Many locally and regionally rare species are not covered by red lists, thus compromising conservation strategies. This is the case with ecotones. After applying three rarity criteria based on both geographic range and on local occurrences to 1755 species of a large transitional zone in South America, we discuss how the priority hierarchy found in the study region can be combined with red books in decision-making to reduce the gaps left by the classification systems adopted by these lists. We point out clear directions about how these species can be used to guide decision making in ecotones, including identifying species of interest for conservation that have not yet been included in red lists, structuring a species group of narrow distribution occurring in areas adjacent to ecological transitions into a hierarchy of priorities for conservation, and using species of the highest hierarchy position in decision making. We believe that the combination of regional lists with national and international red lists is an interesting strategy in the management of species for conservation.
Uncertainties embedded in international classification systems of species, such as the system adopted by IUCN (Akçakaya et al., 2000), can compromise the selection of species of local and regional importance (Lõhmus, 2015). Therefore, alternative systems can be used to select regional priorities (e.g., Gauthier et al., 2010). Among them, the method proposed by Gauthier et al. (2010) is very simple and practical for the evaluation of different types of plant rarities. Basically, this method consists in drawing up a list of priority species for regional conservation from three priority criteria, named, Regional Responsibility, Local Rarity and Habitat Vulnerable. To operate these criteria, the authors propose a scale with five priority classes, in which the scores range from 1 to 5, where 1 means species of lowest priority and 5, species of highest priority. The resulting product is a list of species hierarchically organized according to the degree of priority for conservation. This system has the advantages of (1) flexibility of spatial scale, (2) selecting different forms of species rarity, and (3) being of easy application (Gauthier et al., 2010). This third point is particularly important in reducing the gap that exists between the studies developed in the area of conservation and its practical application, commonly known as the “Knowing-doing gap” (Habel et al., 2013). Furthermore, the classification systems based on regional and local priorities select species that are not covered by international initiatives and combine the results of different systems to cover the largest number of species for conservation management (Mehlman et al., 2004), thereby constituting a powerful strategy. This possibility becomes even more attractive to be used in regions that do not have studies of endangered flora, for example, regions of ecotones and ecological transitions.
