridization. Petunia axillaris and P. exserta Stehmann have recently come into contact in the Serra do Sudeste region of the RS, forming a hybrid zone. Recent contact zones among extant haplotypes for Turnera occurred northeast and southeast of the Iberá system (Mesopotamia/Argentina). Passiflora actinia Hook. and P. elegans Mast. have been in recent contact with the northeastern RS. Calibrachoa heterophylla (Sendtn.) Wijsman populations and Ipheion subspecies suffered recent contact that should have started after marine transgressions approximately 5000 years ago.
Refuges of Podocarpus lambertii and Araucaria angustifolia (Bertol.) Kuntze were reported in the Serra Geral region. High-altitude regions have also been reported as possible refuges for Petunia species from the SBHG, Epidendrum fulgens Brongn., and Turnera sidoides L. from Pampa. More than one refugia was found for Araucaria angustifolia and Petunia axillaris. Petunia integrifolia (Hook.) Schinz & Thell. and Calibrachoa heterophylla occurred in the coastal plain and are thought to have persisted in refuges during the marine transgressions of the Pleistocene (Table S1).
Few studies have reported ancestral areas of occurrence; most were located in the Campos Sulinos, such as the highlands (Mimosa L. species) and the Pampa region (Ipheion uniflorum (Lindl.) Raf. and P. integrifolia subsp. depauperata (R.E.F.R.) Stehmann). Other studies have suggested that the ancestral area is close to the Pampa, such as the Andean region or Pampa for P. axillaris and the western Peripampasic Arc for Turnera sidoides complex (Table S1).
Climatic oscillations of the Pleistocene, together with marine transgression, caused dynamic population expansion and contraction for most species (Table S1). Recent population expansion has been observed in P. bajeensis T.Ando & Hashim., P. integrifolia and P. interior, P. bonjardinensis T.Ando & Hashim., P. reitzii L.B.Sm. & Downs and P. saxicola L.B.Sm. & Downs, P. altiplana T.Ando & Hashim, and P. axillaris, Podocarpus lambertii, Araucaria angustifolia, Turnera sidoides, Calibrachoa heterophylla and Eugenia uniflora L. (Table S1). Even during the LGM, stable populations were found in Tillandsia aeranthos (Loisel.) L.B. Sm. Aechmea calyculata (E.Morren) Baker, Tibouchina hatschbachii Wurdack (Table S1). Petunia inflata, some populations of P. integrifolia, P. axillaris subsp. subandina T.Ando, and Hypochaeris catharinensis Cabrera suffered recent demographic contractions (Table S1). The divergence time was estimated to be mainly during the Pleistocene. Most population fragmentation also occurred during glaciation. Pleistocene climatic changes explained the actual distribution patterns of many species in Campos Sulinos (20 studies). Geographic distance and altitude, bottleneck events, recent geological movements, landscape transformation and cattle grazing, karyotype novelties and river barriers, colonization of new habitats, and changes in the reproductive system appeared, explaining the current distribution in one study each and habitat fragmentation in two studies (Table S1).
