Low seed abundance associated with effect of biological control agents in an invasive shrub Acacia cyclops may limit its seed banks and further spread of the remaining seed crop in the South-western Cape, South Africa. However, there is a limited knowledge on how a reduced seed abundance, and vegetation cover which is positively correlated to seed bank size, affects patterns of granivory in A. cyclops stands. To fill this knowledge gap, granivory rates were measured using seed exclosure cages located both in closed and open A. cyclops tree canopy covers at Langebaanweg. Fresh seeds of A. cyclops were presented in tens per cage, and monitored in four-hour intervals of the day during the seeding season (December–July, 2013). Seed removal by rodents (74%) was not affected by vegetation cover suggesting an increased demand of the scarce seeds of A. cyclops. Conversely, seed removal by invertebrates (16%) was lowest among treatments, and was restricted in low tree canopy cover possibly due to competition for seeds under shady canopy. About 10% of the remaining seeds were consumed by vertebrates during the afternoon times associated with limited dispersal chances. In combination, biological control agents and rodents’ seed predation may effectively reduce seed banks of A. cyclops and invasion of this species in South Africa.
Invasive trees and shrubs represent a major threat to natural resources in South Africa (Richardson & van Wilgen, 2004; Gaertner et al., 2009), and their currently increasing numbers (Richardson & Rejmánek, 2011), suggest that their threat might intensify in future. Of the approximately 70 Acacia tree species that have been introduced into South Africa, predominantly during the 19th Century, at least 13 are currently recognised as invasive (Henderson, 2007; Richardson et al., 2011). Of these, Acacia cyclops commonly known as Rooikrans, was introduced into South Africa in 1835, primarily for stabilisation of sand dunes in the south-western Cape region (Shaughnessy, 1980). It has since become naturalised and has invaded coastal regions across the southern Cape (Henderson, 2007). The proliferation of A. cyclops in South Africa is ascribed to it having been widely propagated as a source of fuel wood for domestic and small-scale commercial purposes (Richardson et al., 2011), and to its prolific seed production (Milton and Hall, 1981; Impson et al., 2011) which increases chances of dispersal by vertebrates. Each seed has a nutritional and orange-reddish aril which attracts vertebrates, especially birds that consume and pass the indigestible seeds and thereby enhance the population dynamics of the plant (Glyphis et al., 1981; Underhill and Hofmeyr, 2007; Mokotjomela and Hoffmann, 2013). Indeed, recent study has shown that several bird species including selected ubiquitous dove species improve germination rates of ingested seeds (Mokotjomela et al., 2015). The seeds are also buried underground in accumulations by ants (Holmes, 1990) and possibly seed-hoarding rodents (Midgley and Anderson, 2005), and thus reducing impacts of predation and incineration of seeds during periodic fires. Holmes (1990) reported that A. cyclops seed banks tend to increase with vegetation cover and thus suggesting dense foliage and tree canopy limit predators’ access for seeds.
Biological control agent species including Melanterius servulus (a seed-feeding weevil) and Dasineura dielsi (a flower-galling midge) have been released on A. cyclops in South Africa in 1991 and 2002, respectively. Both agents were imported from Australia (the native range of A. cyclops), and they are prolific, causing high levels of seed damage in A. cyclops which in turn is expected to limit the invasiveness of the species (Impson et al., 2011; Moran et al., 2013). For instance, M. servulus inflicts seed damaged of up to 97.5%, with an overall average reduction of seed production of 56% between 1998 and 2009 in the South-western Cape (Impson et al., 2011). An earlier study at Koeberg Nature Reserve during the 2012 seeding season showed that invertebrate (e.g. ants) removal of seeds was much lower under dense stands of A. cyclops (Mokotjomela and Hoffmann, 2013) than in the 1980s (Holmes, 1990). However, it is not known how seed reduction imparted by the biological control agents has affected patterns of granivory by other organisms that potentially influence recruitment processes in A. cyclops.