Although transitional regions are sources of diversity and evolutionary novelties, they are neglected by conservation policies (Smith et al., 2001). Our focus here is the transition that occurs between the two largest phytogeographic areas of South America, the Amazon and the Cerrado, which stands out due to its diverse flora (Marimon et al., 2006), low floristic similarity with its adjacent areas (Kunz et al., 2009), and an advancing pattern of the Amazon rainforest into the Cerrado (Marimon et al., 2006). Such issues highlight the importance of this transition in maintaining the biota of both phytogeographic domains (Françoso et al., 2016). However, this transition is the scene of an intense consolidated settlement process (Becker, 2005) and a policy focused on the exploitation of natural resources (Théry, 2005), factors that drive the extinction of rare species. Indeed, rare species with low population, small geographic range and that are restricted to specific habitats deserve special attention in conservation policies (Caiafa and Martins, 2010).
We assume that tree species of a community tend to differ in abundance, geographic range and habitat requirements (e.g., Caiafa and Martins, 2010); in such case, some species would present greater priority for conservation, while others would have lower priority. Otherwise, all tree species would simultaneously occur in their optimal distribution, an unrealistic scenario. Thus, our aim was to investigate how the tree species of the Cerrado-Amazon transition show a hierarchical priority structure for conservation when different forms of rarity are considered in the evaluation of these species. As this list of tree species of hierarchy priority is identified, efforts can be directed into areas that focus on tree species of highest priority for conservation.
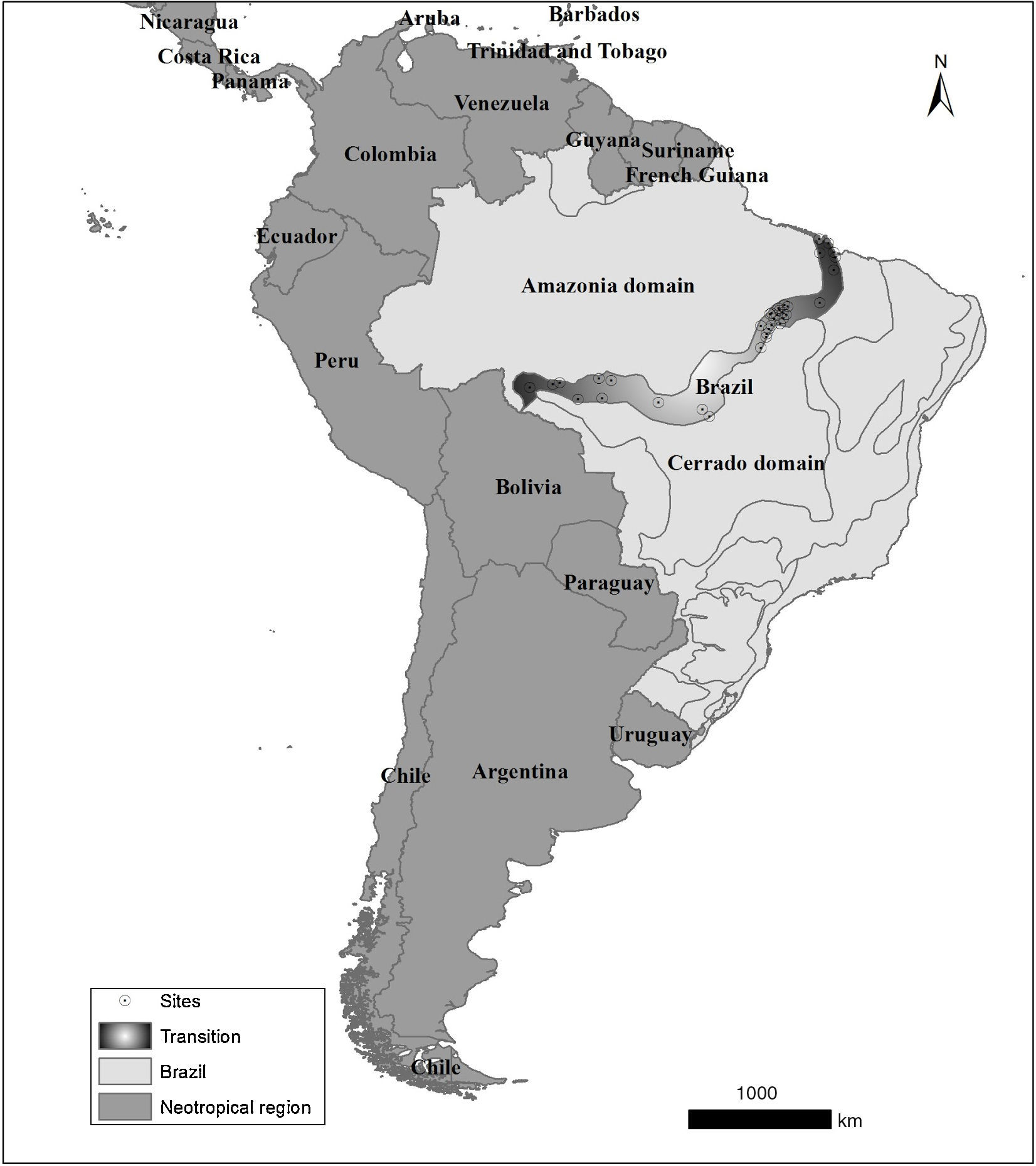
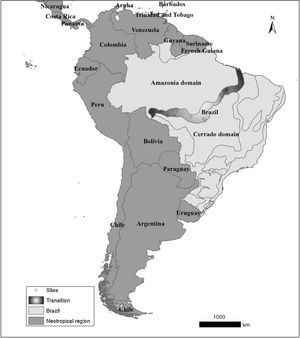
Material and methodsStudy areaWe used the definitions of Ab'Sáber (2003), thus considering the Cerrado-Amazon transition (hereafter, Transition) as part of the states of Pará, Maranhão, Tocantins, Rondonia and Mato Grosso. Records from the SNUC (‘Sistema Nacional de Unidades de Conservação’, the Brazilian system of protected areas) database indicate that the conservation units are unevenly distributed in the Transition - one can find 13 strictly protected areas and 19 areas of sustainable use (http://mapas.mma.gov.br/i3geo/datadownload.htm). Note that these conservation areas are concentrated in extreme portions of this region, specifically in the northern coast of Maranhão state and Rondonia state (Fig. 1).
DatabaseWe used the NeoTropTree database (Oliveira-Filho, 2014) for compiling the species occurring in the Transition (Table S1). The NeoTropTree contains records of native tree species for the entire Neotropical region, providing information for each species by sampling lists organized by sites with a 5-km radius. Each site corresponds to a vegetation type (savanna or forest). We considered the ecoregions that occur in the Cerrado and Amazon areas to verify the extent of the tree species in the Transition. We created a matrix for each site including: (a) occurrence points (geographical coordinates); (b) list of tree species; (c) type of vegetation; and (d) ecoregion.
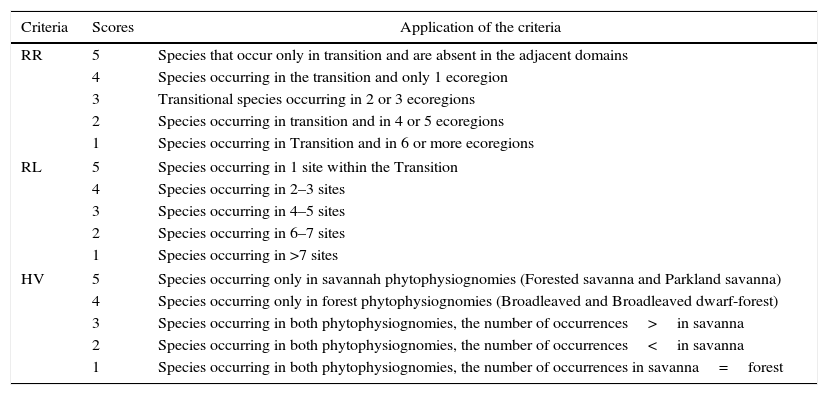
Weighting method for prioritizing speciesTo punctuate the tree species, we used a weighting method based on three priority criteria (Gauthier et al., 2010): Regional Responsibility (RR), Local Rarity (LR) and Habitat Vulnerability (HV). Each criterion was divided into five classes, and each class was assigned with a point value ranging hierarchically from isolated scores of 1 (lowest priority) to 5 (highest priority) for the three criteria assessed (Table 1).
