Functional analysis of secondary succession may allow identifying and predicting processes of community assembly, which can be simultaneously driven by factors related to ecological filters and neutral forces. This study evaluated trait-convergence assembly patterns and trait-divergence assembly patterns in successional areas of Araucaria forest. Plant species were sampled in both the upper and lower strata and were described by 15 functional traits. Data analyses were based on multiplication and Procrustes adjustment of matrices, which permit to discriminate trait-convergence assembly patterns and trait-divergence assembly patterns along the forest succession (our environmental variable), and the influence of phylogeny on these patterns. Initial and late forests were highly different in species composition, but the regenerating stratum was already more similar especially in functional terms. Traits related to the acquisitive-conservative trade-off (wood density, leaf nitrogen content, leaf area, leaf dry matter content) revealed strong convergent patterns of successional changes. Moreover divergence was maximized by specific leaf area, seed mass, deciduousness, and dispersal mode, showing a higher functional diversity in late Araucaria forests.
The analysis of secondary succession may enable the identification and prediction of fundamental processes involved in community assembly (Lebrija-Trejos et al., 2010). The assembly of communities is defined by the colonization and interaction of species coming from a regional pool to form local communities and can be simultaneously driven by factors related to ecological filters (abiotic and biotic environment) and neutral forces (dispersal limitation, ecological drift) (HilleRisLambers et al., 2012; Rosindell et al., 2011), but their proportional importance can vary along the successional time. Meiners et al. (2015) recently presented a conceptual framework of successional drivers that simultaneously accounts for geographic and evolutionary contexts with filter models associated to local dynamics. The framework highlights how each level constrains lower levels (for example, how site conditions constrain species availability) and potential feedbacks among levels (for example, how species performance alters site conditions), considering a broad review of succession studies (Meiners et al., 2015).
Ecological filters in community assembly may result in patterns of both convergence (underdispersion) and divergence (overdispersion) in species traits (Pillar et al., 2009). Trait-convergence is related to a species ability to transpose abiotic filters, driving the assembly to be similar for particular functional traits. Such filters may act at the regional scale (defining the species pool), and then at the local habitat scale, selecting species that are able to get established under the prevailing environmental conditions (Meiners et al., 2015). During the process of succession, early species may change the local environmental conditions (e.g. improving shading) and interact with other species, facilitating or hindering the colonization of new species (Lebrija-Trejos et al., 2010; Schöb et al., 2013).
Pioneer species may facilitate colonization by species that have different traits or strategies, such as shade-tolerant species, influencing, thus, the community assembly of the next successional phase (Verdú et al., 2009). In this case, we can expect a trait divergence pattern between the species that are colonizing and those already established and also among the new recruits, since the species pool of secondary/late forests is larger than the pioneers, increasing the probability of trait-divergence. On the other hand, pioneer species can also delay the colonization of secondary species by, for example, a positive feedback in the recruitment mechanism (‘persistent-monodominance’), hindering the successional process and keeping the community at an alternative state (Norden et al., 2011). This mechanism would maintain a convergence pattern. However, as local environmental conditions change over the time through the increase in vertical structure and canopy, abiotic filters of each successional phase may still lead to patterns of trait convergence within the communities. Secondary and late successional forest trees have evolved to the commonest environmental conditions – shaded habitats – causing species to converge on similar traits (Hubbell, 2005), at least in terms of those associated with light demand.
Considering the above, we would expect patterns of trait convergence along the forest succession process. However, at the scale where the coexisting species are competing for resources, having different strategies to obtain them may be more effective (Silvertown, 2004), leading to patterns of trait divergence. Processes of limiting similarity enhance species diversity through a finer niche partitioning, therefore increasing functional alpha diversity, here measured by the trait dissimilarity between local species (De Bello et al., 2009). As both trait convergence and divergence can be found over forest succession, simple conclusions about patterns of functional diversity and functional composition in forest succession have not yet been consistent (Böhnke et al., 2014). Whereas functional diversity measure the value, range and relative abundance of functional traits in a community, the functional composition can be represented by the community weighted mean value of traits (i.e. CWM) (Díaz et al., 2007). The number and the nature of selected traits strongly influence such functional patterns, and the environmental gradient must be part of this choice (Pillar et al., 2009).