Foraging patterns in vertebrates, especially rodents and other organisms, are influenced by degree of food availability (Bartness and Albers, 2000) and environmental temperatures (Bartness and Albers, 2000; Feldhamer, 2007; Orrock and Danielson, 2009). Consistently, the frugivorous birds in the Mediterranean climate region in South Africa foraged on exotic species with abundant fruit resources (Mokotjomela et al., 2013). Also, foraging patterns of the murid rodent, Ice rat (Otomys sloggetti) in the Drakensberg Alps was restricted to warm parts of the day (Hinze, 2005) while the desert rodents in southern Africa choose to forage during cooler times to avoid thermal stress (Feldhamer, 2007). Because vegetation cover may change the habitat quality for seed predators by modifying, for example, the microclimate (e.g. light, temperature) and soil characteristics (e.g. plant litter), and the risk of predation (Manson and Stiles, 1998), granivory rates tend to be higher under cover than open areas (Meiss et al., 2010). In addition, seed removal and dispersal by ants tend to be more common in hot and semi-arid areas in South Africa than in mesic areas (Bond and Slingsby, 1983). Feeding times of the day on seeds (i.e. morning, midday and afternoons) have important influence on potential dispersal of the ingested seeds in birds (Bibby et al., 2000; Kays et al., 2011), with the seeds of the Panamanian nutmeg tree (Virola nobilis), for example, consumed in the mornings by birds standing a greater chance of dispersal than those consumed later in the day (Kays et al., 2011). Furthermore, feeding peak time in the afternoon have disadvantage since the ingested seeds are then deposited at the roosting sites where seed mortalities are high due to predators and competition between seedlings (Westcott et al., 2005; Kays et al., 2011; Mokotjomela et al., 2013).
Despite the knowledge that birds are important seed dispersers of the highly invasive A. cyclops in South Africa (Glyphis et al., 1981; Underhill and Hofmeyr, 2007; Mokotjomela and Hoffmann, 2013; Mokotjomela et al., 2015), there is a limited knowledge on the temporal patterns of granivory in A. cyclops stands that have seen seed reduction due to biological control agents, and how habitat characteristics such as vegetation cover may influence the patterns of granivory. Granivory of seeds of A. cyclops has important implications