Scores coupled with the three priority criteria, Regional Responsibility, Local Rarity and Habitat Vulnerability used in this study to define priorities for conservation.
| Criteria | Scores | Application of the criteria |
|---|---|---|
| RR | 5 | Species that occur only in transition and are absent in the adjacent domains |
| 4 | Species occurring in the transition and only 1 ecoregion | |
| 3 | Transitional species occurring in 2 or 3 ecoregions | |
| 2 | Species occurring in transition and in 4 or 5 ecoregions | |
| 1 | Species occurring in Transition and in 6 or more ecoregions | |
| RL | 5 | Species occurring in 1 site within the Transition |
| 4 | Species occurring in 2–3 sites | |
| 3 | Species occurring in 4–5 sites | |
| 2 | Species occurring in 6–7 sites | |
| 1 | Species occurring in >7 sites | |
| HV | 5 | Species occurring only in savannah phytophysiognomies (Forested savanna and Parkland savanna) |
| 4 | Species occurring only in forest phytophysiognomies (Broadleaved and Broadleaved dwarf-forest) | |
| 3 | Species occurring in both phytophysiognomies, the number of occurrences>in savanna | |
| 2 | Species occurring in both phytophysiognomies, the number of occurrences<in savanna | |
| 1 | Species occurring in both phytophysiognomies, the number of occurrences in savanna=forest | |
RR is characterized as a biogeographic criteria (Schmeller et al., 2008), and the relevance of each species is considered by comparing its geographic range in the region of interest to its occurrence in an area assumed as a reference. We used ecoregions (sensu Olson et al., 2001) that overlap the areas of the Cerrado and Amazon domains outside the Transition. Ecoregions comprise a set of natural communities and species that occur in a land portion which, when nested within a domain, provide a comparison structure between different units of habitats (see Olson et al., 2001 for more details on ‘ecoregion’ issues). We consider the tree species that occurred in a larger number of ecoregions as a species with broad distribution, thereby having lower priority because they tend to be less vulnerable to stochastic processes. On the other hand, tree species with distribution restricted to one ecoregion tend to be more vulnerable, and are generally considered as priority for conservation (Table 1).
LR is related to the frequency of a species within the region of interest, based on both the number of locations where the occurrence of the species is known and the local tree species abundance (Gauthier et al., 2010). We took the number of sites where the occurrence of the tree species was verified to score their frequency in the Transition. For Gauthier et al. (2010), the fewer the number of sites within a given region where the tree species of interest occur, the rarer this tree species is considered and, therefore, more points this species receives. In contrast, species that present many sites of occurrence are considered as common. Thus, the lower the number of Transition sites in which the tree species occurs, the more locally rare it was considered (Table 1).
HV reflects the risk of decrease of the population in response to events such as loss of habitat or habitat degradation (Gauthier et al., 2010). We considered tree species that occur only in savanna environments as those with highest priority for conservation because they are under-represented in terms of areas and are seen by decision makers as being of less value to the conservation of the forest vegetation types. One can see, for example, the current law for Native Vegetation Protection (Federal Law n° 12,651; see Brancalion et al., 2016), which values forest vegetation types more than savannas regarding the establishment of legal reserves. In addition, as a result of their common features, savannas are the phytophysiognomies that are most vulnerable to human pressures, such as grazing cattle and logging (Mazzetto, 2009). Next, we prioritized species that occur only in forest vegetation types and, then, prioritized the tree species occurring in both savanna and forest physiognomies (Table 1).
Data analysisWe calculated the different proportions of species for the three criteria evaluated in each priority class. For this, we used the chi-square test for proportional comparisons to test the weights of cells through different proportions of tree species in each priority basis, at 95% probability. In addition, the residuals of the test were calculated to demonstrate the importance of each probabilistic cell, since these residuals are obtained from the normalized data of the Gaussian curve. Adjusted residual values equal to zero indicate that the weight of the cell is not different from chance.
Next, we produced a list of priorities for tree species conservation whose weighted average of the three criteria was greater than or equal to four points. We chose four points as the threshold in order to select only the species that reached a large point value. Although the method might seem arbitrary, this was, in fact, necessary to separate the species that are above the average of three points from the others, which allows us to select the species that reach the highest point value. We assigned weight to both RR and LR criterion to select species of low amplitude out of the transition and that were locally rare (Gauthier et al., 2010). By means of the priority list, one can identify the species of highest priority, thereby allowing that conservation management be better directed to a group of tree species. Finally, we verified whether our selected tree species with highest priority for conservation had already been selected by some of the classification systems of endangered flora, which includes national (Giulietti et al., 2009; Martinelli and Moraes, 2013 and Martinelli et al., 2014) and international lists (IUCN, 2015). In cases where species have been identified by another system of classification, we highlighted such species because, as they already have a recognized threat status, they should also be recognized as regionally and internationally important.