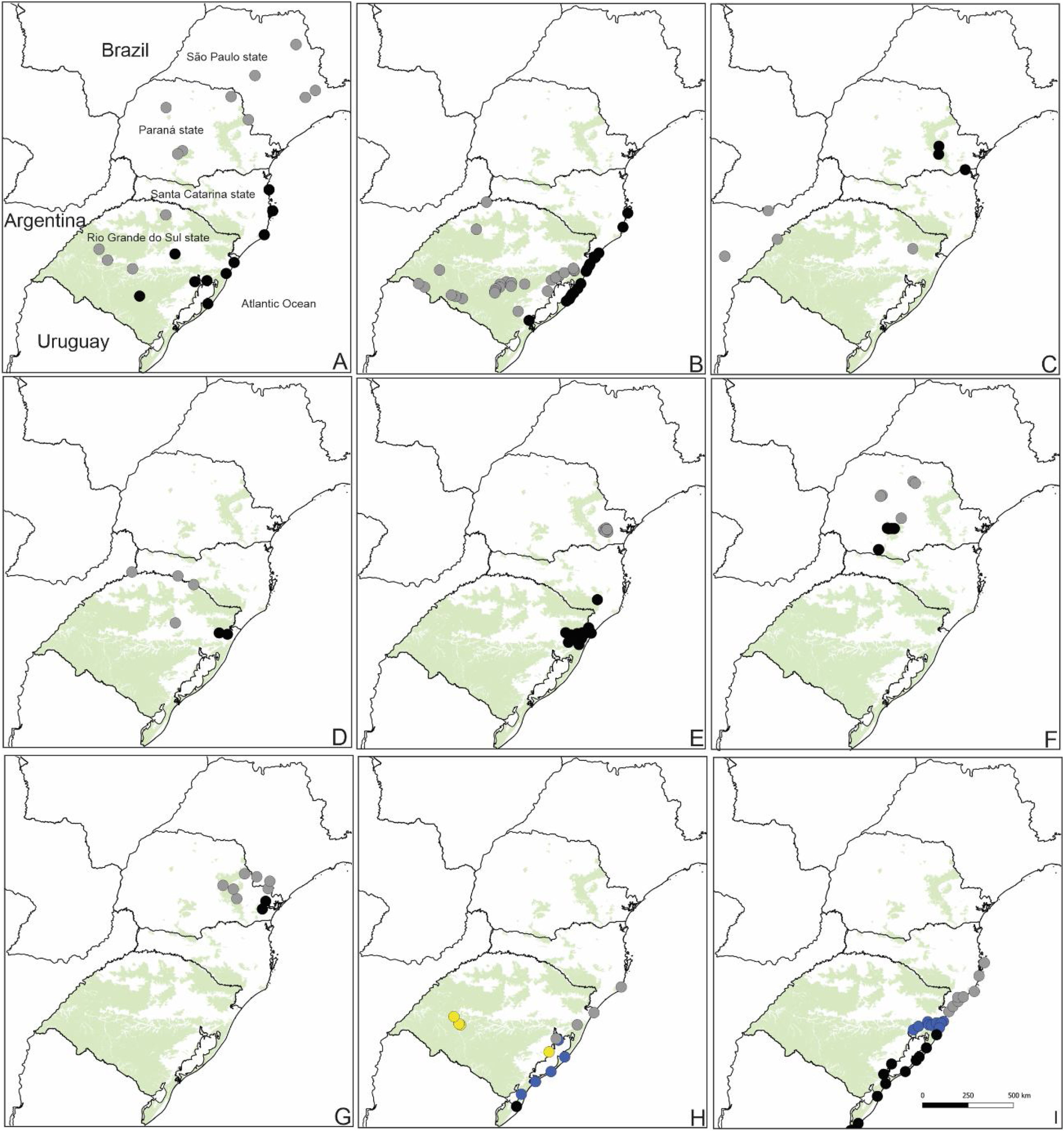
Genetic structure and diversity of populationsApproximately 40 studies reported the population structure at the level of each population or a set of populations with some geographic and genetic isolation, and at least for one of the markers used. In the 14 studies analyzing plants occurring in both Pampa and the SBHG, 8 related population structures and, of those, 4 indicated an east/west geographic pattern of genetic structure (Table S1, Fig. 2A–C). None of these species was found exclusively in Campos Sulinos. Two distinct genetic groups that separate the Caatinga populations from the Atlantic Forest populations were found for Podocarpus lambertii. The degree of genetic differentiation between sample locations increased with the geographical distance between populations of Luehea divaricata Mart. Petunia interior showed a low population structure, and P. inflata revealed moderate to high differentiation at the two collection sites compared with the other sites (Table S1).
Distribution of some species growing in Campos Sulinos that presented population genetic structure. The circles represent the populations and colors of the genetic group identified for each species. Campos Sulinos region is represented in green. A) Cereus hildmannianus K.Schum. ((Silva et al., 2017); SSR data); B) Petunia integrifolia complex (Longo et al., 2014; cpDNA data); and C) Mimosa subser. Dolentes–Brevipedes complex ((Morales et al., 2015); AFLP data) presented an east/west pattern of genetic structure. Gray indicates westerns regions, and black indicates easterns regions. D) Aechmea calyculata (Goetze et al., 2016; cpDNA, SSRs nuclear, nuclear loci); E) Passiflora actinia ((Lorenz-Lemke et al., 2005); ITS data); F) Portulaca hatschbachii ((Feliciano et al., 2022); AFLP data); G) Tibouchina hatschbachii (Maia et al., 2017; cpDNA data), occurring only in SBHG presented a north/south population structuring pattern. Gray indicates east-north populations, and black indicates west-south populations. H) Calibrachoa heterophylla ((Mäder et al., 2013); cpDNA data); and I) Petunia integrifolia subsp. depauperata (Ramos-Fregonezi et al., 2015; cpDNA data) presenting a north/south pattern of population structuration in Pampa. Gray indicates the north group, black indicates the south group, blue indicates the central group, and yellow indicates the mainland group.