on seed banks dynamics which reportedly account for its invasion success, since a larger proportion of the seed crop falls on the ground when pods dry up (Holmes, 1990). The major aim of the present study was to determine how granivory rates in A. cyclops are affected by vegetation cover during different phases of seeding period associated with variable abundance of seeds, and different times of day, which have implications for seed dispersal probabilities. This study tested predictions that rodents’ granivory might be higher in seed cages located under dense tree canopy cover, where predation risk is relatively lower than in those seed cages located in open and low vegetation cove, and that invertebrates’ seed removal might predominate due to semi-arid to arid conditions of the study site.
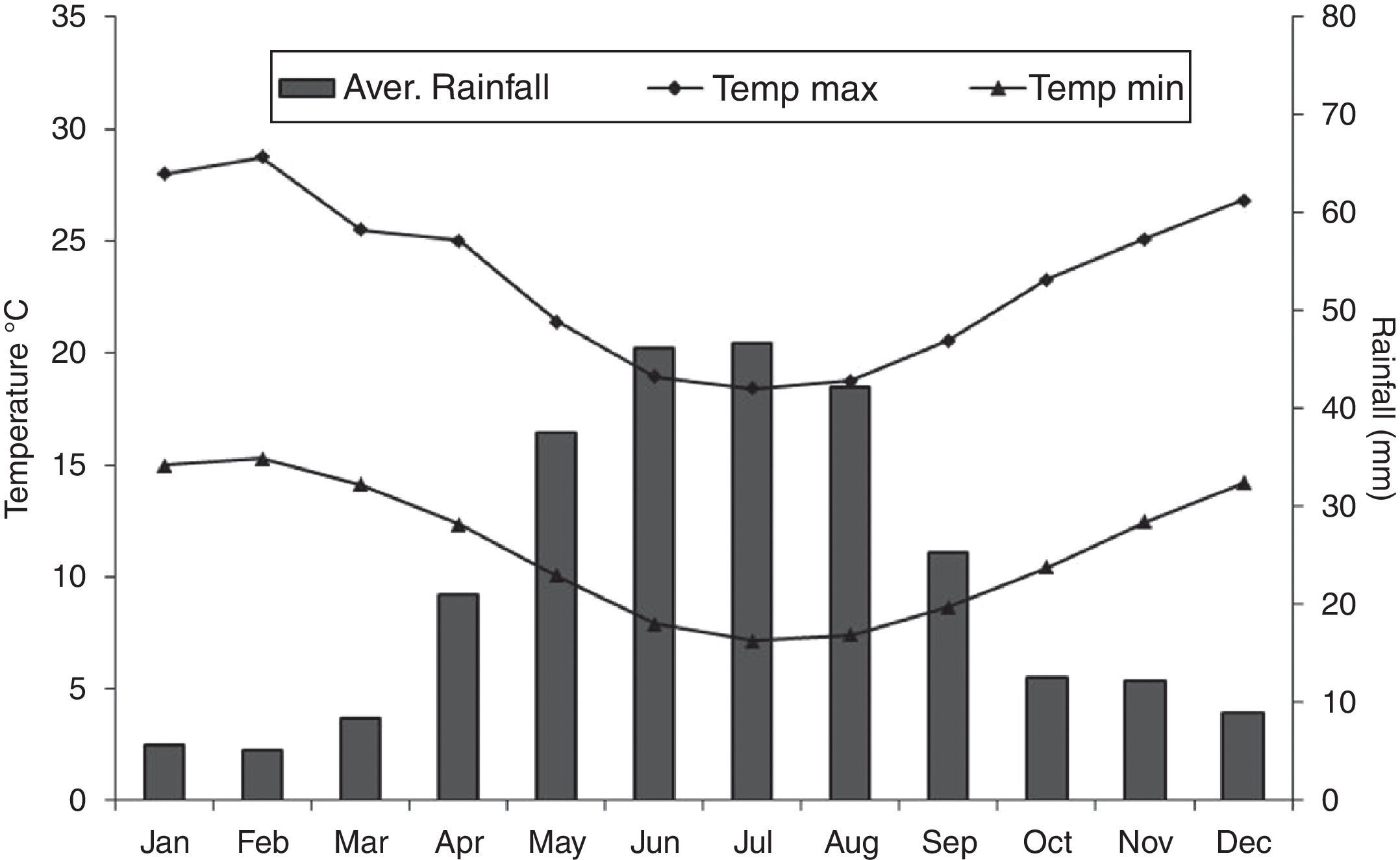
Material and methodsStudy siteThe study area of Langebaanweg (32°57′52.52″ S, 18°7′4.51″ E) is located in the Cape Floristic Region (CFR) of south-west South Africa, recognised as one of the most biologically diverse regions on earth (Goldblatt and Manning, 2002), and thus there is a conservation concern as this region is threatened by invasive trees and shrubs. The predominant vegetation in CFR is a shrubland known as fynbos, which comes from an Afrikaans word meaning “fine bush”. Fynbos primarily comprises four growth forms, namely proteoid, ericoid, restoid and geophyte (Cowling et al., 1996). Langebaanweg is located at the component of the fynbos dominated by dune thickets, although this has been heavily transformed by anthropogenic activities. The climate is Mediterranean with 75% of annual rain falling between April and September, although there is a sharp drop in the average rainfall towards the northwest coast, and thus Langebaanweg is classified as semi-arid (see daily temperature and rainfall variation; Fig. 1).
At Langebaanweg, plants grow in the old phosphate mine dumps where they were originally planted as part of rehabilitation programme for the disturbed landscape. The mean height of adult trees was 5.9±0.3m (n=28) with the canopy cover of approximately 80% in the forest and less than 50% outside the forest. The tree canopy cover was estimated using a modified Braun-Blanquet scale taking into consideration tree branches’ cover per unit area relative to the location of the individual seed trap (Hill et al., 2005).