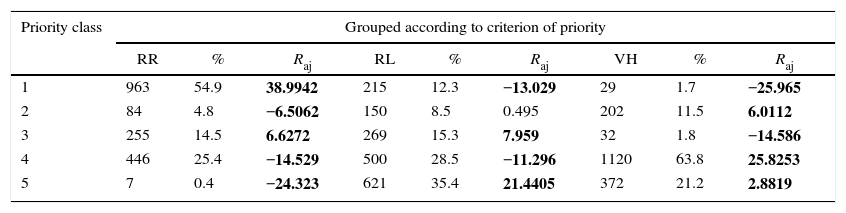
ResultsThe hierarchy of priorities – Our results suggest the existence of a hierarchy of conservation priorities, in which the number of tree species observed in each priority class is associated with the number of their occurrence in the different eco-regions outside the Transition (χ0.05;42=1659.288, p<0.0001), the number of Transition sites where the tree species occur (χ0.05;42=457.897, p<0.0001), and in different habitats (χ0.05;42=2334.64, p<0.0001). The criterion ‘Local Rarity’ had the highest contribution of species to the class with the highest priority, indicating that the locally rare species have the highest proportion among the analyzed criteria (Table 2).
Distribution of 1755 species analyzed in each priority class (1–5), their respective proportions (%) observed for the criterion of Regional Responsibility (RR), Local Rarity (LR) and Habitat Vulnerability (HV) and significant adjusted residuals (Raj), obtained at a significance level of 0.05, deviating more or less from the values expected by chance.
| Priority class | Grouped according to criterion of priority | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | % | Raj | RL | % | Raj | VH | % | Raj | |
| 1 | 963 | 54.9 | 38.9942 | 215 | 12.3 | −13.029 | 29 | 1.7 | −25.965 |
| 2 | 84 | 4.8 | −6.5062 | 150 | 8.5 | 0.495 | 202 | 11.5 | 6.0112 |
| 3 | 255 | 14.5 | 6.6272 | 269 | 15.3 | 7.959 | 32 | 1.8 | −14.586 |
| 4 | 446 | 25.4 | −14.529 | 500 | 28.5 | −11.296 | 1120 | 63.8 | 25.8253 |
| 5 | 7 | 0.4 | −24.323 | 621 | 35.4 | 21.4405 | 372 | 21.2 | 2.8819 |
Numbers in bold correspond to significant values.
RR – From the RR criteria we found that among the 1755 tree species that occur in the Transition, seven (0.4%) were not shared between the transition and the Cerrado and Amazon Domains. These are Alseis pickelii, Erythroxylum timothei, Clusia drouetiana, Retrophyllum piresii, Retrophyllum rospigliosii, Myrcia ilheosensis and Citharexylum krukovii. However, when analyzing the occurrences of these seven species throughout the Neotropics, i.e., in all Brazilian areas and outside Brazil, only E. timothei, C. drouetiana and R. piresii remained as tree species that only occur in the Transition. Tree species that occur in the Transition and in only one ecoregion beyond totaled 446 (25.4%).
LR – Tree species that occur in the transition and in two or three eco-regions totaled 255 (14.5%). The Local Rarity criteria were represented by a high number of species with a single case recorded within the Transition, i.e., species that are registered to a single location. Considering the 1755 species observed, 621 (35.4%) occurred only at a single site within the Transition (Table 2).
HV – Our assessment for Habitat Vulnerability indicates the existence of 372 tree species (21.2%) in the Transition which only occur in savanna physiognomies (savanna woodland or shrub-tree savanna), representing the category of highest priority for the analyzed criteria (Table 2). Following the hierarchy, one can see the tree species within the Transition that only occur in forest formations (broadleaved forests and broadleaved dwarf-forests), totaling 1120 species (63.8%).
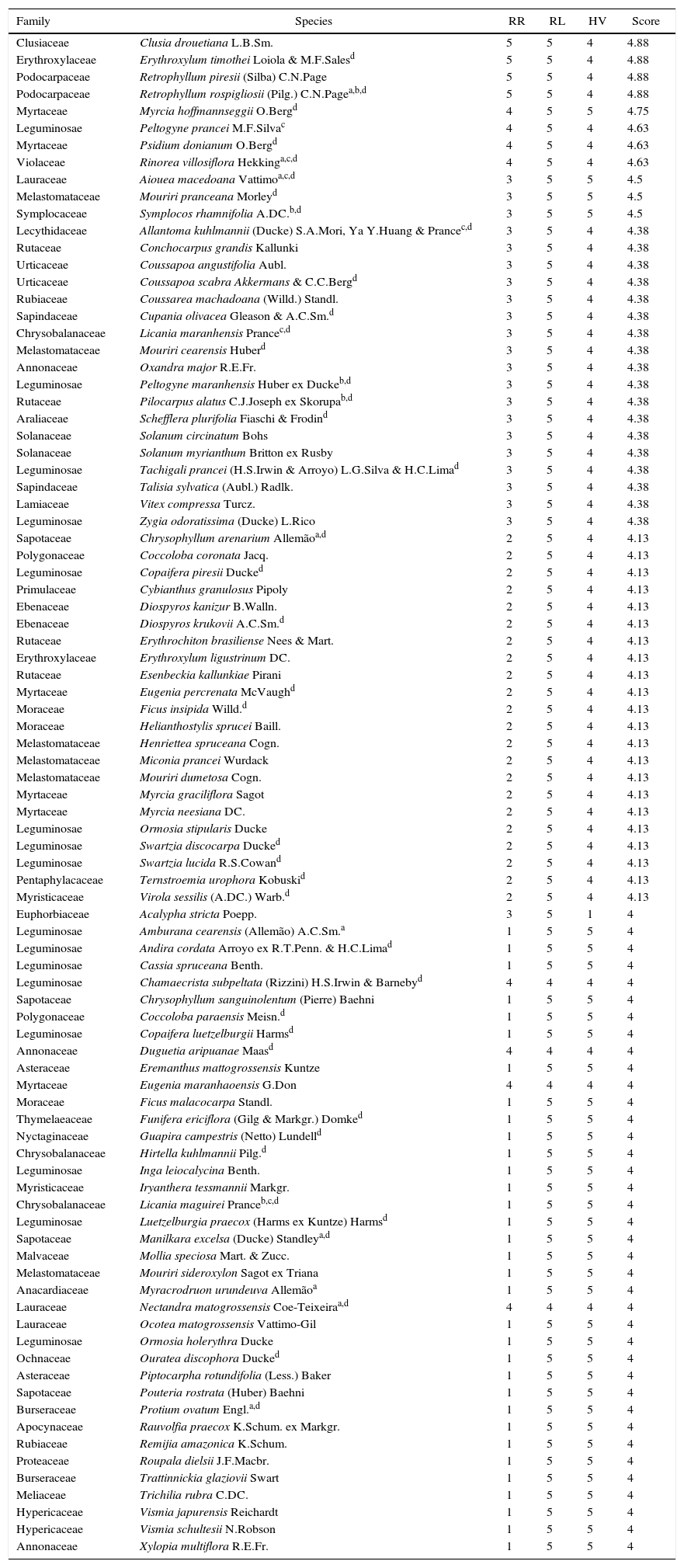
Our list of priority indicates that, from the 1755 tree species that occur in the Transition, 89 are likely to be regionally rare. Note that when the individual criteria scores are analyzed, some species showed a high score for a given criterion, but a low score for another criterion. Indeed, species such as Eremanthus mattogrossensis, for example, received five points for VH and LR, but only one point for RR (Table 3).