In this study we evaluated patterns of convergence and divergence of plant traits, investigating tree species in a successional chronosequence of Araucaria forest plots in southern Brazil, to answer the following questions: (1) How early successional stages differ from late ones in terms of species composition, considering both upper and lower strata? (2) Which functional traits are optimal for revealing patterns of trait-divergence and trait-convergence related to forest development? (3) What patterns of functional composition and functional diversity of both upper and lower strata become evident along forest succession?
Material and methodsStudy siteThe study site was in the Center for Research and Conservation of Nature Pro-Mata (CPCN Pró-Mata; 29°26′27″S and 50°08′ to 50°14′W, 800 to 950m a.s.l.), southern Brazil. The climate is temperate mesothermic super humid (Cfb climate, according to the Köppen classification), with average temperature of the warmest month not exceeding 22°C and the annual isotherm below 18°C.
The site has Araucaria forest in different regeneration stages after early logging activities, from initial to old-growth areas. After the establishment of the conservation area (in 1994), natural regeneration processes started. Late forests (with no signs of clearcutting) and initial successional forests were chosen for this study. Initial forests were under natural regeneration since at least 20 years and are situated very close (nearby) to old-growth forests.
Data collectionThree sites of late forest stage and four sites of initial stage forest were chosen for the vegetation survey. In each of the late forest sites, an area of 1ha was delimited within which 12 random circular plots of 100m2 each were marked, three plots in each ¼ha. These plots were used for sampling the upper stratum, including all individuals with at least 10cm of diameter at breast height (DBH≥10cm). Within each 100m2-plot, four subunits of 1m2 were used to sample the lower stratum (individuals of shrubs and trees with at least 10cm height and <1cm of DBH), systematically placed within each cardinal direction and considering a regular distance (2.8m) from the center of the plot. For the initial stage forests, the sampled area was 0.50ha to preserve the structural homogeneity of early successional stages, with six 100m2-plots. As a result, we had 36 100m2-plots for late forests and 24 for initial forests and for the lower strata we had 144 1m2-plots for late and 96 for early forests.
Based on all sampled species, we selected a subset of the most frequent species (present in at least 10% of the 100m2-plots) to be characterized by plant functional traits. This choice considers the biomass ratio hypothesis (Grime, 1998), that the most frequent and abundant species are expressing the main responses or ecosystem effects related to forest succession (Garnier et al., 2004) – our ecological process of interest.
We used the following plant functional traits: leaf length, leaf width, leaf form (ratio between leaf length and width), leaf area (La), specific leaf area (SLA, mm2/mg), leaf dry matter content (LDMC), leaf nitrogen content (LNC), maximum height (m), wood density (WD), leaf hairiness, leaf deciduousness, zoochoric dispersal, diaspore size, mean number of seeds per fruit, and seed mass (see Table S1). These traits were chosen due their importance for ecosystem processes concerned with forest succession, such as primary production, carbon and nutrient cycling, and litter decomposition (leaf traits, plant height and wood density), as well as dispersal and establishment in new areas (dispersal type, diaspore and seed features) (Chave et al., 2009; Cornelissen et al., 2003). The seven first traits were measured or calculated considering local field collections, following main recommendation of Cornelissen et al. (2003), while all others were compiled from literature and databases. Leaf traits were based on a sample of four adult individuals per species, taking at least 10 leaves of each individual. These individuals were from the studied sites, attending plots from early and advanced forest whenever possible. The leaves were kept fresh (within humid plastic bags) and were weighted in the laboratory (most on the same day). After, they were scanned for further evaluation of leaf area (with the Image J software) and then dried until weight stabilization. Following the measurements, average values were calculated per species. Further details about the choice and the measurements of traits are given in Table S1.
Data analysisFor analytical purposes we considered each three 100m2-plot (inside one ¼ha) as one sampling unit (SU) describing the local community, so that the species abundance was arranged together (i.e. upper stratum=300m2; lower stratum=12m2), resulting in 12SU of late forest and 8SU of initial successional forest. We first compared the similarities between stages and strata using the Jaccard index, considering the stage classification performed a priori.
The community composition data (matrix W) were then organized into two matrices: one for the upper stratum and another for the lower stratum. Only species for which traits were measured were considered in these matrices W, in order to match the same species considered in the traits matrix (matrix B) comprising the species described by their traits (Table S3). Furthermore, we used an environmental matrix (matrix E) to follow the methodology proposed by Pillar et al. (2009) and analyze which set of functional traits maximize the congruence with the environmental gradient of interest, in terms of trait-convergence and divergence of assembly patterns (respectively labeled as TCAP and TDAP). We used for E the total basal area of the upper stratum, as a proxy of forest development through succession (Leithead et al., 2012). A synthesis of this variable according to our prior classification of forest plots can be seen in Fig. S1 and we used this proxy as a predictor for further analyses. Additionally, we used the community phylogenetic information (matrix P), which is based on the species phylogenetic distances weighted by species community abundance to infer about the influence of phylogeny on the paper questions two and three.
