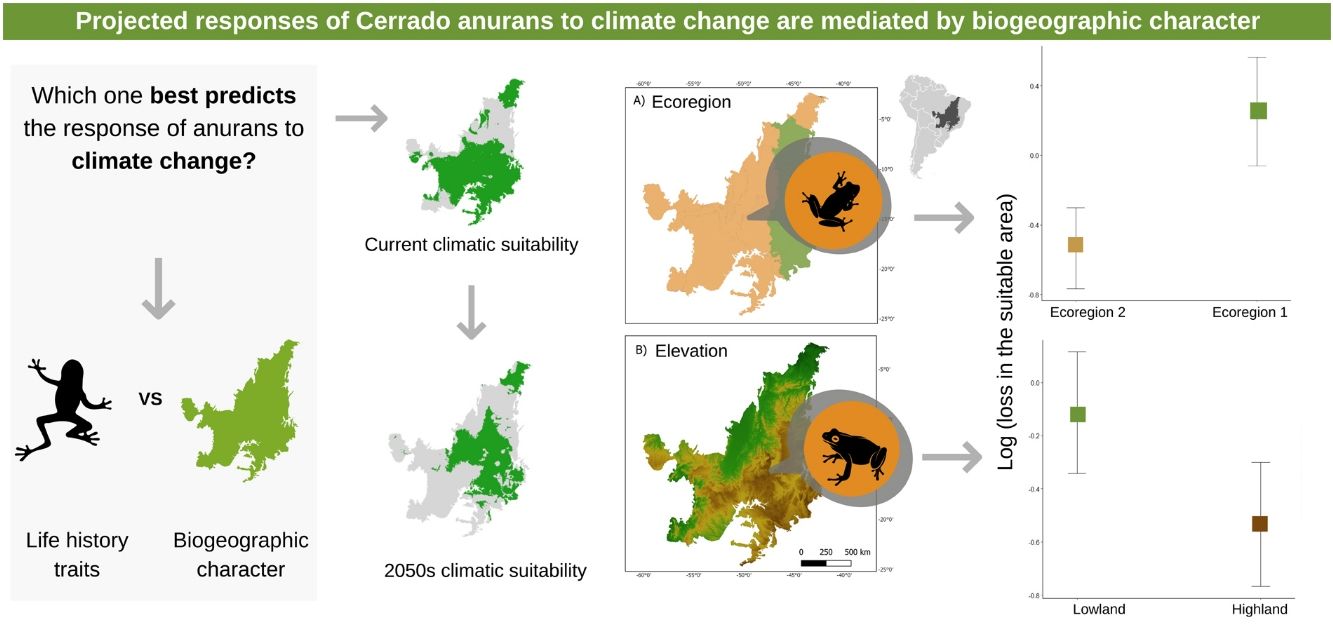
Climate change has affected the distribution and phenology of several species. However, due to narrow ecological and physiological tolerances, not all species are affected in the same way. Here, we test whether life history characteristics and biogeographic character influence the response of Cerrado anurans to climate change using ecological niche models. Our results demonstrate that a reduction in the potential distribution area is expected for most species in both optimistic (∼ 70%) and pessimistic (∼ 65%) emission scenarios. At first glance, this result might seem illogical, but when we assessed the magnitude of the losses, we found that even though more species lost area in the optimistic scenario, the amount of area lost was less than in the pessimistic scenario. Our findings also show that species that inhabit higher-altitude areas and the Western Cerrado will be more vulnerable to climate change. This shared “destination” in the face of climate change is associated with the sharing of some climate niche attributes among species in the same region, leading to similar responses. This data can be used to support biodiversity protection measures, such as the creation of a system of ecological corridors that allows the species tracking optimal climatic requirements in space.
In the last century, the Earth’s average temperature has increased by about 0.6 °C (IPCC, 2013) and current forecasts suggest an increase of 1.5 °C for the 21st century (IPCC, 2018). There is growing evidence that all levels of biodiversity are already being impacted by global warming, from organisms to ecosystems level (Parmesan, 2006). Some known effects include changes in phenology, physiology, range size, range localization and in interspecific relationships, modifying community structure and ecosystem functions (Bellard et al., 2012). Understanding the responses of species to this threat, however, is still a major challenge for the scientific community.