Most plants found only in the SBHG (11 of 14) showed genetically structured populations; for some species, a north/south pattern was found (Table S1; Fig. 2D–G). Most species in the Pampa presented structured populations (19 of 30). Coastal populations frequently formed an isolated genetic group, and a north/south pattern of structuration was also observed (Table S1; Fig. 2H, I). The species distribution of Herbertia darwinii Roitman & J.A.Castillo, H. pulchella Sweet, and H. quareimana Ravenna extend to the Río de la Plata grasslands; the structure found for the Pampa show an east/west pattern.
Considering species from the Río de la Plata grasslands (sensuSoriano, 1992), populations of the widespread species Petunia axillaris and Turnera sidoides in Brazil, Argentina, and Uruguay constituted different genetic groups (Table S1; Fig. 1B, C).
Genetic diversity was considered high-to-moderate in 38 studies. Nine studies on Campos Sulinos plants reported high or moderate genetic diversity. Haplotype diversity was frequently greater than nucleotide diversity (Table S2). Among the species occurring in the SBHG, only Portulaca hatschbachii D. Legrand had low levels of genetic diversity. Only one Pampas species presented low levels of genetic diversity (Petunia integrifolia), and the same pattern of haplotypic diversity greater than nucleotide diversity was found. Eugenia uniflora and Epidendrum fulgens had the widest geographical distribution throughout the Atlantic Forest, but were not identified in the SBHG, and presented contrasting patterns of diversity, with Pampa populations more structured and genetically diverse than the rest of their distribution. Both species possibly had multiple regions inside the Pampa as possible refuges during the Pleistocene climatic oscillations (Tables S1 and S2).
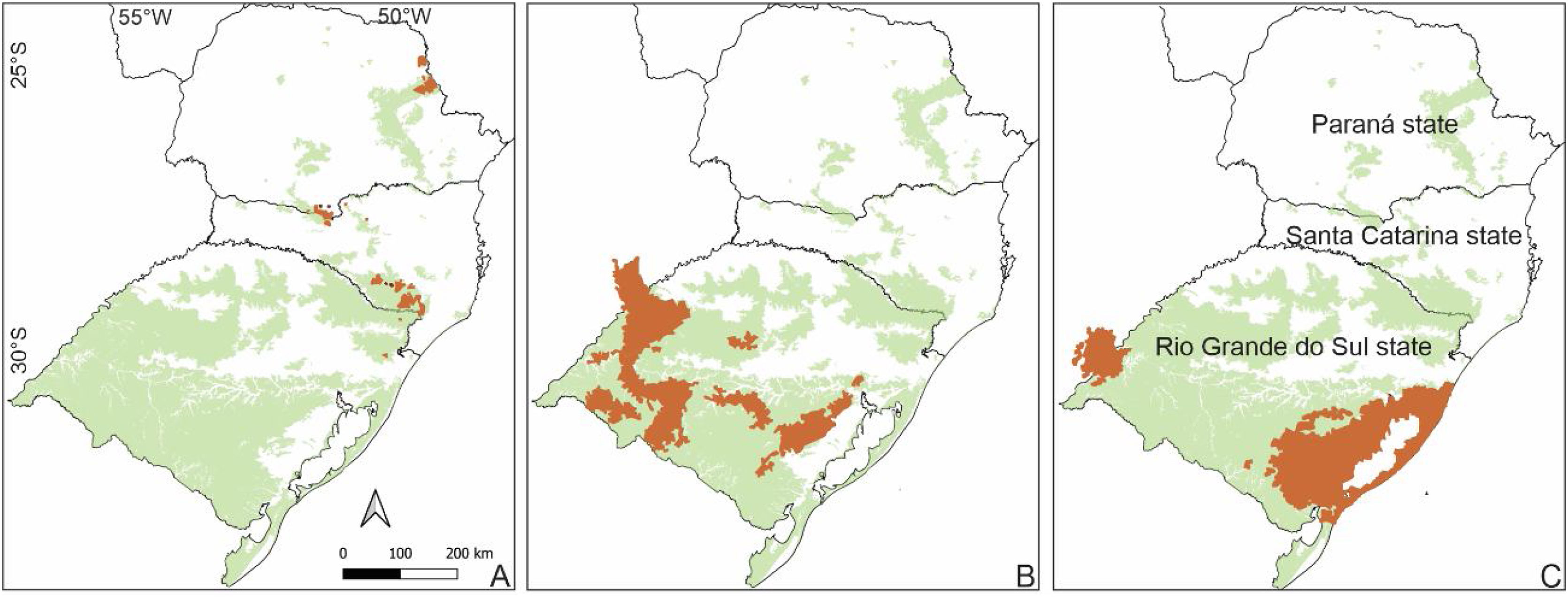
Diversity mappingThe highest genetic diversity regions in the SBHG and Campos Gerais were in the north of Campos Gerais (PR state) and in the northeast of the SBHG (border region of RS and SC). The small SC/PR border region also showed high genetic diversity (Fig. 3A). The regions with the highest diversity in the Pampa were in the center and northwest of the RS, extending toward northeast Argentina (Fig. 3B). Considering the widespread species, the southeastern region, including the Atlantic Ocean coast and the northwest of the RS, were the most genetically diverse regions in Campos Sulinos (Fig. 3C). The metrics for each marker are shown in Fig. S2.