To answer the questions about divergence and convergence assembly patterns along forest succession, we submitted the data to the analytical method proposed by Pillar et al. (2009), which is based on matrix multiplication and Procrustes adjustment procedures among the matrices W, B, E, and P (Pillar et al., 2013). The influence of the phylogeny on the assembly patterns revealed by the trait subsets maximized for TDAP and TCAP was analyzed by matrix correlations (Duarte et al., 2016). Details can be seen in the supplementary material.
Functional composition based on the results of TCAP was explored through regression models of community-weighted mean traits (CWM) according to Garnier et al. (2004), which may be arranged in matrix T, against the environmental variable (forest development, matrix E). Species and functional composition patterns related to the set of traits maximized for TDAP were explored by using principal coordinates analysis (PCoA) on the matrices X of both strata. Matrix X contains fuzzy weighted community composition, which is based on the trait similarities between the species that were recorded in a given community and between them and the other species in the species pool. The computation of matrices T and X is explained in the supplementary material. As matrix X expresses both TCAP and TDAP patterns (even when calculated from those traits that maximized TDAP), we projected the community-mean value of traits maximized in both analyses of each stratum to the ordination diagram, considering their correlation with the axes. Functional diversity was estimated considering the trait subsets maximizing TDAP, by applying the Rao's quadratic entropy index, to express divergence within communities.
ResultsWe recorded 94 species in the plots, among which 36 species had more than 10% of frequency, and were included in the functional analyses (Table S2). The upper stratum was mainly characterized by Tibouchina sellowiana in the initial successional areas (47% of sampled individuals) and Myrcia retorta (19%) in the late forests. Daphnopsis fasciculata (58%) and