Predation risk is perceived by prey and mesocarnivores through risk signals given by large carnivores. These signals can be manipulated without exposing mesocarnivores to real risk, creating landscapes of fear through perceptual traps, altering behavior. Olfactory signals like urine and feces have been used to deter carnivores that predate on livestock, but a more biologically meaningful cue could be more effective. Livestock guardian dogs (LGD) deter carnivores and reduce predation, so using their whole-body odor as a risk signal in a livestock system could contribute to reduce livestock-carnivore conflict. We tested LGD whole-body odor effect on Patagonian foxes (Lycalopex culpaeus and Lycalopex griseus) present in sheep production in three different habitats—forest, scrubland and pastureland—and analyzed behavioral changes. The presence of LGD whole-body odor reduce the presence of foxes in scrubland habitats and could increase fear behavior and reduced food consumption compared to non-scented places. This technique could act as a complement to LGD, amplifying its effect, but the habitat characteristics must be considered to make it effective. LGD whole-body odor, a more realistic risk signal, representing the presence of LGD without exposing mesocarnivores to a real encounter, i.e. a perceptual trap, could contribute to reduce livestock losses and carnivore threats from livestock owners, aiming to facilitate coexistence between livestock production and native carnivores.
Perceptions of predation risk, as conveyed through auditory, visual, and olfactory cues by large predators, can significantly influence the behaviors of prey and mesopredators. Such cues are interpreted as signals of heightened risk, which can adversely affect survival and reproduction chances, directly reducing fitness (Linnell and Strand, 2000; Creel and Christianson, 2008). These signals instantiate a 'landscape of fear'—areas where the risk of injury or death increases significantly during encounters between smaller predators and their larger counterparts (Glen and Dickman, 2005). The landscape of fear, a heterogeneous three-dimensional space defined by the level of risk perception, is affected by changes that may occur in the habitat, hence influencing the lethality of predators to smaller animals (Laundre et al., 2010). This scenario forces animals to adjust their behavior according to perceived predation risk, decreasing time allocated to foraging and increasing time spent on vigilance and affecting spatial selection (Brown et al., 1999; Laundre et al., 2001; Lodberg-Holm et al., 2019; Suraci et al., 2016). Hence, the landscape of fear offers a conceptual framework for species management by manipulating the risk perceived by animals. This approach may be useful in preventing focal species from using the habitat or segregating them, such as mesopredators engaged in livestock predation (Atkins et al., 2017).
One way to manipulate fear in landscapes to mitigate conflicts between livestock production and conservation of wild carnivores can be achieved through strategic use of perceptual traps for mesopredators (Bacon and Boyce, 2016). A perceptual trap occurs when a high-quality habitat is undervalued, so low-quality habitats are preferred (Patten and Kelly, 2010) and could be produced by falsely indicating high predation risk, suggesting the habitat should be avoided. Signaling higher predation risks in areas where carnivore-livestock conflicts are critical might reduce visitation or predation on livestock by mesocarnivores, facilitating land-sharing between carnivores and livestock (Crespin and Simonetti, 2020).
Olfactory signals are important for communication among carnivores; they detect information about conspecifics, prey, competitors and potential predators through odors (Vanak et al., 2009) and habitat characteristics can interfere with this communication (Verdolin, 2006). The persistence and dispersion of olfactory cues depends on environmental variables such as temperature, air humidity, wind speed, and vegetation, influencing communication by odors (Müller-Schwarze, 2006; Finelli et al., 2011). Trees and shrubs act as obstacles to wind, becoming barriers for signals to travel and allowing odors to stay longer in a spot. In contrast, areas with little or no vegetation have higher wind speeds and allow olfactory signals to travel and disperse faster (Müller-Schwarze, 2006). Thus, to manage wild carnivores with scent-based repellents to discourage them from approaching and moving through areas where livestock graze or give birth, ecological aspects such as animal behavior, complexity of odor cues and habitat characteristics must be considered.
Olfactory repellents such as urine and feces of large-bodied carnivores have been used to simulate their presence as a management technique to prevent livestock predation (Masini et al., 2005; Shivik et al., 2011), but the evidence of their effectiveness is variable (Miller et al., 2016). These excretions might not be the best cue of the presence of a large predator (Mason, 1998). They could only be perceived as waste produced by an individual’s occasional passage, and not necessarily as an intentional territorial mark, thus failing to indicate the constant presence of a predator in the area (Leo et al., 2015).