Seed removal in A. cyclopsSeeds that were used for feeding trials were harvested from A. cyclops populations at Langebaanweg. Exclosure cages were used to measure removal rates of seeds by different combinations of invertebrates, rodents and birds. Each cage covered a 0.25m2 area of the soil surface and was 5cm high. The cages consisted of metal frames which were covered with different configurations of mesh to allow different levels of access to seeds. To expose seeds to invertebrates only, the sides and top of the cages were entirely enclosed in mesh. To expose seeds to rodents and invertebrates but exclude birds, the top and two opposite sides of the cage were enclosed with mesh, leaving two sides open. To determine combined seed removal rates by birds, rodents and invertebrates together, the patch was left uncaged and therefore ‘exposed to all’, which was also used as experimental control.
In preparation for placement of seeds, both within the caged exposure and in the ‘open to all’ treatment, a sheet of polyester gauze ‘shade cloth’ was laid out and covered with a 2cm thick layer of sand. This formed an isolating layer which ensured that extraneous seeds from the seed bank already in the sand did not become incorporated in the trials, and that placed-out seeds that became covered with sand could be recovered before counts were made at the end of the exposure periods. The cages were pegged down within the patch of sand covering the shade cloth.
Ten sets of cages were placed at 10m intervals along a 100m long transect located under tree canopy cover of A. cyclops thickets, and in low vegetation cover areas at the periphery of the thickets. Each set comprised an ‘exposed to all’, an ‘exposed to invertebrates only’, and an ‘exposed to rodents and invertebrates’ seed traps, placed 3m apart. At the start of each trial, 10 arilate seeds were uniformly spread within the confines of each cage and in the ‘open to all’ seed trap. Seed removal was monitored at four-hour intervals starting from sunrise (morning, midday and afternoon) over three consecutive days during different phases of seeding season (beginning, middle, late and end of seed production). Overall, at least 300 seeds were placed out every day. Since the length of the fruiting season for A. cyclops spans ∼ eight months in South Africa (Mokotjomela et al., 2015), and because seed abundance changes progressively with the season, the season was divided into two-month seeding phases. Seed removal was defined by the difference between the original number of seeds and the number of seeds remaining after five hours. After the counts were made, fresh seeds were added to the trap to replenish those that had been removed and to start each four-hour monitoring period with a full complement of 10 seeds (Mokotjomela and Hoffmann, 2013).
To determine seed removal by rodents only, the numbers of seeds removed from the ‘exposed to invertebrates only’ treatment was deducted from that of the ‘exposed to rodents and invertebrates’ treatment. To determine seed removal by birds and large mammals, the numbers of seeds removed from the ‘exposed to rodents and invertebrates’ treatment was deducted from that of the ‘exposed to all’ treatment (Mokotjomela and Hoffmann, 2013).
Statistical analysesBecause of pseudo-replication and unbalanced sample sizes and thus inequality of variance, data were analysed as the averages of individual treatment replicate using a General Linear Model Analysis of Variance (GLM – ANOVA) in SPSS version 22.0 (IBM Corp., Armonk, NY, USA), and the Type 3 sum of squares were used. Variation between the means of seed removal rates of A. cyclops from different seed cages located under and outside tree canopy cover was measured, spanning different phases of seed season that were measured at four-hour intervals of the day (i.e. designated the morning, midday and afternoon). The percentage numbers of seeds consumed from different treatments was fitted as response variables, while vegetation cover, seeding phases, times of the day and treatments were fitted as predictor variables. The Dunnett post hoc test was used to distinguish significantly different seed removal rates between two treatments (i.e. “exposed to rodents” and “exposed to invertebrates only”) with the experimental control represented by a treatment designated “exposed to all”. The Duncan's post hoc test was applied to distinguish significantly-different mean percentage seed removal rates during different seeding phases and different time of the day. Means are presented ±1 se.