The 89 most important species for the conservation of the Amazon-Cerrado Transition, followed by scores achieved in each priority criteria, Regional Responsibility (RR), Local Rarity (LR) and Habitat Vulnerability (HV).
| Family | Species | RR | RL | HV | Score |
|---|---|---|---|---|---|
| Clusiaceae | Clusia drouetiana L.B.Sm. | 5 | 5 | 4 | 4.88 |
| Erythroxylaceae | Erythroxylum timothei Loiola & M.F.Salesd | 5 | 5 | 4 | 4.88 |
| Podocarpaceae | Retrophyllum piresii (Silba) C.N.Page | 5 | 5 | 4 | 4.88 |
| Podocarpaceae | Retrophyllum rospigliosii (Pilg.) C.N.Pagea,b,d | 5 | 5 | 4 | 4.88 |
| Myrtaceae | Myrcia hoffmannseggii O.Bergd | 4 | 5 | 5 | 4.75 |
| Leguminosae | Peltogyne prancei M.F.Silvac | 4 | 5 | 4 | 4.63 |
| Myrtaceae | Psidium donianum O.Bergd | 4 | 5 | 4 | 4.63 |
| Violaceae | Rinorea villosiflora Hekkinga,c,d | 4 | 5 | 4 | 4.63 |
| Lauraceae | Aiouea macedoana Vattimoa,c,d | 3 | 5 | 5 | 4.5 |
| Melastomataceae | Mouriri pranceana Morleyd | 3 | 5 | 5 | 4.5 |
| Symplocaceae | Symplocos rhamnifolia A.DC.b,d | 3 | 5 | 5 | 4.5 |
| Lecythidaceae | Allantoma kuhlmannii (Ducke) S.A.Mori, Ya Y.Huang & Prancec,d | 3 | 5 | 4 | 4.38 |
| Rutaceae | Conchocarpus grandis Kallunki | 3 | 5 | 4 | 4.38 |
| Urticaceae | Coussapoa angustifolia Aubl. | 3 | 5 | 4 | 4.38 |
| Urticaceae | Coussapoa scabra Akkermans & C.C.Bergd | 3 | 5 | 4 | 4.38 |
| Rubiaceae | Coussarea machadoana (Willd.) Standl. | 3 | 5 | 4 | 4.38 |
| Sapindaceae | Cupania olivacea Gleason & A.C.Sm.d | 3 | 5 | 4 | 4.38 |
| Chrysobalanaceae | Licania maranhensis Prancec,d | 3 | 5 | 4 | 4.38 |
| Melastomataceae | Mouriri cearensis Huberd | 3 | 5 | 4 | 4.38 |
| Annonaceae | Oxandra major R.E.Fr. | 3 | 5 | 4 | 4.38 |
| Leguminosae | Peltogyne maranhensis Huber ex Duckeb,d | 3 | 5 | 4 | 4.38 |
| Rutaceae | Pilocarpus alatus C.J.Joseph ex Skorupab,d | 3 | 5 | 4 | 4.38 |
| Araliaceae | Schefflera plurifolia Fiaschi & Frodind | 3 | 5 | 4 | 4.38 |
| Solanaceae | Solanum circinatum Bohs | 3 | 5 | 4 | 4.38 |
| Solanaceae | Solanum myrianthum Britton ex Rusby | 3 | 5 | 4 | 4.38 |
| Leguminosae | Tachigali prancei (H.S.Irwin & Arroyo) L.G.Silva & H.C.Limad | 3 | 5 | 4 | 4.38 |
| Sapindaceae | Talisia sylvatica (Aubl.) Radlk. | 3 | 5 | 4 | 4.38 |
| Lamiaceae | Vitex compressa Turcz. | 3 | 5 | 4 | 4.38 |
| Leguminosae | Zygia odoratissima (Ducke) L.Rico | 3 | 5 | 4 | 4.38 |
| Sapotaceae | Chrysophyllum arenarium Allemãoa,d | 2 | 5 | 4 | 4.13 |
| Polygonaceae | Coccoloba coronata Jacq. | 2 | 5 | 4 | 4.13 |
| Leguminosae | Copaifera piresii Ducked | 2 | 5 | 4 | 4.13 |
| Primulaceae | Cybianthus granulosus Pipoly | 2 | 5 | 4 | 4.13 |
| Ebenaceae | Diospyros kanizur B.Walln. | 2 | 5 | 4 | 4.13 |
| Ebenaceae | Diospyros krukovii A.C.Sm.d | 2 | 5 | 4 | 4.13 |
| Rutaceae | Erythrochiton brasiliense Nees & Mart. | 2 | 5 | 4 | 4.13 |
| Erythroxylaceae | Erythroxylum ligustrinum DC. | 2 | 5 | 4 | 4.13 |
| Rutaceae | Esenbeckia kallunkiae Pirani | 2 | 5 | 4 | 4.13 |
| Myrtaceae | Eugenia percrenata McVaughd | 2 | 5 | 4 | 4.13 |
| Moraceae | Ficus insipida Willd.d | 2 | 5 | 4 | 4.13 |
| Moraceae | Helianthostylis sprucei Baill. | 2 | 5 | 4 | 4.13 |
| Melastomataceae | Henriettea spruceana Cogn. | 2 | 5 | 4 | 4.13 |
| Melastomataceae | Miconia prancei Wurdack | 2 | 5 | 4 | 4.13 |
| Melastomataceae | Mouriri dumetosa Cogn. | 2 | 5 | 4 | 4.13 |
| Myrtaceae | Myrcia graciliflora Sagot | 2 | 5 | 4 | 4.13 |