In contrast, the use of “whole-body odor”, a complex odor mixture from skin, saliva, feces, urine, dandruff and glandular secretions, offers an alternative. In areas treated with whole-body odor from dingoes (Canis lupus dingo), red foxes (Vulpes vulpes) changed their feeding behavior, reducing food consumption when predator scents were present (Leo et al., 2015). The use of whole-body odor from livestock guardian dogs (Canis lupus familiaris) (hereafter LGD) elicited the avoidance of European hares (Lepus europaeus) (Ugarte et al., 2021). LGD are utilized as a non-lethal technique to manage wild carnivores predating on livestock and influence wildlife, acting as a surrogate top-predator to both herbivores and mesopredators (van Bommel and Johnson, 2016; Ugarte et al., 2021). Mechanisms behind their effectiveness could lie in a variety of signals they exhibit toward other carnivores. LGD usually do not confront predators physically, rather they act as a territorial deterrent to other carnivores through visual, auditory and olfactory signals (Allen et al., 2017; van Bommel and Johnson, 2017; Bromen et al., 2019), which would explain their effectiveness as a non-lethal technique (van Bommel and Johnson, 2016).
This evidence suggests that a more complex and biologically meaningful arrangement of odors is required to elicit an aversive response instead of only single excretion odors, providing more information that can be encoded by the signal receiver (Apfelbach et al., 2015; Gorman and Trowbridge, 1989; Nolte et al., 1994). Such a complex odor cue represents the presence of large carnivores more realistically, hence an imminent threat by proximity (Leo et al., 2015; Carthey and Banks, 2016; Ugarte et al., 2021). In these conditions, mesocarnivores should act to reduce the risk of being preyed upon or injured, even when food is available (Lima and Dill, 1990), reducing foraging times, increasing surveillance and eventually abandoning a food patch, evaluated as the density of abandoned food or giving up density (GUD) (Brown, 1988; Verdolin, 2006; Haswell et al., 2018). By manipulating this odor, a landscape of fear can be created, modifying mesocarnivores’ behavior (van Bommel and Johnson, 2016).
Understanding how LGD olfactory signals behave in different habitats would allow them to be manipulated with a specific environmental context approach, potentially creating perceptual traps, triggering avoidance of spatial overlap between livestock and wild carnivores. Thus, incorporating our knowledge about mesocarnivore behavior and its variation according to vegetation, we could amplify LGD effects with a low-cost non-lethal technique such as whole-body odor, inducing predator-livestock temporal segregation in the habitat, decreasing possibilities of encounter and thus reducing risks of conflicts (van Bommel and Johnson, 2016).
The Chilean Patagonia region contributes 75% of national sheep production in Chile (ca. 1.5 million head INE, 2017), but 44% of livestock ranches are affected by predation on lambs and ewes by pumas (Puma concolor) and foxes, including chilla (Lycalopex griseus), and culpeo (L. culpaeus) (Soto-Volkart, 2001; INE, 2017). These foxes, described as troublesome mesocarnivores as they prey on livestock (Lucherini and Merino, 2008; Silva-Rodríguez et al., 2009), are responsible for lambs and sheep deaths in 54% of the affected farms (INE, 2017). This results in significant economic loss, to which many farmers respond with lethal control, whereas only few producers use LGD.
An adult male LGD can weigh up to 49.8 kg and an adult puma 72 kg, while the largest culpeo weighs up to 10 kg and the largest chilla 4.5 kg (Iriarte and Jaksic, 2017; Lorenz, 1989). This weight difference creates a disadvantage to culpeo and chilla (called foxes from now on), because facing an LGD carries a potentially high risk of being severely injured or killed in a confrontation. In this scenario, we would expect the whole-body odor of LGD to reinforce risk cues that might convey LGD presence, triggering a landscape of fear for foxes, which ought to modify local fox distribution and behavior, hence reducing the chances of attacks on livestock and potentially reducing conflict (Crespin and Simonetti, 2019).