Myrceugenia myrcioides (30%) best represented the lower stratum of initial and late forests, respectively. The Jaccard similarity between upper and lower strata within each forest stage presented higher values for late forests than initial forests (mean±SD for sampling units: 0.25±0.10 and 0.18±0.15, respectively). In addition, the lower stratum of initial forests was more similar to the lower stratum (0.30±0.15) than to the upper stratum (0.24±0.13) of late forests.
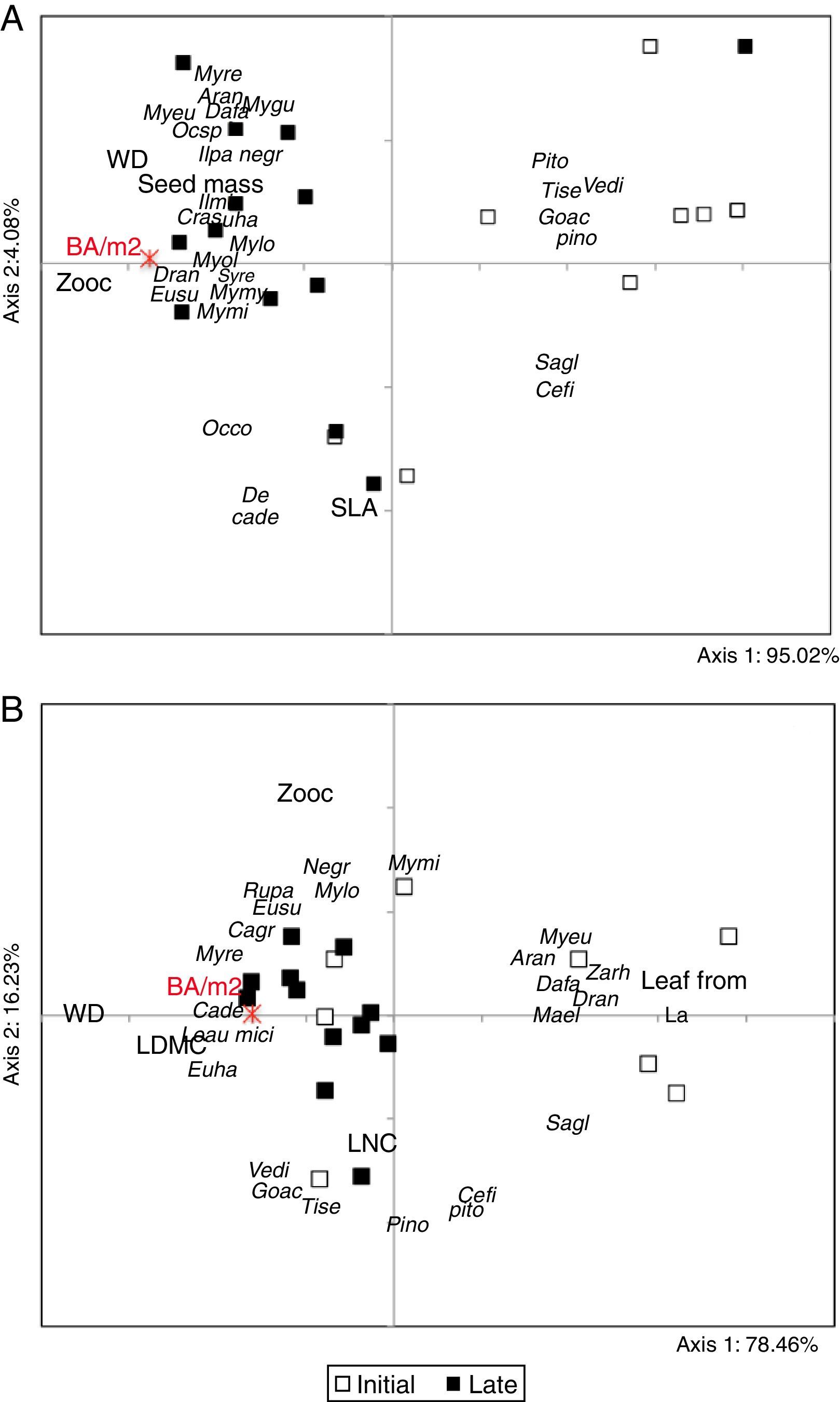
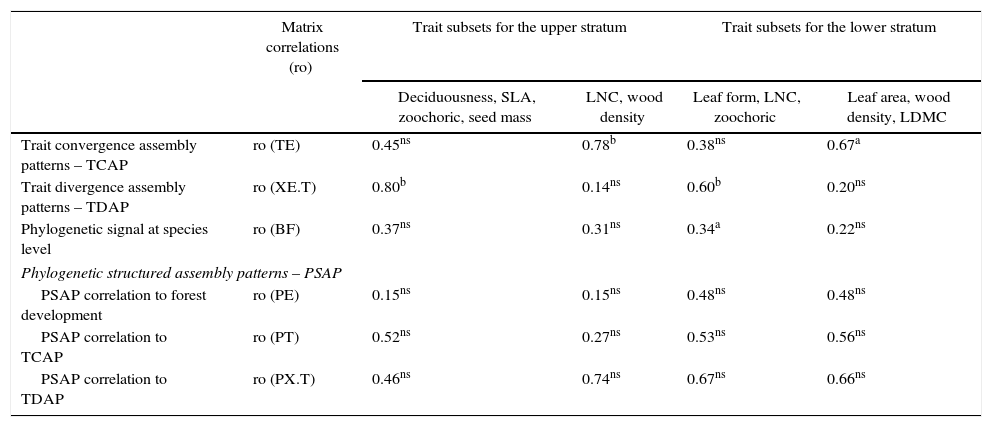
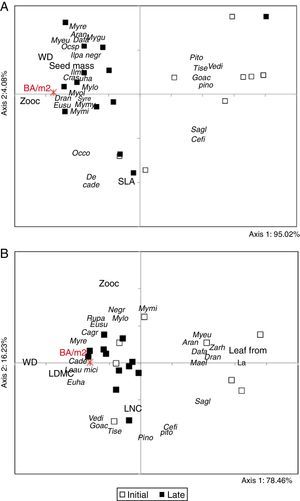
Table 1 presents all trait subsets that maximized either TDAP or TCAP for the upper stratum and the lower stratum of the communities, according to the forest development gradient (matrix E). Divergence and convergence patterns expressed by the PCoA ordinations of the fuzzy-weighted community composition matrix (X) clearly show differences between late and initial forests for both strata (Fig. 1). Individuals present in the upper stratum of late forests are mainly represented by zoochoric species with higher seed mass and wood density in comparison to those present in initial forest areas. Some areas had a higher participation of deciduous species with high SLA and LNC values (lower portion of Fig. 1A) and might be representing an intermediate successional stage (intermediate values of total basal area of trees). Lower stratum patterns (Fig. 1B) still discerned the successional stage of forests, but four initial areas were already more similar to late forests. The initial areas that stayed separated in the diagram were mostly represented by individuals of species with long, narrow leaves and with less influence of zoochoric dispersal compared to late successional forests. We did not see any significance of phylogenetic structured assembly related to the trait-divergence and convergence patterns for the selected set of traits (Table 1). The only influence of phylogeny was at the level of species for the set of traits that maximized TCAP of the lower stratum (leaf form, LNC, zoocoric).