| Myrtaceae | Myrcia neesiana DC. | 2 | 5 | 4 | 4.13 |
| Leguminosae | Ormosia stipularis Ducke | 2 | 5 | 4 | 4.13 |
| Leguminosae | Swartzia discocarpa Ducked | 2 | 5 | 4 | 4.13 |
| Leguminosae | Swartzia lucida R.S.Cowand | 2 | 5 | 4 | 4.13 |
| Pentaphylacaceae | Ternstroemia urophora Kobuskid | 2 | 5 | 4 | 4.13 |
| Myristicaceae | Virola sessilis (A.DC.) Warb.d | 2 | 5 | 4 | 4.13 |
| Euphorbiaceae | Acalypha stricta Poepp. | 3 | 5 | 1 | 4 |
| Leguminosae | Amburana cearensis (Allemão) A.C.Sm.a | 1 | 5 | 5 | 4 |
| Leguminosae | Andira cordata Arroyo ex R.T.Penn. & H.C.Limad | 1 | 5 | 5 | 4 |
| Leguminosae | Cassia spruceana Benth. | 1 | 5 | 5 | 4 |
| Leguminosae | Chamaecrista subpeltata (Rizzini) H.S.Irwin & Barnebyd | 4 | 4 | 4 | 4 |
| Sapotaceae | Chrysophyllum sanguinolentum (Pierre) Baehni | 1 | 5 | 5 | 4 |
| Polygonaceae | Coccoloba paraensis Meisn.d | 1 | 5 | 5 | 4 |
| Leguminosae | Copaifera luetzelburgii Harmsd | 1 | 5 | 5 | 4 |
| Annonaceae | Duguetia aripuanae Maasd | 4 | 4 | 4 | 4 |
| Asteraceae | Eremanthus mattogrossensis Kuntze | 1 | 5 | 5 | 4 |
| Myrtaceae | Eugenia maranhaoensis G.Don | 4 | 4 | 4 | 4 |
| Moraceae | Ficus malacocarpa Standl. | 1 | 5 | 5 | 4 |
| Thymelaeaceae | Funifera ericiflora (Gilg & Markgr.) Domked | 1 | 5 | 5 | 4 |
| Nyctaginaceae | Guapira campestris (Netto) Lundelld | 1 | 5 | 5 | 4 |
| Chrysobalanaceae | Hirtella kuhlmannii Pilg.d | 1 | 5 | 5 | 4 |
| Leguminosae | Inga leiocalycina Benth. | 1 | 5 | 5 | 4 |
| Myristicaceae | Iryanthera tessmannii Markgr. | 1 | 5 | 5 | 4 |
| Chrysobalanaceae | Licania maguirei Pranceb,c,d | 1 | 5 | 5 | 4 |
| Leguminosae | Luetzelburgia praecox (Harms ex Kuntze) Harmsd | 1 | 5 | 5 | 4 |
| Sapotaceae | Manilkara excelsa (Ducke) Standleya,d | 1 | 5 | 5 | 4 |
| Malvaceae | Mollia speciosa Mart. & Zucc. | 1 | 5 | 5 | 4 |
| Melastomataceae | Mouriri sideroxylon Sagot ex Triana | 1 | 5 | 5 | 4 |
| Anacardiaceae | Myracrodruon urundeuva Allemãoa | 1 | 5 | 5 | 4 |
| Lauraceae | Nectandra matogrossensis Coe-Teixeiraa,d | 4 | 4 | 4 | 4 |
| Lauraceae | Ocotea matogrossensis Vattimo-Gil | 1 | 5 | 5 | 4 |
| Leguminosae | Ormosia holerythra Ducke | 1 | 5 | 5 | 4 |
| Ochnaceae | Ouratea discophora Ducked | 1 | 5 | 5 | 4 |
| Asteraceae | Piptocarpha rotundifolia (Less.) Baker | 1 | 5 | 5 | 4 |
| Sapotaceae | Pouteria rostrata (Huber) Baehni | 1 | 5 | 5 | 4 |
| Burseraceae | Protium ovatum Engl.a,d | 1 | 5 | 5 | 4 |
| Apocynaceae | Rauvolfia praecox K.Schum. ex Markgr. | 1 | 5 | 5 | 4 |
| Rubiaceae | Remijia amazonica K.Schum. | 1 | 5 | 5 | 4 |
| Proteaceae | Roupala dielsii J.F.Macbr. | 1 | 5 | 5 | 4 |
| Burseraceae | Trattinnickia glaziovii Swart | 1 | 5 | 5 | 4 |
| Meliaceae | Trichilia rubra C.DC. | 1 | 5 | 5 | 4 |
| Hypericaceae | Vismia japurensis Reichardt | 1 | 5 | 5 | 4 |
| Hypericaceae | Vismia schultesii N.Robson | 1 | 5 | 5 | 4 |
| Annonaceae | Xylopia multiflora R.E.Fr. | 1 | 5 | 5 | 4 |
Scores are assigned from the weighted average of the three criteria
Our results indicate that the taxonomic composition of species in our priority list comprises 39 families and 69 genera (Table 3). In summary, these results show that Leguminosae (16) and Melastomataceae (6) are the most representative families in this list. Among the main genera composing priority species that were not listed as threatened, one can find Virola, Potueria, Duguetia, Hirtella and Iryanthera, among others (Fig. 2a–e).
Representatives of some of the genera that comprise the 89 tree species of high priority: Virola (a); Pouteria (b); Duguetia (c); Hirtella (d); Iryanthera (e) and representative of one of the genera classified as threatened: Nectandra (f). Photo credits: Maciel, E.A,.; Silveira, A.; Santos, J.P.