In this study, we test fox risk perception related to LGD presence by providing a risk signal to foxes immersed in livestock systems. In a first experiment, we aim to determine behavioral changes (e.g., visitation rate, time spent foraging and in vigilance) produced by a risk signal, the whole-body odor of LGD, on native mesocarnivores immersed in livestock systems, and to verify if there is a difference between the three habitats present in these lands (pastureland, scrubland and forest) with available food. Due to the influence of vegetation on the perception of olfactory cues, in the presence of LGD whole-body odor we expect visitation rates to be highest in grassland, intermediate in forests, and lowest in scrublands. When foxes visit these habitats with available food, we expect changes in behaviors associated with risk perception between scented and non-scented places; an increase in time dedicated to vigilance and a reduction in time dedicated to foraging when the treatment is present.
In a second experiment, we aim to test if the LGD whole-body odor induces fear in foxes, so the energy reward of available food should be less than the cost perceived of being injured or preyed upon due to the presence of the odor signal, and foxes’ GUD will be less when the odor is present compared to control stations.
If odor is perceived as a risk, it would reinforce the role LGD plays in protecting livestock, preventing predation and thus reducing chances of carnivore-livestock conflicts. In the first experiment, our goals were: (1) to measure differences in visit rates to experimental stations with and without whole-body odor in the different habitats, and (2) to estimate differences in time allocated to vigilance or foraging to analyze fear behaviors in the different situations proposed. In the second experiment, we aimed (3) to evaluate energetic costs by measuring GUD in scrublands with and without the treatment.
MethodsStudy AreaFieldwork was conducted in Anita Beatriz sheep farm, Riesco Island, Río Verde, Magallanes and Chilean Antarctic Region, Patagonia, Chile (52°52'01.2"S 71°33'16.5" W) during May, 2017 and May and June, 2018 (austral autumn, no lambs present). Anita Beatriz farm covers 729 ha, supports ∼1500 sheep and presents landscapes with different vegetation cover, including subpolar forests with mainly Nothofagus pumilio and N. antartica, scrubland with Berberis microphylla and Chiliotrichum diffusum as main species, and pastures with grass species for sheep consumption. Three Pyrenees shepherds and one Pyrenees mastiff have been used in this farm since 2009 as a management tool to reduce sheep predation by pumas and foxes, which allows evaluating risk perception behavior such as vigilance or food consumption, among others, of mesocarnivores who have previous experience with LGD in the area.
LGD whole-body odor extractionTo extract the whole-body odor of LGD, we followed Leo et al. (2015). Clean 100% cotton towels of 700 gr/cm2 were left for at least 1 month in LGD kennels, which allowed extracting odors from skin, saliva, feces, urine, dandruff and other substances that dogs excrete. Afterward, the towels were stored in airtight bags at -20 °C until use. As control, we used clean cotton towels stored in the same way. All towels were always manipulated with surgical gloves and masks to avoid contamination with other odors.
Habitat classificationWe assessed vegetation structure by measuring horizontal and vertical cover using a five-meter tape deployed in four cardinal points around the center of each randomly selected site. To quantify horizontal cover, we measured the total amount (in centimeters) in which the tape was covered by shrubs or trees. For the vertical cover, the height of the vegetation was measured every one meter of the tape. Vegetation coverage clearly differed between habitats (Kruskal–Wallis H = 23.9, p < 0.001). Scrubland has the widest horizontal coverage and forest has the highest vertical cover. Pastureland has no horizontal nor vertical vegetation cover (Figure S1).
Experiment 1Fox presenceTo test the effect of olfactory signals on patch usage, we installed experimental stations at 30 sites separated by at least 500 meters, 10 sites in forest, 10 in scrubland and 10 in pastureland. At each site, we set up an experimental station with four towel pieces of 12 × 12 cm. Due to the announced presence of snow, odor signals were placed inside white, square plastic dispensers with holes in the base and held by a 60 cm wood stake, so the sent disperse downwards and the towel stays dry. Because of the body size of the largest fox species in the area, approximately 1 m from head to the base of the tail; wood stakes were separated by at least one and a half meter, so stakes would not interfere with the foxes' passage. Finally, a container with 200 pellets of Purina Pro Plan® adult dog food for medium breeds, recommended by National Metropolitan Zoo professionals as an alternative to raw meat, was located at the center of each station (Figure S2). The stations were randomly assigned either to treatment (odor) or control (five or each). No stations were installed within the bushes or with bushes covering the dispensers. Therefore, there was no obstruction by bushes within the food patch. All stations had one camera trap set to film 60-second videos separated by at least 30 min, to reduce the probability of recording the same individual passing by. We consider videos separated by 60 min or more as independent events.