Summary of the highest genetically diverse areas (at 95% quantile) in Campos Sulinos based on genetic diversity indices of species growing in South Brazilian highland grasslands and Campos Gerais, Pampa, or all regions (widespread species: Pampa, SBHG and Campos Gerais, for details, see Table S2) including all types of markers. A) South Brazilian Highland Grasslands and Campos Gerais. B) Pampa. C) Widespread species. Green indicates Campos Sulinos, and brown indicates the most genetically diverse areas.
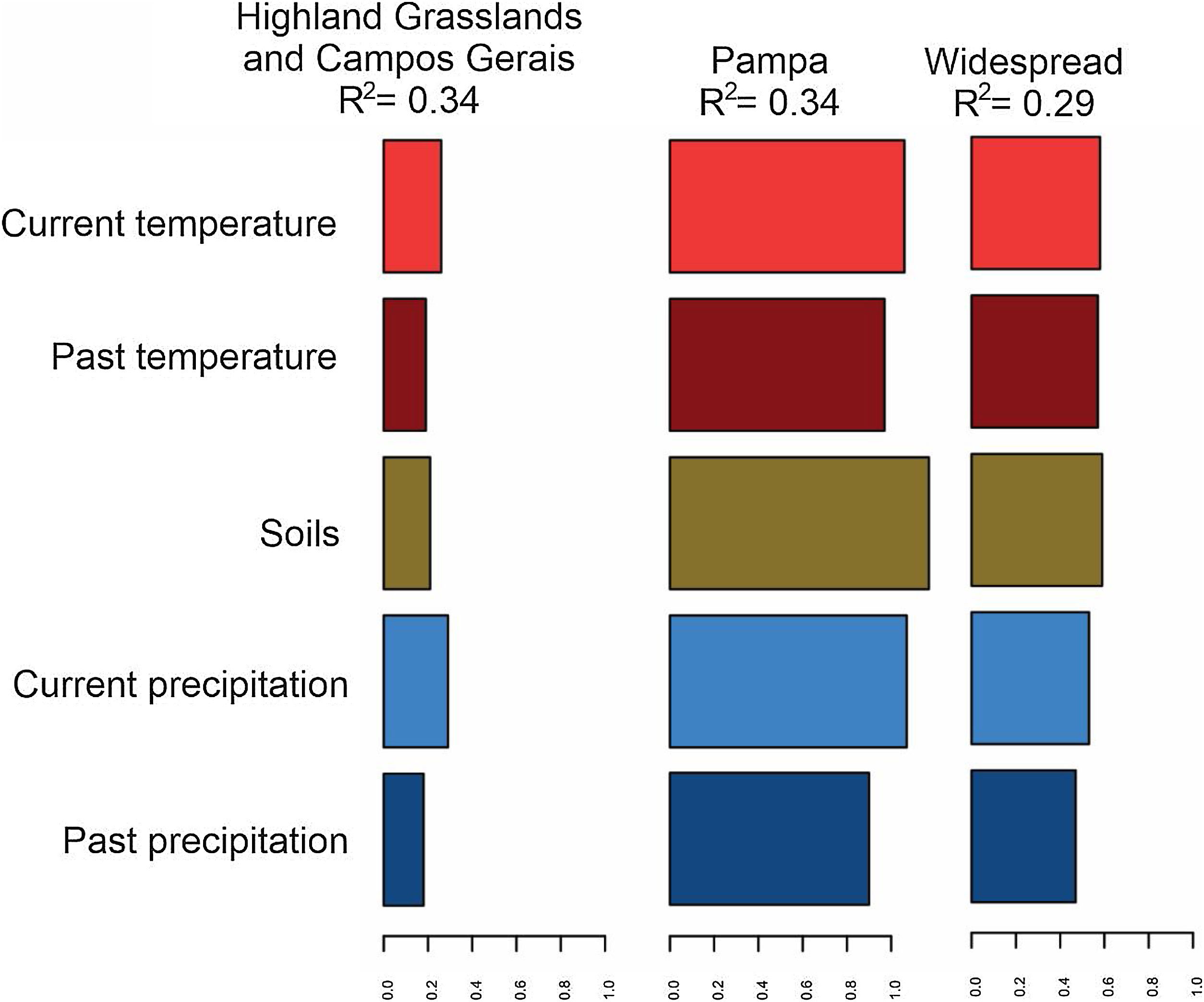
Model predictions of the genetic diversity metric reflected intermediate correlation values for all subsets of species occurring in Pampa (R2 0.34), SBHG (R2 0.34), and widespread species (R2 0.29). Environmental variables (past and current climatic variables and soil traits) affected the range-wide genetic diversity differently when the subregions were considered. Current precipitation in the SBHG and Campos Gerais was more important in the species genetic diversity model, whereas in the Pampas and for widespread species, soil features were important (Fig. 4).
Environmental variable importance analysis using the scaled mean local genetic diversity across all markers. Five groups of environmental predictors were used: current and past temperature and precipitation (19 bioclimatic descriptors each) and soil descriptors (11 variables). The values of these descriptors were presented in an aggregated format.
The number of plants with genetic diversity data and information on pollinators and seed dispersal was limited (Tables S1 and S2). However, we observed some general patterns: (1) bees were the most cited pollinators (22 studies), followed by hummingbirds (10), and hawkmoths (9); (2) most plants presented specialized pollination syndromes; and (3) autochory was the most frequent dispersal mode.
DiscussionThe high genetic diversity and low number of species studied in Campos Sulinos indicate an immense challenge for efficient conservation efforts that consider intraspecific genetic diversity. Genetic data at the intraspecific level provide valuable information to increase conservation success (Hohenlohe et al., 2021; Andrello et al., 2022) and are also highly relevant for ecological restoration (e.g., Mijangos et al., 2015; Durka et al., 2017). The study region is a key area for biodiversity conservation in Brazil (Guerra et al., 2020; Overbeck et al., 2022). In addition to the small number of studied species, our compilation also highlighted a bias toward a few taxa that are usually used as models by certain research groups.