ResultsRemoval of A. cyclops seedsOverall, there was a highly significant difference between the treatments and the experimental control “exposed to all” (F(2, 717)=66.7; p<0.001). The Dunnett post hoc test showed that seed removal from “exposed to rodents” cages and the experimental control were non-significantly different, but greater than the amount of seeds removed by invertebrates. Vegetation cover did not have a significant influence on seeds removal rates from three different treatments (F(1, 216)=0.5; p=0.461). However, both the seeding phases and time of the day had significant effects with Duncan's post hoc test showing greatest seed removal during the late phase of the seeding period (F(3, 216)=5.6; p=0.001), and during the afternoons (F(2, 216)=11.0; p<0.001). There were significant two-way interactions between vegetation cover and phase seeding season (F(3, 648)=18.9; p<0.001).
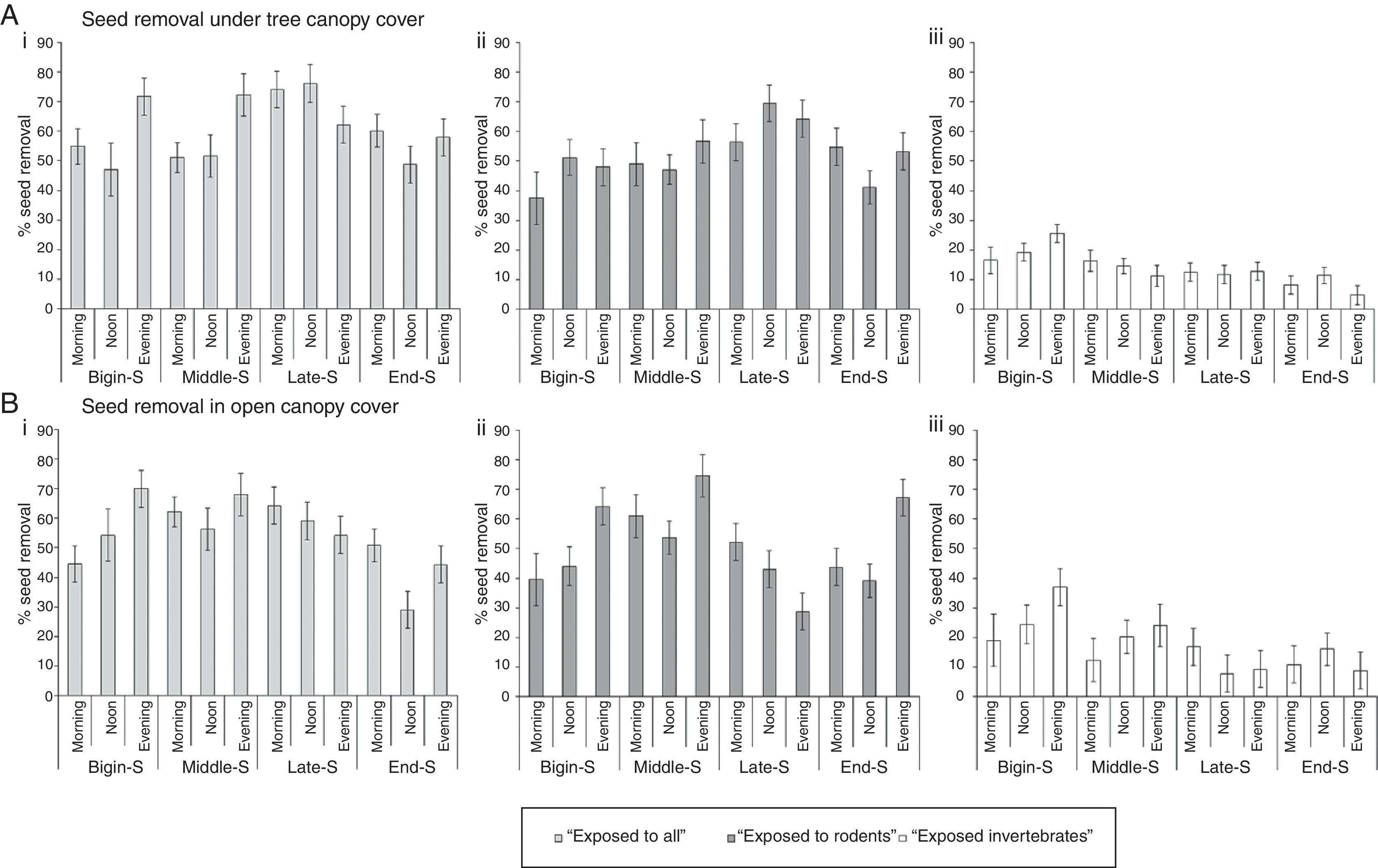
There were non-significant differences in the effects of vegetation cover on seed removal from the cages accessed by all organisms (‘exposed to all’), and those accessed by rodents (‘exposed to rodents’) (F(1, 216)=2.8; p=0.095; F(1, 216)=0.74; p=0.390; Fig. 2A – i, ii and B – i, ii), respectively. However, invertebrates (‘exposed to invertebrates only’) removed significantly greater number of seeds from the cages under the sparse tree canopy than under the dense tree canopy (F(1, 216)=13.6; p<0.001; Fig. 2A – iii and B – iii).