To allow fox habituation and avoid neophobia towards the stations (Travaini et al., 2013; Leo et al., 2015), empty plastic dispensers, the food patch and the camera traps were installed for 48 h before the experiment. From the third day on, towels with or without the whole-body odor were placed and renewed daily. With the same frequency, the food consumed in each container was measured and replenished, so it was available for the next 24 h. This procedure was carried out for 7 days in fields without livestock, to avoid the extra risk of predation on livestock due to the possible carnivore attraction.
To determine differences in visitation rates to stations, we counted the number of visits every 24 h and standardized it by the total operating time of each camera trap. A general linear model with negative binomial distribution was used to analyze the effect of habitat, treatment, horizontal vegetation cover, vertical vegetation cover and distance to the nearest field with livestock on the number of visits. Model assumptions were assessed using a residual diagnosis for hierarchical regression models with the R package DAHRMa (Hartig and Hartig, 2017).
Fox behaviorTo evaluate behavioral variation of foxes, an ethogram based on literature, field and video observation of fox behavior was conducted (Iriarte and Jaksic, 2017; Colque, 2018; Guntiñas et al., 2020). We included the total time an individual was seen in camera during a recording, and time allocated to foraging, vigilance (upright position, attentive, without eating, observing the surroundings), exploring (sniffing the station), marking (rubbing the body against ground or food container, or urinating), grooming (scratching or licking their fur) and resting, during that time in camera. Videos with carnivores rapidly passing by were excluded, because we cannot differentiate if the station was not detected or if it was being actively avoided. Some individuals were distinguishable by their body marks; however, since it was not possible to differentiate them all, we decided to consider videos with a minimum 60 min apart from each other separate events.
We used the Behavioral Research Interactive Software (BORIS) (Friard and Gamba, 2016) to keep track of time-recorded events. Parametric distribution and homoscedasticity of residuals were tested by applying a logarithmic transformation. To determine statistical differences in behavior in stations with LGD whole body odor and control stations, a multivariate analysis of variance (MANOVA) with treatment and habitat as factors was conducted, and a post-hoc test was used to examine further differences in behavior between habitats and between scented and unscented towels.
Experiment 2Food consumptionTo evaluate if LGD whole-body odor changes food consumption, we installed nine stations with the same design as Experiment 1, but only in scrubland. To assess the effect of whole-body odor on foraging, each experimental station had a food source (plastic container staked to the ground) with approximately 500 g (646 pellets) of Purina Pro Plan® Adult food for Medium Breeds, twice the suggested portion traditionally used for foxes in captivity, presuming free animals could have greater energy expenditure. Food choice was a recommendation from National Metropolitan Zoo professionals; we consulted them to find alternatives to raw meat, because of the effects of strong winds, rain and snow on meat weight. Additionally, we counted pellets as an alternative to estimate food weight. Food was renewed daily for eight days; during that period, food left behind on the previous night was collected, to establish the number of pellets not consumed as an approximation of GUD instead of food weight, because of the humidity absorbed in extreme weather conditions. We applied a log function to normalize residuals and performed a paired t-test to explore differences among odor treatments.
Fox presenceTo analyze the influence of odor treatment and vegetation cover within scrubland on fox presence, a General Linear Model with binomial distribution was used. Model assumptions were assessed using a residual diagnosis for hierarchical regression models with the R package DAHRMa (Hartig and Hartig, 2017). All statistical analyses were performed in the R platform (R Core Team, 2017).
ResultsExperiment 1Fox presenceA total of 729 videos were obtained in the 30 stations, of which 211 were of chilla, 9 of culpeo (220 videos with foxes pooled and considered in subsequent analyses), and 4 of puma (Figure S3). The remaining videos recorded native birds (e.g., Curaeus curaeus, Milvago chimango Caracara plancus), European hares (Lepus europaeus), native rodents, horses and LGDs. Only foxes’ videos were included in analyses, i.e. chilla and culpeo, and we did not register more than one fox in the same video.