Almost all studies using genetic data in Campos Sulinos have been based on one or a few markers but have also combined molecular data with different information, such as morphometry and crossing experiments. While the combination of different types of evidence reinforces these conclusions, high-throughput sequencing allows the analysis of many loci in a large number of individuals using less time and laboratory work with high statistical power and resolution. Additionally, genomic data allow us to assess both neutral and adaptive variants with different implications for the conservation and restoration of ecosystems, such as identifying genetic loci associated with diseases or environmental adaptive characteristics that underpin the resilience of populations and communities and, consequently, ecosystem functions and services (Funk et al., 2012; Hohenlohe et al., 2021; Heuertz et al., 2023). A joint effort by government agencies (e.g., funding agencies) and research groups to increase plant genomic studies in Campos Sulinos for genomics-informed management is necessary.
Contact zones, refuges, and population expansion and contractionBoth ancient and recent contact zones have been reported in our literature review of the Campos Sulinos population genetics. The LGM period is cited as the cause of the diversification/isolation and contact of lineages in most studied species (e.g., Lorenz-Lemke et al., 2010; Turchetto et al., 2014; Giudicelli et al., 2019, 2022). The climate oscillations between cold and warm periods led to dynamics between grasslands and forests; as the climate became warmer and more humid approximately 5000 years ago, grasslands in this region were maintained by herbivory and fire (Behling, 2002; Andrade et al., 2015). Refuges in Campos Sulinos were reported mostly in higher elevation regions, following the dynamics of increase and retraction of open vegetation during the Pleistocene period. Even though climatic oscillations of the Pleistocene caused population expansion and contraction dynamics in many organisms, some species were stable, even during the LGM (Goetze et al., 2016; Maia et al., 2017; Aoki-Gonçalves et al., 2020), evidencing the importance of genetic studies to capture the particularities of each species.