Due to narrow ecological and physiological tolerances, some vertebrate groups are likely to be more vulnerable to climate change (Blaustein et al., 2010). Species living at environmental temperatures very close or above their thermal optimum, as tropical amphibians, are expected to be more affected by climate change because any small increase in temperature should affect it disproportionately, reducing their thermal performance and the probability of these species being rescued evolutionarily (Tewksbury et al., 2008; Huey et al., 2009). Moreover, amphibians are ectothermic and have life-history traits such as a complex life cycle (at least for most species) and reproductive specifics that makes them highly dependent on the current temperature and precipitation regimes (Huey et al., 2012). Although most amphibians are impacted by climate change, not all species are likely to be equally affected (Foden et al., 2008). Life history characteristics (e.g. distribution pattern, habitat specialty, reproductive strategy, body size), for example, can be indicators of species vulnerability to climate change (Borges et al., 2019; Foden et al., 2013).
Species that need specific environmental triggers to initiate breeding events, for instance, may be more vulnerable (Foden et al., 2013). Anurans with an explosive reproductive strategy may eventually have their reproductive events more affected by climate change than species with an extended reproductive strategy, because they need specific amounts of precipitation to initiate reproduction (Wells, 2007). It also makes sense to expect amphibians’ body size to reflect their vulnerability to climate change, as body size is directly related to thermal balance and can lead to specific sensitivities to climate (Olalla-Tárraga and Rodríguez, 2007). Larger sized ectotherms, for instance, are less susceptible to hot and dry environments and might experience higher body temperatures than smaller sized species (Rubalcaba and Olalla-Tárraga, 2020). This can be explained by the increase in the convective boundary as the animal’s size increases (Stevenson, 1985). Consequently, heat loss by convection will be lower in larger individuals and the operating temperature should increase if solar radiation is absorbed. Thus, it is expected that larger individuals will be able to withstand higher temperatures (Seebacher et al., 1999, 2003). Therefore, several life-history traits can be used to identify species that are at greatest risk from climate change, allowing for better targeting of conservation efforts to the most vulnerable species.
Biogeographic character can also be a good indicator of species vulnerability to climate change (e.g. elevation, ecoregion). The relationship between spatial distribution and climatic niche can be explained by the fact that not all areas are equally affected by global change, as some areas may experience a much more accentuated climatic anomaly, as demonstrated by Borges and Loyola (2020) for the region north and northwest of the Cerrado. Furthermore, climate change may extinguish specific climatic conditions in some regions in the future. In this way, species with restricted distribution or those that, although locally abundant, are strongly associated with specific geographic regions (e.g. high altitude endemic, specialists in forest habitats and rupestrian grasslands), tend to be more vulnerable because they occupy a limited range of microhabitats (Foden et al., 2013). With the advance of global warming, it is expected that the climatic conditions suitable for certain organisms will be shifted upwards (Enriquez-Urzelai et al., 2019), and therefore, species that occupy higher altitudes will be the most affected (Nori et al., 2016), causing a potential reduction in the range of the species (Blaustein et al., 2010). Generally, these species have a low capacity to recover in the face of large population reduction events and can easily reach local extinction (Foden et al., 2013).
Few studies, until now, include specific traits (such as body size, breeding pattern, habitat specialization) in the assessment of the impacts of climate change on species (e.g. Borges et al., 2019; Foden et al., 2013). In this study, we addressed that gap evaluating whether life history characteristics (distribution pattern: endemics or widespread, habitat specialization, reproductive strategy, body size) and biogeographic character (elevation and ecoregion) influence the response of Cerrado anurans to climate change. For amphibians, this approach may be particularly important because more than 40% of the species in the group are in some IUCN threat category (IUCN, 2021). Furthemore, species that are already experiencing declines due to other factors, such as emerging infectious diseases or habitat loss, are likely to be more heavily affected by climate change (Foden et al., 2008).
Material and methodsOccurrence dataWe gathered occurrence records for 63 species, of which 17 are endemic to the Brazilian Cerrado. We obtained records of anurans occurrence through the following databases: Global Biodiversity Information Facility (GBIF: http://www.gbif.org); Biodiversity Information Serving Our Nation (BISON: https://bison.usgs.gov); Vertnet database (http://vertnet.org/), speciesLink (http://www.splink.org.br/) and Integrated Digitized Biocollections (IDGBIO: https://www.idigbio.org). See detailed information about the method of filtering records in the supplementary material (SM).