Our results also indicate that the taxonomic composition of species of our priority list comprises 11 families (for instance, Chysobalanaceae, Leguminosae and Lauraceae) and 13 genera (for instance, Myracroduon, Licania, Amburan and Nectandra) (Fig. 2f) that have already been classified by other systems, such as IUCN or Biodiversitas (Table 3). We noted that only 14 species that comprise the priority list had already been detected as threatened by some of the specialized classification systems (Table 3).
DiscussionThe hierarchy of priorities – We demonstrated that the tree species of the Transition have a hierarchical structure of priorities for conservation. Some of them were recorded only once in the Transition, while others have restrictions to habitats and others appear to occur only within the Transition. Our results, as well as those of other studies carried out in this region (e.g., Marimon et al., 2006), converge to a central point, which is the importance of the Transition for the conservation of tree diversity. In fact, we found that the tree species that occur in this region have low geographic distribution range in the adjacent domains, Cerrado and Amazon. Some of those species have low population rates, while others are restricted to savanna or forest vegetation types. Thus, our findings resulted in a combination of species with high regional responsibility, high local rarity and high vulnerability to habitat.
RR – Our results indicate well defined species groups with respect to the pattern of rarity, which was characterized by the fact that 25.4% of the species occurred in only one ecoregion out of the Transition and, at the other extreme, 54.9% of the species occurred in six or more ecoregions, on one or both adjacent areas. RR is a geographical criterion, and this type of rare species, i.e., based on biogeographical amplitude, results from historical processes (Pärtel et al., 2005). In fact, paleoclimatic processes that occurred during the Quaternary period are expected to have shaped the vegetation patterns of the South Amazonian border (Méio et al., 2003 and Haffer, 2008). Such processes may have dramatically affected the niche of many species and contributed to the emergence of new evolutionary lineages (Aleixo et al., 2010) and, thus, to the high diversity of existing forests (Haffer, 2008).
The species that comprise Southern Amazonia underwent long adaptation processes that allowed them to occupy marginal areas with different conditions than those from which they originated, which also resulted in different patterns of species richness (Ivanauskas et al., 2004). These events of species adaptation to the unique habitats of the transition have been suggested as an explanation of the floristic dissimilarity that is observed between Transition areas and core areas of adjacent domains (Kunz et al., 2009). Thus, the biogeographic gradient formed by rare species found here suggests that the Transition shelters species with high regional responsibility. Such a gradient results from long periods of adaptive responses that occurred over the paleoclimate processes that characterized the Transition (Pärtel et al., 2005). We believe that to be effective, regional conservation policies should consider various strategies, including biogeographic gradients (Gustafsson et al., 2014) recorded in ecological transitions, as noted here.