Trait subsets with maximum congruence for trait convergence assembly pattern (TCAP) and trait divergence assembly pattern (TDAP) related to the forest development gradient (E) for each forest stratum. The values represent congruence based on Procrustes trace between matrices (“ro”, see methods for details), after the use of respective trait subsets. Congruence values for the phylogenetic signal at species level and the phylogenetic structured assembly patterns (PSAP), considering each subset of traits, are also given.
| Matrix correlations (ro) | Trait subsets for the upper stratum | Trait subsets for the lower stratum | |||
|---|---|---|---|---|---|
| Deciduousness, SLA, zoochoric, seed mass | LNC, wood density | Leaf form, LNC, zoochoric | Leaf area, wood density, LDMC | ||
| Trait convergence assembly patterns – TCAP | ro (TE) | 0.45ns | 0.78b | 0.38ns | 0.67a |
| Trait divergence assembly patterns – TDAP | ro (XE.T) | 0.80b | 0.14ns | 0.60b | 0.20ns |
| Phylogenetic signal at species level | ro (BF) | 0.37ns | 0.31ns | 0.34a | 0.22ns |
| Phylogenetic structured assembly patterns – PSAP | |||||
| PSAP correlation to forest development | ro (PE) | 0.15ns | 0.15ns | 0.48ns | 0.48ns |
| PSAP correlation to TCAP | ro (PT) | 0.52ns | 0.27ns | 0.53ns | 0.56ns |
| PSAP correlation to TDAP | ro (PX.T) | 0.46ns | 0.74ns | 0.67ns | 0.66ns |
Traits acronyms: SLA, specific leaf area; LNC, leaf nitrogen content; LDMC, leaf dry matter content.