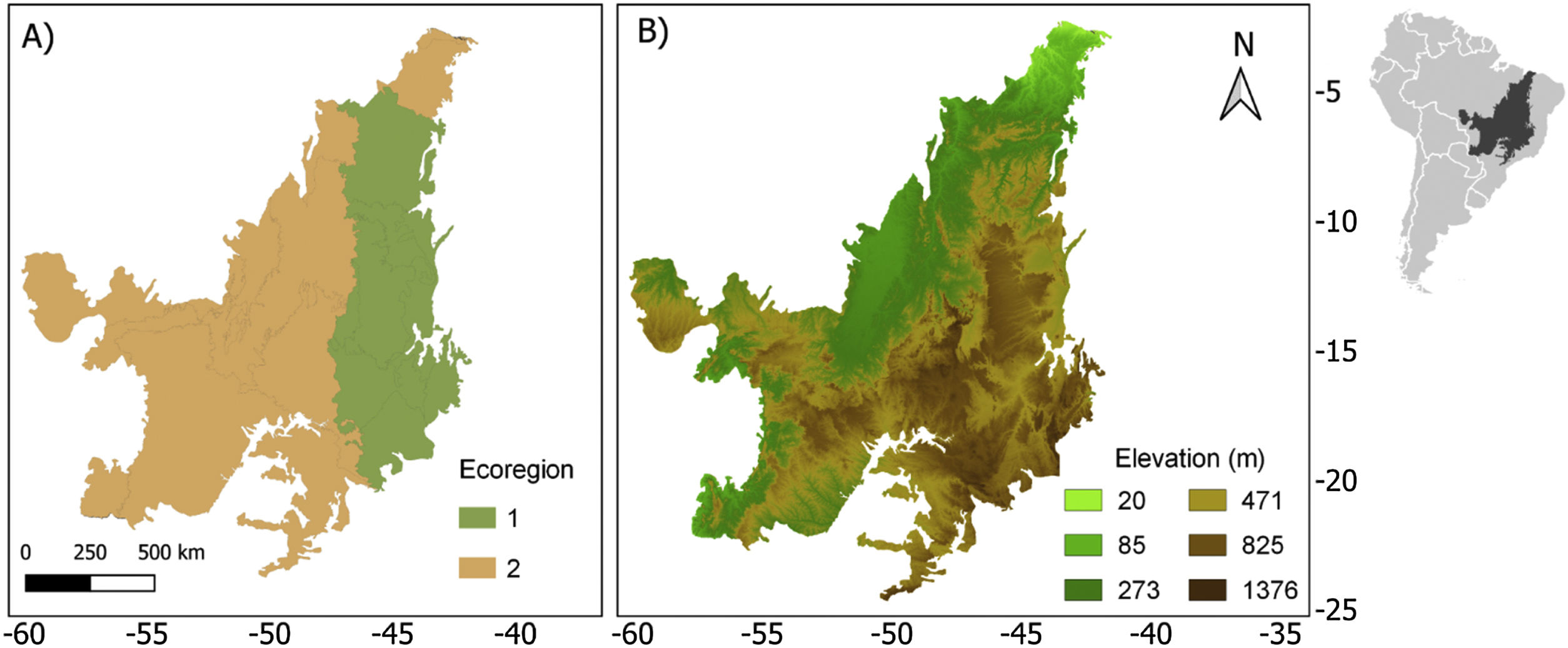
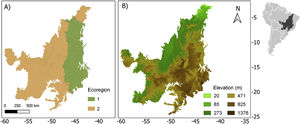
Life-history traits and biogeographic characterThe 63 anuran species that occur in the Cerrado were classified according to the following traits: body size (adult snout-vent length-SVL), habitat (open areas, forest areas, open and forest areas, rupestrian fields), breeding pattern (prolonged, explosive, both), distribution pattern (endemic or widespread). In addition, we classified the species according to the ecoregion (1 and 2) and elevation (highland and lowland) that they occur in the Cerrado (Fig. 1). We grouped the most similar ecoregions based on a cluster analysis provided by the author (Personal communication. Sano, 2021), but with a coarser hierarchical level. We classified the highland and lowland regions using a cutoff value (471.5 m) represented by the median of the altitude range for the species studied (Sano et al., 2019). See SM for more details.
Ecoregion (a) and elevation (b) maps for the Brazilian Cerrado (sensu Sano et al., 2019). On map (b) the green colors represent the lowlands and the brown ones represent the highlands.
We obtained a set of 19 bioclimatic variables through WorldClim database 1.4 (Fick and Hijmans, 2017) with a spatial resolution of 2.5 arc minutes. To reduce problems with collinearity, we calculated a correlation matrix, using Pearson's correlation coefficients with a cut-off value of 75%. After this procedure, we evaluated each variable considering its biological relevance and selected those that were less correlated. We considered seven variables as predictors: BIO1, BIO2, BIO15, BIO17, BIO18, BIO19 and “BIO20” = duration of the rainy season. The “BIO20” were calculated from the raw monthly precipitation data available on Worldclim. We consider the rainy period as the number of months with precipitation greater than 170 mm. This variable was included as a predictor in the modeling process along with the other bioclimatic variables.