LR – We recorded high spot rarity, with 35.4% of the species occurring only once in the Transition. This may result from species with low population rates (Caiafa and Martins, 2010) as well as species that are poorly sampled, a gap that is commonly referred to as ‘Wallacean shortfall’ (see Brito, 2010). Both reasons have direct implications for conservation, because species with low population rates deserve greater conservation care (Caiafa and Martins, 2010). If, on the other hand, local rarity of these species results from sampling deficit, this implies a worrying gap for regional diversity, because the Transition is at the center of high human pressure, which makes improving the knowledge of its flora urgent. However, it has been suggested that data deficiency most often reflects rarity and, thus, higher vulnerability (Corlett, 2016).
HV – We have demonstrated that in the Transition, many species occur only in forest habitats, while others occur in savanna habitats (Table 3). While the current law of native vegetation protection establishes 80% and 35% Legal Reserve in the Amazon to areas of forests and savannas, respectively (Brasil, 2012), we believe that this code has serious implications for the conservation of these species, which, in the Transition, occur in unique habitats (Haidar et al., 2013). We therefore suggest that the areas of Legal Reserve are equivalent to 80% regardless of the physiognomy.
The priority method applied to the 1755 species that occur in the Transition revealed the existence of a regional priority list containing 89 species, whose weighted average of the three criteria was equal to or larger than four points (Table 3). Of these 89 priority species for conservation, 39 were endemic to Brazil (Lista de Espécies da Flora do Brasil, 2015) and only 14 had already obtained a national or international conservation status (Giulietti et al., 2009; Martinelli and Moraes, 2013; Martinelli et al., 2014; IUCN, 2015). Our priority list was also composed of species from the Cerrado, such as Myracrodruon urundeuva, E. mattogrossensis, Amburana cearensis, Mouriri cearensis and others. Evidence shows that these species have low relative dominance in the Cerrado (Françoso et al., 2016). Likewise, species from the Amazon, such as Licania maranhensis, Nectandra matogrossensis and Rinorea villosiflora comprised our priority list. These species are among those with reduced geographic distribution in the Amazon (ter Steege et al., 2016). This fact shows that our set of priority tree species may have limited occurrences in both Amazon and Cerrado domains.
We demonstrated, by means of a weighting method based on three criteria, that it is possible to score tree species priorities in order to conserve transitional zones. Considering that ecological transitions are areas that concentrate a high diversity of organisms, but which attract little interest for conservation (Smith et al., 2001), and that simple approaches can be used to enable access to scientific results by conservation managers (Habel et al., 2013), we suggest that this approach could be replicated in other transition regions to identify priorities. This idea can even be extended to other groups such as herbs and epiphytes.
We found that only 14 of the 89 species on our list of priority species were covered by any protection category (Table 3). Although this fact may seem contradictory, the differences between the numbers of species selected for each system are due to the particularities presented by each one of them. If, on the one hand, the method of priorities based on three criteria used here is proposed to prioritize rare species that express different forms of rarity in local and regional levels (Gauthier et al., 2010), the IUCN system, on the other hand, prioritizes species of international importance, through population rates and the known occurrence areas of species. However, note that species chosen here as having priority for conservation, both of which were shown by the IUCN as being unlisted, presented equal importance to regional conservation if one considers the weighted average of the three adopted criteria. “Alternative” systems, such as that presented here, should be used with caution, because the lack of knowledge about the distribution of many taxa can compromise such approaches. In addition, we are dealing with a single source of geographic data, and including some criteria that may be valid only at restricted scales. Thus, to the extent that a combination of the listed species is possible, with threatened plant species already listed by other classification systems forming a single list (Mehlman et al., 2004), the gaps left by a particular classification system (Akçakaya et al., 2000; Lõhmus, 2015) can be reduced.
The method adopted here proved to be flexible even considering a larger scale, since it was possible to select species with the highest priority from the two largest phytogeographic areas of South America. We believe that the method can be easily applied not only by researchers, but also by managers of protected areas because it is a quick and convenient method that does not require a lot of computational effort. Thus, the approach based on regional responsibility, local rarity and vulnerable habitat (Gauthier et al., 2010) used here could help to reduce the gap between the results produced by conservation science and the professionals who deal with management of biodiversity, as suggested by Habel et al. (2013).
The results of this study indicate that our priority list may be used for future conservation strategies to be carried out both for the Transition, and for the geopolitical units that cover this region. We suggest that future flora assessments – at national, regional and local levels – more closely evaluate the species selected here as priorities. We also suggest that priority-setting initiatives start to consider rating systems that select species of local and regional interest. We believe that the combination of regional lists with national and international red lists is an interesting strategy in the management of species for conservation.
Conflicts of interestThe authors declare no conflicts of interest.
We are indebted to CAPES, which granted E.A. Maciel with a graduate scholarship, and to FAPEMAT (Process #224333/2015), for the financial support.