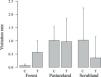
Fox visits to scrubland were 37.2% higher in stations without treatment, but in pastureland and forest, visits were higher in scented stations (Fig. 1). After the installation of the experiment, fox dens were found in the immediate vicinity of two forest stations, biasing fox visitation rate. Fox dens fulfill a crucial role in the survival and daily routines of foxes, providing shelter from predators, relief from extreme environmental conditions, and a secure place for resting (Moehrenschlager et al., 2004; Kanwar and Jaipal, 2023). The exclusion aims to mitigate potential biases linked to den-associated behaviors, ensuring that the observed responses of foxes to the olfactory treatments are not confounded by their natural inclination to seek proximity to their dens for safety and rest. Therefore, 67 visit records were removed from this analysis, and 153 visits records were considered.
Fox visitation rate was negatively affected by horizontal vegetation being lower in patches with higher horizontal vegetation cover (β = 0.97, z = −3.17, p < 0.01) (Table 1). Proximity to livestock had a significant positive effect on fox presence ( β = 1.01, z = 2.20, p < 0.01) (Table 1).
General linear model for fox visits to stations.
| Parameter | Estimate | SE | z-value | β coefficient | p-value |
|---|---|---|---|---|---|
| (Intercept) | −3.709 | 1.601 | −2.317 | 0.021 | |
| Habitat-Pastureland | −0.695 | 1.558 | −0.446 | 0.498 | 0.655 |
| Habitat-Scrubland | 2.227 | 1.447 | 1.539 | 9.276 | 0.123 |
| Treatment-Odor | 0.395 | 0.427 | 0.925 | 1.485 | 0.355 |
| Horizontal vegetation cover | −0.036 | 0.008 | −3.167 | 0.974 | 0.002 |
| Vertical vegetation cover | −0.011 | 0.003 | −0.413 | 0.998 | 0.679 |
| Proximity to livestock | 0.001 | 0.001 | 2.200 | 1.001 | 0.027 |
Time allocated to different behaviors was significantly influenced only by treatment (MANOVA: df = 1, F = 2.99, p = 0.05 [Table 2]). Vigilance was 1.33 times higher in patches with whole-body odor treatment than in unscented stations (df = 1, F = 5.60, p = 0.02) (Figure S4a). On other behaviors there was no significant effect although foraging absolute numbers suggest an apparent reduction (0.18 times lower at scented places), and there was a slight tendency to increase exploration and marking in the presence of the treatment (Figure S4b and S4c–d).
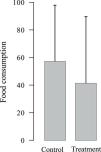
Experiment 2Food consumptionAverage food consumption showed a slight 27.7% decrease in stations with LGD whole-body odor (Fig. 2), but the difference between LGD odor treatment and control was not statistically significant (T = 0.44, p-value = 0.66). The non-significant tendency was probably due to small sample size, with only 6 food consumption counts for the LGD odor treatment and 6 counts for the control. In fact, the power of the test was only 0.39; at least two equal sized groups of n = 13 would be required to achieve a statistical power of 0.80.
Fox presenceFox presence was recorded at 9 stations, a total of 19 times during this experiment, with an average of 2.1 visit per station (min = 0, max = 6). The GLM analysis showed no evidence of odor treatments influencing fox arrival (β = 0.19; Z = 0.34; p > 0.05). Nevertheless, horizontal vegetation cover had a significant negative effect (β = −0.01; Z = −2.14; p < 0.05) (Table 1S) on fox visitation rate at experimental stations in scrubland (Fig. 3).
DiscussionLivestock guardian dogs could be acting as a large predator to foxes (Lima and Dill, 1990; van Bommel and Johnson, 2016; Crespin and Simonetti, 2019; Gaynor et al., 2019). Exposing foxes to LGD odor cues could trick them, making them suppose that the dog is near, inducing them to avoid places with the signal, and triggering a perceptual trap through the landscape of fear.