Ecological niche models (ENMs)We build the ENMs using the MaxEnt algorithm (version 3.4.1, Phillips et al., 2017). This algorithm is based on the maximum entropy method and uses only known presence data and environmental variables to model species distribution (Phillips et al., 2017). We followed an acknowledged routine and recommendations to build ENMs (e.g. Bagley et al., 2020, see SM for details) using ENMwizard package (Heming et al., 2019).
Statistical analysesTo test the hypothesis that life history traits and biogeographic character may mediate the response to climate change, we used generalized linear models (GLMs). The best model is the one with the lowest AICc and the highest loglik and weight. We use average estimates from the models for inference, once this approach has better accuracy and less bias than the best-model selection approach (Anderson, 2008). All analyzes were performed on the R software (R Core Team, 2021).
ResultsMost species show a projected reduction in their potential distribution area for 2050, considering both the pessimistic scenario (N = 41, 65.07%) and the optimistic scenario (N = 45, 71.42%). At first glance, this result might seem illogical, but when we assessed the magnitude of the losses, we found that even though more species lost area in the optimistic scenario, the amount of area lost was less than in the pessimistic scenario. The results show that in a pessimistic scenario, anurans should lose an average of 742,781 km (SD ± 371,235 km) in their potential distribution area, while in the optimistic scenario, the loss should be an average of 607,714 km (SD ± 340,448 km). The extreme loss in the optimistic scenario was −1,462,964 km, while in the pessimistic scenario it was −1,627,318 km.
Although most species suffer range contractions, a small fraction seems to tend to gain new climatic adequate areas in the future (N = 18, 28.57%) for the optimistic scenario and (N = 22, 34.92%) for the pessimistic scenario (SM Table 2). In the pessimistic scenario, anurans should gain an average of 616,381 km (SD ± 719,261 km) of climatically suitable areas. In the optimistic scenario, the gain should be on average 729,458 km (SD ± 735,946 km). The extreme gain in the optimistic scenario was 2,830,538 km, while in the pessimistic scenario it was 2,823,006 km.