PCoA ordination triplot of successional Araucaria forests in southern Brazil. The analyses were based on matrices X of 20 plots described by the species composition of the upper stratum (A) and the lower stratum (B) after fuzzy-weighting by species similarities in terms of functional traits that maximized TDAP (Upper stratum: deciduousness, SLA, zoochoric, seed mass; Lower stratum: Leaf form, LNC, zoochoric). Functional traits were projected on the diagram based on their correlation with the ordination axes, considering their community-weighted mean (CWM) values. The total basal area of trees of the upper stratum (BA/m2) was also projected based on their correlation values with the axes. Communities are identified according their priori successional stage classification and species labels can be seen in Table S1.
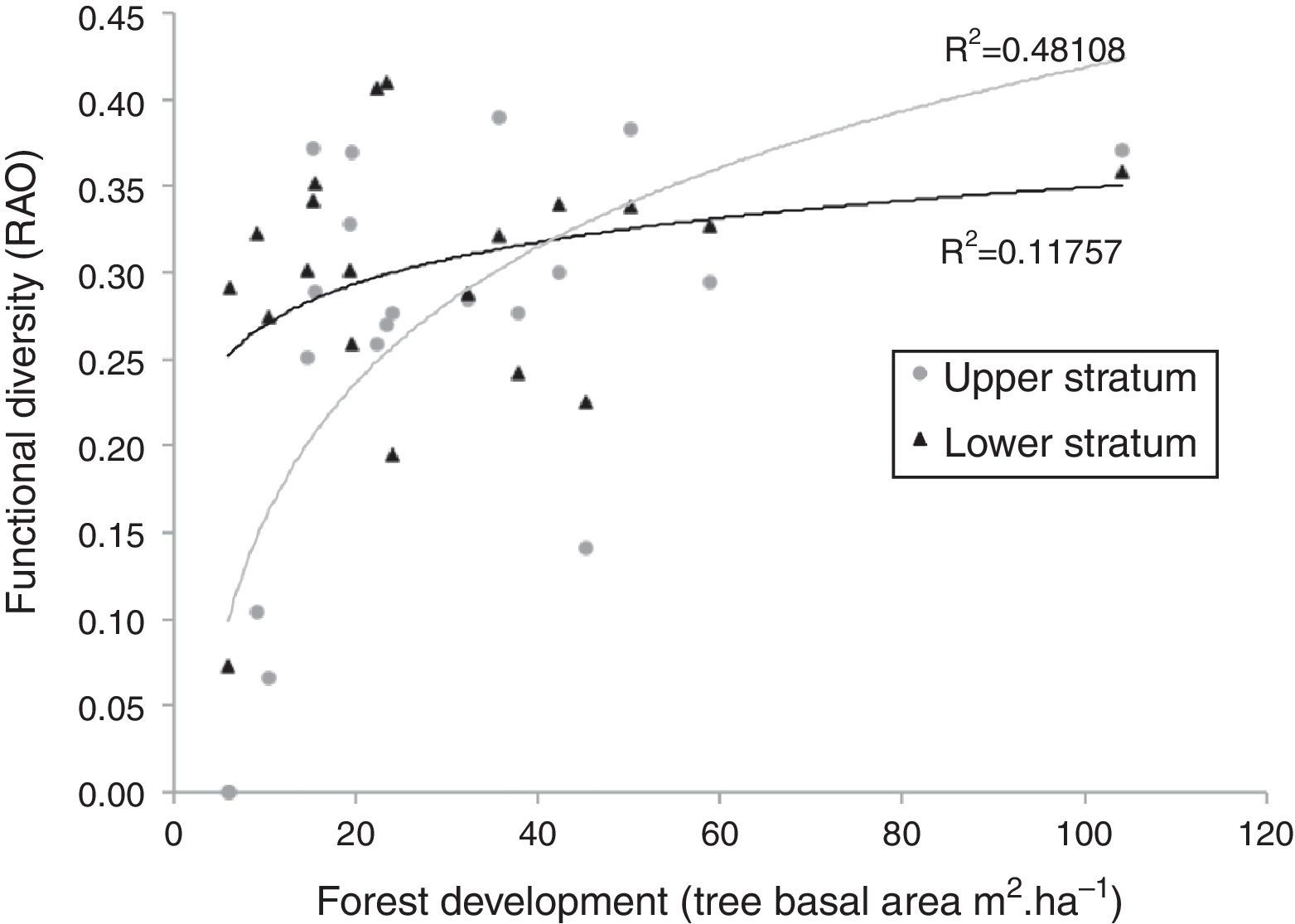
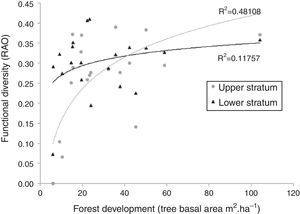
Functional diversity based on trait subsets that have maximized TDAP (Table 1) showed a logarithmic pattern of increase with the forest development, stabilizing the values for areas of 30–40m2ha−1 of tree basal cover (Fig. 2). This pattern, however, was much less prominent in the lower stratum than in the upper.
Functional diversity (Rao Entropy) of upper and lower strata of Araucaria forest according to the forest development, measured as the stem cover of trees with at least 10cm DBH (see Fig. S1 for this relation and the priori stage classification). Trait subset used for estimating was that with higher congruence for TDAP in each stratum (see Table 1).