Foxes exposed to LGD whole-body odors seem to avoid habitats with the olfactory signal, being less frequent on patches with the treatment and higher horizontal coverage. This could be partially explained by the horizontal coverage, which interferes with the visual perception of the experimental station, but bushes are dense vegetation that may also favor the permanence of the fearsome signal in the near surroundings; and the obstruction of the fox’s view may make scrubland a riskier habitat (Thorn et al., 2012).
In contrast, the experimental stations in patches with no horizontal vegetation are more exposed, and foxes could be more likely to have a higher occurrence; because although they have flexible habitat use, they prefer open areas with greater visibility for hunting and fleeing from risky situations (Thorn et al., 2012; Iriarte and Jaksic, 2017; Santo Domingo et al., 2021). In addition, odors in these places could be lost faster that in areas with a dense horizontal coverage, affecting the effect of repellents to prevent fox arrivals.
Nevertheless, vegetation cover is a manageable variable in livestock production (Crespin and Simonetti, 2020); hence, we could control odor perception to enhance a riskier habitat through management of cover. As livestock breeding takes place in several vegetation configurations, and perception of olfactory signals depends on air flow, the efficiency of repellents depends on vegetation structure. This is important to consider, especially in areas with high horizontal coverage such as scrubland and forest with abundant undergrowth vegetation, where preliminarily, treatment presence could decrease mesocarnivore arrivals. A pending task is to explore more efficient formats to deliver the olfactory signal in an effective intensity and durability.
We conducted the experiments in places without livestock presence, but next to fields with sheep (between 0.25 and 2.34 km away from livestock) and fox presence increased with proximity to livestock. We believe there are three possible explanations for this increasing in fox presence: (1) livestock can act as an attractor for predators by increasing prey availability and accessibility (Trainor and Schmitz, 2014), yet we made the experiments during no-lamb season and most videos were of chilla, who only predate upon lambs, so we think there was not an actual increased prey availability or accessibility; (2) foxes might be being attracted by sheep carcasses, since the highest mortality occurs during winter due to low temperatures, but we did not observe carcasses next to livestock during the experiments; and (3) there was a difference in wild prey availability, which was higher in sites closer to sheep. Foxes are opportunistic carnivores and their presence is associated with prey and livestock (García-Solís et al., 2021). Lamb presence, carcasses and wild prey availability are important to explain fox habitat use and could be useful for feature experiments. Nevertheless, testing LGD odor as a deterrent in fields with livestock could control those possible confounding variables and add realism to the technique.
When foxes are present in scented stations, they are supposed to increase risk perception, allocate more time to fear behavior and reduce time spent on behavior that exposes them to higher risk (Lima and Dill, 1990; Suraci et al., 2016). Vigilance and exploration increase with the presence of LGD whole-body odor; they are more heedful to possible danger as an adaptive response to risk (Brown et al., 1999; Creel and Christianson, 2008). Our results show indications that foraging would be reduced by the LGD odor, so its potential use to discourage predation, by amplifying the perceived presence of LGD, should also be considered in future studies.
Despite the small sample size in Experiment 2, we also found that average number of visits per stations decreased with horizontal vegetation in scrubland, which is consistent with what was found during Experiment 1, but on a smaller scale. This would reinforce the idea that management of vegetation cover could be accompanied using olfactory signals to improve a landscape of fear for foxes, allowing management in a non-lethal way, but further studies are required to confirm LGD whole-body odor’s effect in foraging costs and habitat selection in Patagonia. This region is a challenging environment because of climate conditions; dog pellets have easy storage and transportation but were not easy to manipulate due to environmental moisture. In future studies we suggest adapting the containers so that, as much as possible, they do not retain excess moisture as in Diserens et al. (2022), or using alternative foods, suitable for counting in units or that might better moisture absorption resist and function as s bait to reach an adequate sample size to analyze GUD, we would also suggest extending the time of the experiment to face different climatic conditions and replicate it in different seasons. We believe that Jensen et al. (2023) show a suitable design for carcass deployment in behavioral experiments, which could be weighed to measure GUD and also implies a higher cost to the act of foraging compared to dog pellets, adding reality to future experiments to test this technique.