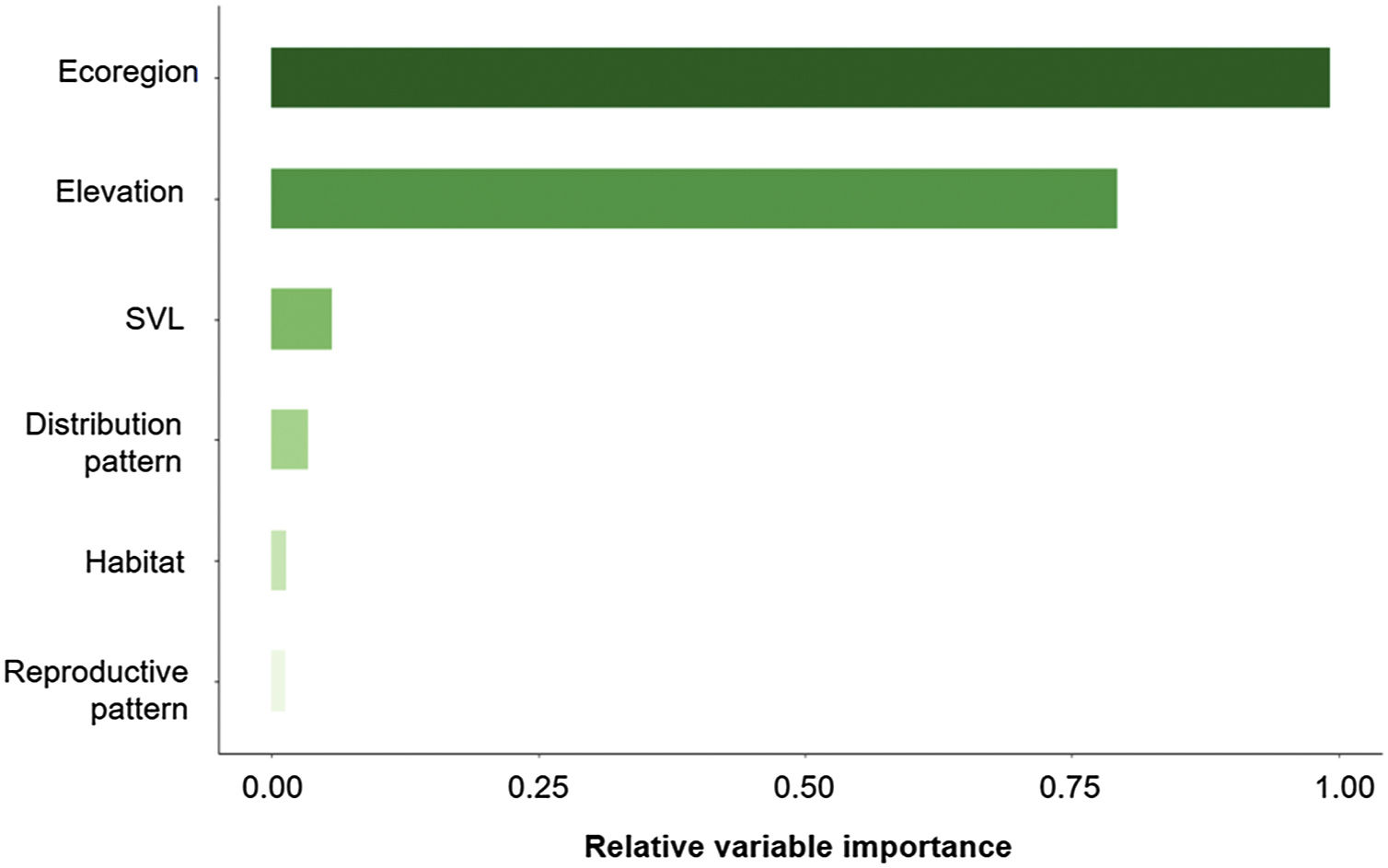
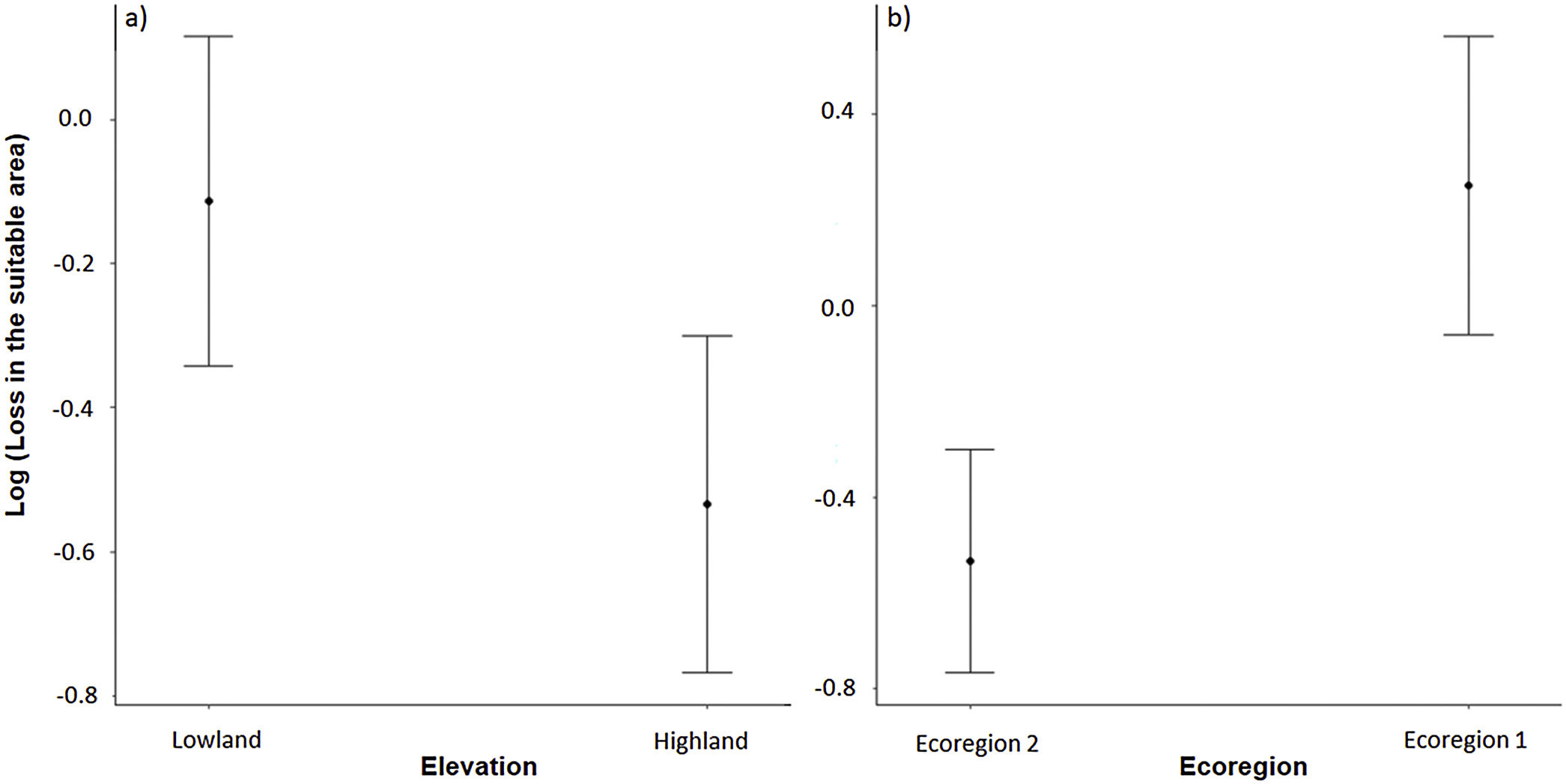
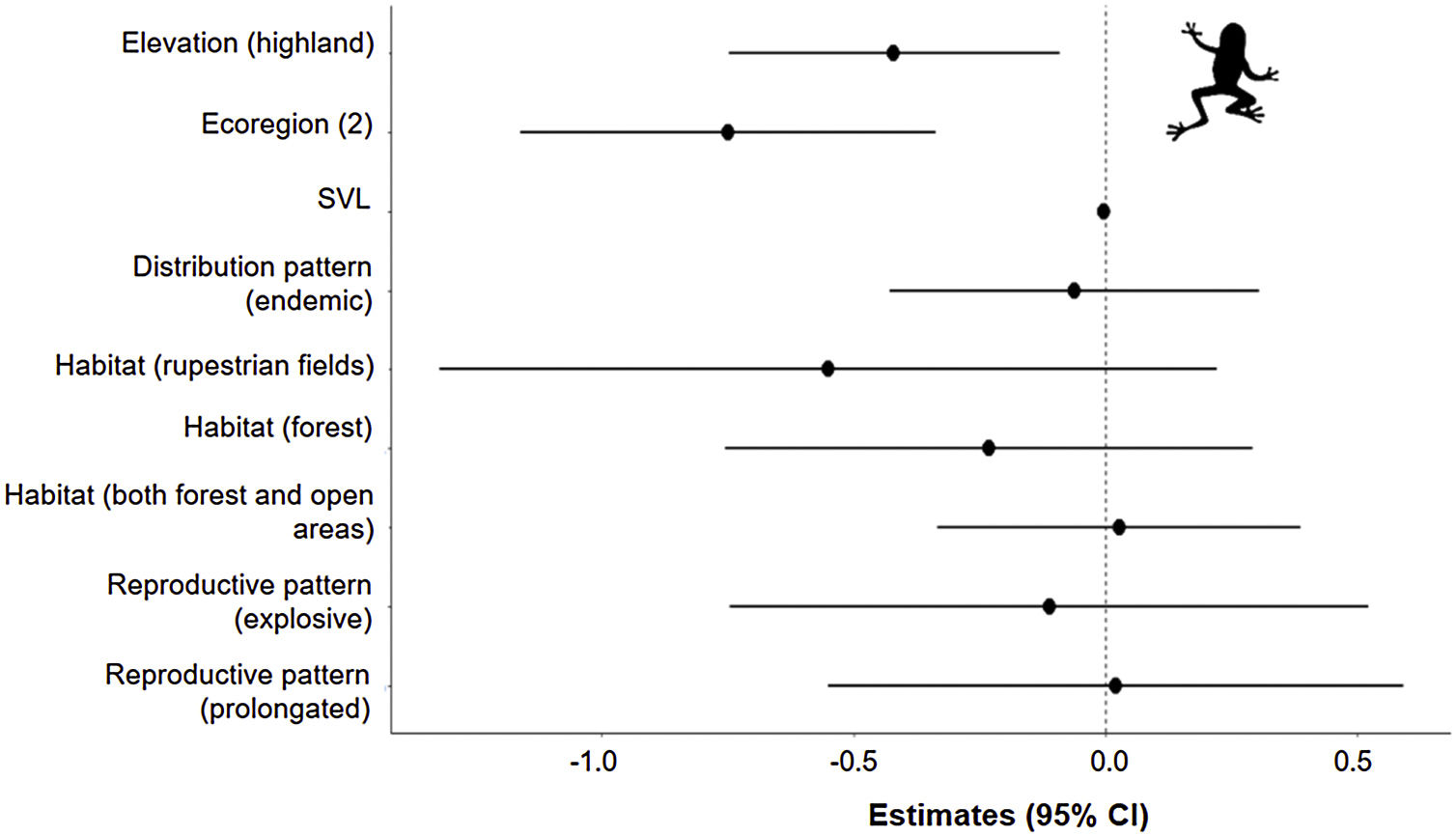
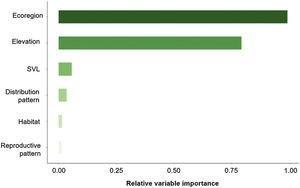
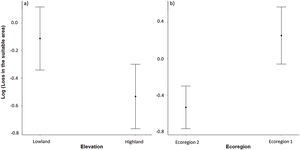
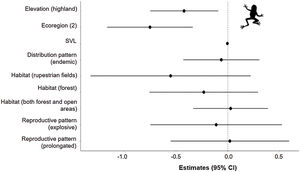
The three best models, according to the lowest AICc, weighted 0.88, 0.05, and 0.02, in this order (Table 1). These models included ecoregion, elevation, distribution pattern and snout-vent length (SVL) as predictors for changes in species distribution in response to changes in climate. The variables with the greatest relative importance were ecoregion (W = 0.99) and elevation (W = 0.79, SM Table 3) while the other variables had relative importance less than 0.03 (Fig. 2). The average model coefficients showed that the ecoregion and elevation can significantly affect its vulnerability to climate change. Species with occurrences concentrated at higher altitudes (highland) and in the western region of the Cerrado tend to present greater projected losses in their potential distributions in the future (SM Table 4, Figs. 3 and 4).