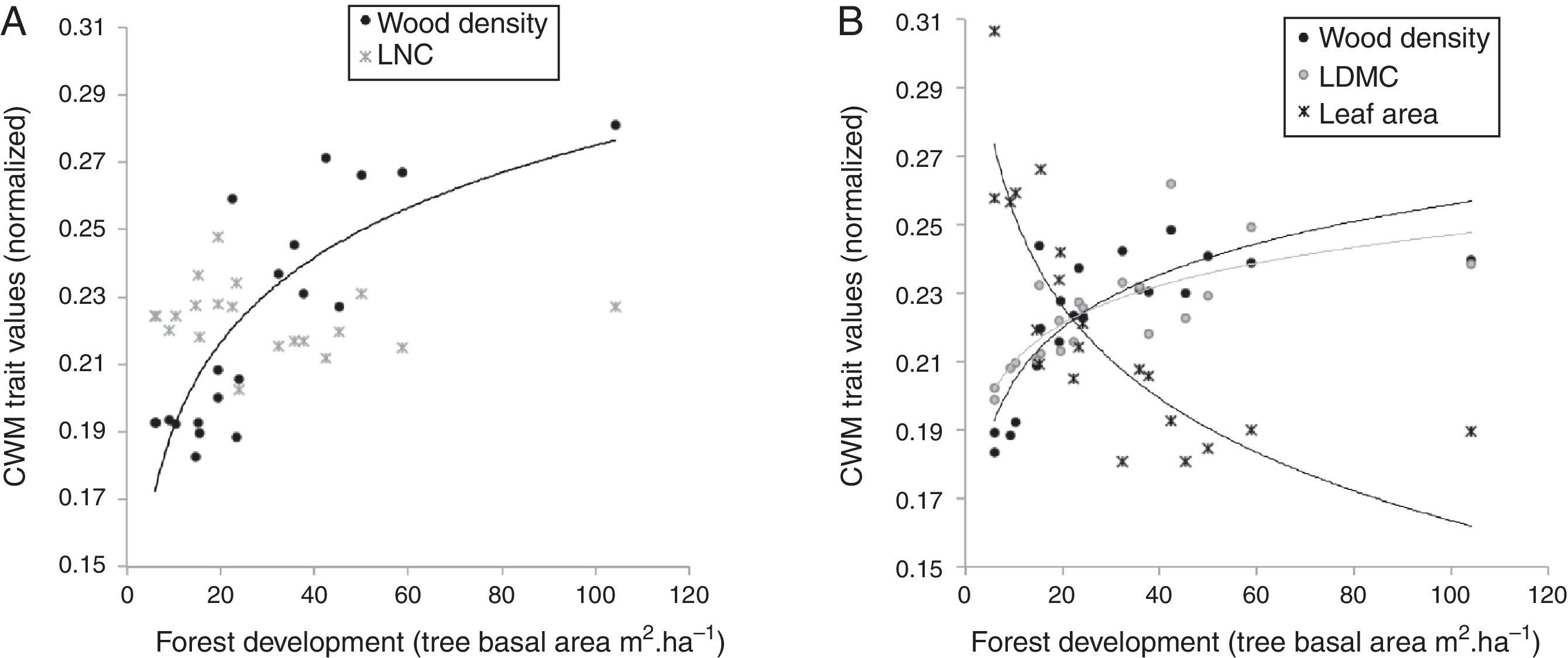
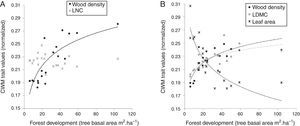
Convergence pattern in the upper stratum was maximized by wood density and leaf nitrogen content (LNC), but only wood density CWM values have shown a sharp relation with the forest development (Fig. 3A). In the lower stratum, wood density, leaf dry matter content (LDMC), and leaf area composed the subset best related with the environmental gradient (r=0.67) and all of them showed clear patterns of community convergence along the forest development (Fig. 3B).
Relation between CWM trait values and forest development in Araucaria forest, considering functional traits that maximized the congruence for convergence patterns of upper (left) and lower (right) strata. Logarithmic line tendency in (A) is y=0.0364ln(x)+0.1075, r2=0.68012 for wood density and in (B) are y=0.0224ln(x)+0.1525, r2=0.69715 for wood density; y=0.0162ln(x)+0.1725, r2=0.60655 for LDMC; y=−0.039ln(x)+0.3431, r2=0.74141 for leaf area.
Our results indicate strong trait-convergence and divergence in the assembly of the communities, revealing the relationship between patterns of the selected traits (optimal trait subsets) and the environmental gradient established over the forest development, not associated to the phylogeny of species. Successional changes linked to convergence patterns were mostly related to the acquisitive-conservative gradient (Chave et al., 2009): late forests had in average more individuals of species with higher wood density and LDMC, and lower leaf area and LNC (examples of conservative plant strategies) than those communities at initial succession. Conversely, an increase in trait-divergence through the development of forest was mainly related to deciduousness, specific leaf area, leaf form, dispersal by animals and seed mass. Potential associations between these traits and successional processes are discussed below.