Patterns of population structureA pattern of genetic differentiation could be identified in the Campos Sulinos based on the revised studies: phylogenetic and phylogeographic studies with species of broad distribution in Campos Sulinos indicate a lineage division between SBHG and Pampa (Lorenz-Lemke et al., 2010; Fregonezi et al., 2013; Acosta et al., 2016), in agreement with plant community data (Andrade et al., 2019). Thus, considering the species and genetic diversity, heterogeneity is a hallmark of Campos Sulinos.
Our results on the population genetic structure in the region reinforced the need for more studies on phylogeography and population genetics to guarantee that conservation efforts will be able to consider different evolutionary lineages (Hoban et al., 2021) and establish effective ecological restoration (Mijangos et al., 2015). Population genetics information is important to guide decision-making in restoration, for example, to define the geographic delineation of seed transfer zones based on spatial genetic differentiation among populations of a species (Durka et al., 2017; Carvalho et al., 2020). Currently, this is not considered in restoration actions and planning; the seeds of native species are not easily available (Rolim et al., 2022). We recommend that future research on plant species introduction include aspects of population genetics from the early stages. Therefore, including species from families of high relevance to the region, such as Poaceae Barnhart and Asteraceae Bercht. & J.Presl, which have been relatively understudied, is important.
Mapping genetic diversity and conservation effortsVisual inspection of the genetic diversity maps provided here (Fig. 3) revealed that, to the largest extent, genetic hotspots were located outside the protected areas (Fig. S3). In addition, the regions where agriculture and planted forests have expanded during the last few years, such as the northwestern and central regions of the RS, have no protected areas (Fig. S3). The area covered by natural open formations decreased from 9.3 million ha in 1985 to 6.6 million ha in 2020 (MapBiomas, 2021, Fig. S3). These numbers highlight the importance of increasing the coverage of conservation areas in Campos Sulinos, particularly in areas with high diversity.
We found that current precipitation and soil variables were important drivers of genetic diversity in Campos Sulinos. Precipitation influences the genetic structure of Petunia and Calibrachoa species (Barros et al., 2020; Pezzi et al., 2022). The importance of soil features has been highlighted in previous studies on Petunia axillaris (Turchetto et al., 2014; Giudicelli et al., 2022). The intrinsic combination of different biotic and abiotic factors makes it difficult to establish common drivers of diversity in Campos Sulinos but also results in high biodiversity (Overbeck et al., 2022; Andrade et al., 2023).
We did not find a relationship between the pollination systems and diversity. Most references used to indicate pollination syndromes here are not pollination biology studies, and studies of plant-pollinator interactions in Campos Sulinos that establish the association between genetic diversity and pollination modes are lacking.
ConclusionCampos Sulinos has high genetic diversity and population genetic structure for most of the studied plant species. Both diversity and structure are often associated with the present or past environmental variations. In Campos Sulinos, which is characterized by high heterogeneity as noted in species and plant community surveys, our study on genetic diversity and population structure highlights the importance of advancing conservation and ecological restoration efforts by explicitly incorporating genetic aspects. We also found a lack of comparative phylogeography studies, which could indicate patterns of genetic diversity and structure across different species throughout the landscape, and studies using high-throughput sequencing strategies, which could facilitate a detailed picture of the distribution of genetic diversity and contribute to modeling the evolutionary potential of species in the face of environmental changes. More integrative studies on microevolution are necessary to understand the dynamics of this region and preserve this process.
We live in an era of ongoing global change, in which the resilience of ecosystems to environmental changes is paramount. Therefore, conservation and restoration must consider the genetic diversity of plant populations. Research on this topic should be incentivized and should receive adequate support from financing agencies.
Declaration of competing interestThe authors declare that they have no conflict of interest.
This paper was motivated by the collaboration in the project ‘GrassSyn - Biodiversity of Brazilian grasslands and savannas: patterns and drivers, ecosystem services, and strategies for conservation and restoration’ (SinBiose - Centro de Síntese em Biodiversidade e Serviços Ecossistêmicos, Brazil; CNPQ grant 442348/2019-3 to GEO).
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant 309797/2022-5 to CT), Universidade Federal do Rio Grande do Sul (UFRGS), Programa de Pós-Graduação em Botânica (PPGBOT-UFRGS) and Programa de Pós-Graduação em Genética e Biologia Molecular (PPGBM-UFRGS). IVQ was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior -Brasil (CAPES) – Finance code 001. We would like to thank Editage (www.editage.com.br) for English language editing.