Six models with the highest rankings among the 23 candidate models that assessed the predictive ability of regional traits and life history traits of the species as correlates of changes in distribution in response to global warming. Second order Akaike information criterion values (AICc), AICc weights (Weights), AICc differences (Delta), log-likelihood (loglik) and degrees of freedom (df). The best model is the one with the lowest AICc, the highest LogLik and weight (the first model).
| Models | df | LogLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
| Elevation + Ecoregion | 4 | −55.025 | 118.7 | 0 | 0.789 |
| Ecoregion | 3 | −58.314 | 123 | 4.29 | 0.092 |
| SVL + Ecoregion | 4 | −57.717 | 124.1 | 5.38 | 0.053 |
| Ecoregion + Distribution pattern | 4 | −58.24 | 125.2 | 6.43 | 0.032 |
| Habitat + Ecoregion | 6 | −56.703 | 126.9 | 8.17 | 0.013 |
| Ecoregion + Reproductive pattern | 5 | −58.063 | 127.2 | 8.44 | 0.012 |
Estimates (black points) of the effects of climate change on the potential distribution of anuran species from the Cerrado (63) with distinct characteristics. Confidence intervals (black horizontal lines) that overlap with the null effect line (vertical dashed line) demonstrate the absence of significant effect.
Our findings show that species that inhabit higher-altitude areas and the Western Cerrado will be more vulnerable to climate change. This shared “destination” in the face of climate change is associated with the sharing of some climate niche attributes among species in the same region, leading to similar responses. From this result, it is possible to identify specific regions of the Cerrado that have more vulnerable species and need to be prioritized. One of the most widely used conservation strategies on a global scale has been the establishment of protected areas (PAs) (Drummond et al., 2009). The relevance of these areas to promote the maintenance of biodiversity is unquestionable, but they alone do not guarantee the persistence of populations. The system of conservation units (UCs) currently existing in Brazil is composed of protection areas with static and immutable limits, surrounded by several matrices of soybean, cane and corn crops, especially in the Cerrado (Carvalho et al., 2009). Given that, the most common response of species to climate change is the range shift to track most appropriate climatic conditions, a more efficient conservation strategy could be the establishment of ecological corridor systems, at the landscape level, that allow optimal habitat tracking between patches of natural vegetation.
Our results showed that about 70% of the anuran species studied will lose part of their climatically suitable areas in response to climate change (e.g. Rhinella rubescens, Scinax squalirostris), but some species will have significant potential distribution increases (Leptodactylus cunicularius). Many species are unable to track suitable climatic conditions in space due to low dispersal capacity or the existence of barriers that limit movement (Urban et al., 2013). For these species, phenotypic plasticity and genetic adaptation offer the only way to maintain viable populations and dampen the effects of climate change, providing a means to persist in their current distributions (Urban et al., 2014; Diniz-Filho et al., 2019).
One of the consequences of climate change both for species that lose areas and for species with large potential expansions in the future is the restructuring of anuran communities in the Cerrado (Vasconcelos et al., 2018). This restructuring of communities caused by global warming can lead to changes in the dynamics of competition and interaction between species (Tylianakis et al., 2008). Therefore, identifying species for which climate change will have a lesser potential impact can be of high importance, once the resources for the establishment of conservation actions are limited and need to be effectively directionated to protect the most vulnerable species.
It was also demonstrated that the ecoregion 2 of the Cerrado where we find the greatest loss in the potential area of species distribution concentrates a high peak of anuran richness (Vasconcelos et al., 2018). The high species richness may be related to the environmental heterogeneity found in these areas. The central-west region of the cerrado, for example, includes high altitude areas such as the Chapada dos Veadeiros and lowland areas such as the Araguaia River (Françoso et al., 2020). In the southwest region, there is a strong influence of the Amazon in the floristic composition, while the northwest region has more than 70% of its native vegetation and includes an important portion of the protected areas of Jalapão (Françoso et al., 2020). Despite the high richness, the western region will possibly be exposed to high climatic anomaly values in precipitation patterns considering the difference between the current climate and 2050, as well as the north and northwest regions (Borges and Loyola, 2020).