Plant species assembled in the understory of the studied initial forests were more similar to the upper stratum of late forests than to their own, which may indicate turnover of species within the ongoing successional process. Adult individuals of pioneer species (e.g. T. sellowiana) may be favoring the colonization of secondary species in the understory (e.g. Myrceugenia spp.). Successional changes in the forest structure led to changes in the understory environment (Lebrija-Trejos et al., 2010), allowing the assembly of regenerating species to be more similar to the advanced areas. We could also observe some communities previously considered ‘initial’ very close to late forests, when regarding community similarity based on species composition fuzzy-weighted by the functional traits (matrix X, Fig. 1B). Over the successional time, an increased phylogenetic evenness has been linked to the recruitment of late-successional species (e.g. Letcher, 2010), which could, in turn, present functional convergence in traits, conferring adaptive value for regeneration in shade conditions (Hubbell, 2005). In the present study, we saw an increase of trait-divergence along the forest development, not related to the phylogeny.
The results of chosen trait subsets for trait-divergence assembly patterns (TDAP) may express one or both alpha and beta divergence along the environmental gradient (Pillar et al., 2009). Various leaf traits were selected for maximizing divergence patterns along the forest development studied here, i.e. species inside the same community and/or species in communities that are at similar successional stages seem to be overdispersed in terms of trait states of deciduousness, SLA, leaf form, and LNC. Such traits are related to the species fitness and, consequently, competition, especially in terms of resource acquisition under light limiting conditions and nutrient demand (e.g. Wright et al., 2010). Considering these leaf traits, we can assume that coexisting species should be more successful when leading different (contrasting) strategies for acquiring and stocking energy, resulting in higher alpha diversity with the ongoing succession (Fig. 2). Additionally, we can also assume that communities along the forest development gradient diverge more as they stray in terms of successional time, which results in a higher beta divergence (Silvertown et al., 2006).
Some reproduction traits were also associated with divergence patterns – seed mass and zoochory. They should be related to the high variability of seed attributes among the species established in the upper stratum of late forests and to a higher proportion of zoochory (since the state ‘1’ of our study expresses the amount of zoochoric individuals in the community) in both strata of advanced forests, when comparing to initial successional forests (Duarte et al., 2007; Zanini et al., 2014). Divergence pattern of seed mass was mainly associated to overdispersion inside the communities of intermediate development, where coexisting species presented greater amplitude of states (higher functional diversity when considering only the seed mass trait – data not shown). Despite the predominance of zoochoric dispersal in advanced areas, we saw that species assembled in initial areas were also represented by anemochory and autochory.
Convergence patterns, here expressed by the community-weighted mean of traits that maximized TCAP (LNC and wood density for the upper stratum; leaf area, wood density, and LDMC for the lower stratum), revealed the expected change from acquisitive to the predominance of conservative plant strategies in late forests. The acquisitive-conservative gradient reflects the resource economy and growth strategy of plant species and can be recognized by the leaf economic spectrum, which runs from fast to slow return on investments of nutrients and dry mass in leaves (Wright et al., 2004), and by the continuum between early and late species, which further includes reproductive features (e.g. from small to big seeds) (Lohbeck et al., 2013). The decreasing logarithm of leaf area, as the forest development increase, agrees with the forest type – Araucaria forest, where many typical species are small-leaf species, as Araucaria angustifolia itself, and other abundant Myrtaceae species (Bergamin et al., 2012). Such decrease in leaf area was also observed along succession in dry forests, but not in wet tropical forests (Lohbeck et al., 2013), which is related to different trade-offs either associated to water economy or light availability among the species in each forest type. The Araucaria forest distribution is mainly related to low mean annual temperature, frost frequency and cloud cover (highland conditions in southeastern Brazil), which in turn favors small-leaf species, with high dry matter content (LDMC) and high wood density. We can make up this picture by looking at the regenerating stratum in late forests, where we can expect to find a well-developed Araucaria forest in the future.
Initial and late Araucaria forests are highly different in terms of species composition, but the regenerating stratum of initial forests (20 years old) was already more similar to advanced forests, especially in terms of functional traits. Species assembly along the forest development was clearly related to functional traits that express acquisitive-conservative strategies (wood density, leaf nitrogen content, leaf area, and leaf dry matter content), which maximized convergence patterns. On the other hand, trait-divergence patterns were also significant. Specific leaf area, seed mass, deciduousness and dispersal mode were the traits that maximized divergence, expressing a logarithmic relationship between functional diversity and forest development. Therefore, successional communities have been assembled through the influence of environmental and biotic filters, but limiting similarity processes seem to increase with the advance of the succession, as shown by the higher trait divergence in late Araucaria forests.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Brazilian Research (CNPq) and Education (CAPES) Councils for research grants and financial support of projects.