Variation in % mean (±SE) ground seed removal rates for A. cyclops from traps accessed by all organisms (‘exposed to all’), rodents (‘exposed to rodents only’) and invertebrates (‘exposed to invertebrates only’) under tree canopy cover (A – i, ii and iii) and in open canopy cover (B – i, ii and iii) during different phases of the seeding season: beginning of seeding (Begin-Seeding – December-January), middle of the season (Middle-Seeding – February-March), late seeding (Late-Seeding – April-May), and end of seeding (End-Seeding – June-July). Seed removal was measured at Langebaanweg. Error bars represent the standard errors of means.
For cages accessed by all organisms and rodents, seeding phases had a highly significant influence, with greater numbers of seeds removed during the late phase of the seeding period (F(3, 216)=13.5; p<0.001; F(3, 216)=5.3; p=0.001; Fig. 2A – i, ii and B – i, ii) and in the afternoon (F(2, 216)=7.5; p=0.001; F(2, 216)=5.3; p=0.005; Fig. 2A – i, ii and B – i, ii). Similarly, both the seeding phases and the times of the day had a significant influence on invertebrate activity, with greater numbers of seeds being removed during the beginning and middle of the seeding phase (F(3, 216)=4.7; p=0.003), and during the noon to afternoon times (F(2, 216)=8.7; p<0.001; Fig. 2A – iii and B – iii).
Rodents, ground foraging birds and large mammals, and invertebrates removed 74%, 10% and 16% of presented seeds of A. cyclops respectively.
DiscussionSeed consumption by vertebrates has important implications on seed fate and recruitment in plants (Nathan and Muller-Landau, 2000), and thus understanding granivory rates might be important for conservation management where invasive alien plants threaten indigenous species. This study investigated how vegetation cover affected granivory patterns and rates over different phases of the seeding season (e.g. bi-monthly) and times of day in an invasive shrub A. cyclops stands under biological control in the South-western Cape, South Africa.
Contrary to the study prediction, vegetation cover did not influence seed removal by vertebrates including rodents. An equivalent seed removal by rodents both under and outside tree canopy might reflect a limited seed availability, associated in part with biological control effect (Impson et al., 2011), and partly due to competition for seeds resource by other organisms whose foraging activity is restricted in the forest either due to fear of predation or extreme temperatures during the daytime. Thus, rodents seeking seeds extend their foraging range beyond the forest canopy and thus confound the reported effects vegetation cover on their foraging patterns (Meiss et al., 2010). The relatively low seed removal by rodents during the early phases of the seeding period in this study is a likely result of high abundance of A. cyclops seeds, of which Holmes (1990) reported to be causing predator satiation. It is therefore logical that the peak seed removal by rodents occurred during late phases of the seeding period when the number of available seeds was declining.