Other studies suggest that mesocarnivores respond differently to large carnivore whole-body odor in different contexts. At sheep-raising ranches in Australia, Leo et al. (2015) found that dingo whole-body odor changes foraging and drinking behaviors of red foxes, and suggest that dingo odor gives a perception of a potentially dangerous encounter to foxes. In contrast, in one of the best-preserved forests in Europe, Diserens et al. (2022) found that wolf whole-body odor mainly does not inspire fear in mesocarnivores. Racoon dogs (Nyctereutes procyonoides) increased GUD when the odor was present only at locations where the encounter rate with wolves was high and red foxes did not show variation in foraging cost when the odor was present, suggesting that the simulation of large carnivore presence with odor cues is context-dependent. The context of our study was similar to that of Leo et al. (2015), but simulating the presence of a large carnivore that do not facilitate mesocarnivores by carrion provisioning, so foxes did not have to face the trade-off between risk avoidance and food acquisition (Lima and Dill, 1990). We expected LGD odor to induce fear because foxes do not associate dogs with carrion, and the same should happen in livestock farms with mesocarnivore predation problems. Livestock conditions must be considered as well; younger domestic animals suffer more predation (Moreira-Arce et al., 2018), and birth time is especially risky due to predation by mesocarnivores (Thorn et al., 2013; Gervasi et al., 2014), so testing this technique during the birthing season could determine if LGD whole-body odor is a good non-lethal option to avoid lamb predation by mesocarnivores.
LGD whole-body odor as a management technique should be used with some considerations; prior experience with an LGD is also important, besides landscape features like vegetation cover. Naïve animals could not respond with antipredator behaviors to this new olfactory cue (Cox and Lima, 2006; Carthey and Banks, 2016). Another consideration should be the freshness of the treatment; we renewed the treatment every day, so our results do not encompass this factor. Fresh cues could be indicating predator proximity, and if they are not renewed constantly, the effect of predator whole-body odor could decrease (Bytheway et al., 2013). Finally, if this technique is used without LGD presence, habituation could be a problem. Thompson (2009) mentioned this important characteristic as the eighth of nine parameters of habituation: “Presentation of another (usually strong) stimulus results in recovery of the habituated response (dishabituation)”. We propose that LGD is the strong stimulus that recovers the fear response, so this technique would be acting as a risk perception effect amplification along with LGD, not as a single technique to increase fear.
Foxes with previous experience with LGD showed signs of fear with a single signal, indicating LGD could be around without requiring their presence, perceiving high predator density, and proving that affects fear response on subordinate species (Scheinin et al., 2006; Shapira et al., 2008; Gaynor et al., 2019; Ugarte et al., 2021). Therefore, we are possibly creating a perceptual trap to conflictive foxes in a livestock farm, which could play an important role in habitat selection for these mesocarnivores and result in a decrease of the likelihood of encounter between livestock and these predators (Gilroy and Sutherland, 2007; Karlsson and Johansson, 2010; Patten and Kelly, 2010). This possible perceptual trap would be reinforcing the effectiveness of LGD, which also deter hares (Ugarte et al., 2021), creating a landscape of fear for both predators and livestock competitors.
Creating perceptual traps through the landscape of fear offers a non-lethal technique that could avoid or decrease livestock predation, amplifying LGD effects in places that these dogs do not cover, reaching livestock that moves away from the herd and the dog is not able to protect, especially in bush environments. The whole-body odor could be useful as a complementary-amplifier non-lethal technique to the use of LGD, preventing livestock predation. With this management technique we can contribute to diminish carnivore hunting, aiming to reduce livestock-carnivore conflict.
Authors’ contributionCSU, CS and JAS conceived and designed this investigation. CSU and CS performed the data collection and analyzed the data. CSU, CS and JAS wrote the paper.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are grateful to: (a) Gabriela Simonetti-Grez and Gregor Stipicic for their hospitality; (b) Estancia Anita Beatriz for allowing us to do the fieldwork inside the farm; (c) Programa de Apoyo a la Investigación from the Facultad de Ciencias, Universidad de Chile for their support; (d) ANID and the National PHD scholarship 21220797 to CSU (2022-2026) and; (f) ANID and the Fondecyt Regular 1220424 to JAS for their partial support. This research is part of the Program ‘Ganadería sustentable’ of Asociación Kauyeken.