In spite of the biological control agents that have substantially reduced reproductive output in A. cyclops stands in South Africa, seed removal and dispersal still occur (Mokotjomela et al., 2015). This report was confirmed by the finding that rodents (e.g. striped mice Rhabdomys pumilio), which are key seed consumers of A. cyclops (Holmes, 1990; Mokotjomela and Hoffmann, 2013), removed fewer seeds than were removed from the experimental control seed cages. Indeed, additional organisms including birds and large mammals that are known to be effective seed dispersal agents were documented feeding on A. cyclops post-dispersal seeds at Koeberg Nature Reserve (Mokotjomela and Hoffmann, 2013). Nevertheless, birds and mammals removed relatively fewer seeds (10%) compared to a previous report showing 15% removal (Mokotjomela and Hoffmann, 2013). A possible explanation is high competition for scarce seed resource in combination with low abundance of granivorous organisms imparted by the heavily fragmented local environment at Langebaanweg. This finding also suggests that a limited number of seeds might be dispersed by birds from A. cyclops stands having seed production reduced by biological control agents (Mokotjomela et al., 2015). Removal of seeds during the cooler afternoon times might limit long distance dispersal, and increase mortality, since Kays et al. (2011) reported that birds which are effective dispersal agents, tend to be most active in the mornings.
That invertebrates removed the fewest seeds among the treatments, and that this happened in seed cages located in open/sparse canopy cover, contradicts the second study prediction that invertebrates’ seed removal especially by ants would be predominant due to prevalent aridity of the Langebaanweg (Bond and Slingsby, 1983), and a previous study (e.g. Holmes, 1990) showing that ants remove more seeds under dense vegetation cover. This finding could be explained by interference competition by multiple organisms taking shade cover under the trees during extremely hot daytimes. Trampling of the seed aril which is a target resource for ants, by larger organisms (Mokotjomela and Hoffmann, 2013), might render the aril unfavourable to ants. Also, the seed aril deteriorates rapidly due to microbial effect in relatively moist ground conditions under dense canopy cover (Meiss et al., 2010; Fricke et al., 2013), thereby losing chemical compounds which Midgley and Bond (1995) suggested play an important role in attracting ants and thus mediating their seed removal activity in Acacia species. To confirm the importance of fresh nutritious A. cyclops’ seed aril to ants and other invertebrates, greater numbers of seeds were removed during the beginning of the seeding phase (i.e. after pods’ opening) in this study.
In conclusion, the study has shown that uniform granivory patterns of rodents under closed and open canopy covers might be an indication of reduced seed production. This, however, contradicts reported trends showing importance of vegetation cover as refugia for foraging rodents (Andrzejewski, 2002; Meiss et al., 2010). Because a large portion of A. cyclops seed crop falls on the ground, where dispersal and ground seed bank recharging occur (Holmes, 1990), the observed high rodents’ seed predation may be a limiting factor to both processes, and thus complementing the biological control agents. Seeds of A. cyclops contribute more than 50% of the daily stripped mice's diet in the South-western Cape (Holmes, 1990). Alternatively, invertebrates continue to constantly remove and bury seeds, since different studies (e.g. Holmes, 1990; Mokotjomela and Hoffmann, 2013 and this study) have reported equivalent seed removal rates of approximately 13–16% at Table Mountain and Koeberg Nature Reserve and Langebaanweg respectively. Taken together, these studies suggest that ants’ seed dispersal at least for A. cyclops might not be significantly influenced by environmental conditions, including moisture and temperature. Although these results are site-specific, they provide preliminary highlights on success of biological control method of controlling invasive alien plants in South Africa. Indeed, Richardson et al. (2011) predicted that Australian acacias have potential to invade about a third to global terrestrial environment and threaten biodiversity of which suggest they require stringent management and control approaches such biological control agents to pro-actively suppress their invasions where they are being used for commercial purposes.
Conflicts of interestThe author declares no conflicts of interest.
TMM is funded by the Global Change and Sustainability Research Institute, University of the Witwatersrand. National Research Foundation (NRF) and University of Cape Town (URC) provided funding during data collection. Fossil Park managers at Langebaanweg also provided access to the study site. Prof. John Hoffmann provided insightful initial comments on the manuscript. David Maphisa, Tsepo Sefako and Toka Mokotjomela assisted in collecting data.