The scenario could get even worse when we consider that other drivers (such as habitat loss and fragmentation) may interact with climate change (Borges and Loyola, 2020; Borges et al., 2019) through a wide range of mechanisms (Oliver and Morecroft, 2014). For example, fragmentation can make it difficult for species to move across the landscape and thus prevent them from tracking optimal climate conditions in space (Thomas et al., 2004). In the Cerrado, around 30% of the native vegetation is expected to be eliminated by 2050 (Soares-Filho et al., 2016; Strassburg et al., 2017). The Cerrado southern, where the highest anuran richness is found (Diniz-Filho et al., 2004), is one of the regions with the highest rate of conversion of native vegetation into agricultural systems (Strassburg et al., 2017) and with less coverage by environmental protection areas (Françoso et al., 2020). Therefore, the anurans that occur in this region should be affected both by habitat loss and climate change, which should increase the risk of extinction of these species (Colli et al., 2020).
For species that no longer have climatic areas available within the Cerrado, it would be necessary to migrate to the transitional zone between Cerrado and Atlantic forest. In this sense, priority strategies for the conservation of these anurans in the Ecoregion 2 and highlands would be the creation of ecological corridors (several small reserves) to facilitate the movement of species through the landscape. According to the dynamics of metapopulations, keeping several small reserves can reduce the risk of extinction, as it allows the recolonization of new environments (Ovaskainen, 2002). In this case, if a population from a small reserve suffers some type of local disturbance, it is possible that populations from other small reserves will recolonize the area (Tjørve, 2010). For ecoregion 1 and lowland areas, where species must maintain a large part of their climatically adequate area, larger and more spaced conservation areas would be better, because with larger reserves, it is possible to maintain larger populations that are more likely to adapt to environmental changes. For regions that do not have native vegetation, it is necessary to focus on restoration measures to increase habitat and connectivity between areas, preventing a possible homogenization of communities (Borges and Loyola, 2020).
Our results also indicated a reduction in suitable climatic conditions in the future for species at higher altitudes. Species that occur at intermediate and high altitudes can be particularly sensitive, because predicted climate change should shift adequate environmental conditions to ever-increasing altitudes, hampering the dispersal of species to more suitable climates (Enriquez-Urzelai et al., 2019). In this scenario, it is expected that the higher the altitude the species occupies (e.g. mountain top species), the greater the effect of climate change, as the available climatic area tends to reduce (Nori et al., 2016). These areas receive an even higher priority when considering the high species richness they harbor when compared to neighboring areas. Furthermore, due to the restriction of environmental conditions in high altitude areas, most species that inhabit these regions tend to be endemic and depend exclusively on these habitats to persist (Graham et al., 2014). Thus, if they fail to adapt or disperse to new areas, these species can suffer population reduction until they become locally extinct (Pacifici et al., 2015), leading to the loss of a rich portion of biodiversity.
We found a low explanatory power of life-history traits to predict changes in the potential distribution of the species we studied. Contrasting results have been found when using the vulnerability approach based on life-history traits. For instance, several studies have already shown that biological traits can be good predictors of the vulnerability of a large number of species (e.g. Foden et al., 2013; Dawson et al., 2011). On the other hand, Ortega et al. (2019) found that changes in the potential distribution of birds are barely predicted by life-history traits. Despite the low explanatory power for life-history traits, our results provide evidence that the biogeographic character is very important to explain the changes in the distribution of anuran species. To our knowledge, we are one of the few studies that compare the predictive importance of biological traits and biogeographic character in the face of climate change. This approach has important implications in terms of conservation as it indicates whether future strategies should target specific species or regions. Our results indicate that greater attention should be given to species in higher altitude areas and in the western region of the Cerrado, as these are the regions where there should be greater loss of potential anuran area due to climate change and represent critical points for the biodiversity conservation.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. GAF thanks the Modelagem de Nicho e Distribuição de Espécies course at the Universidade Estadual de Santa Cruz (UESC) for their helpfull comments that originin and improved the initial idea of this manuscript. GAF also thanks to the Para Modelagem studies group (UESC) for all discussions about niche models. MS acknowledges funding through a research grant (309365/2019-8) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). DSF received a technological development scholarship from MCTIC/CNPq (Process 465610/2014-5). NMH acknowledges his post-doc fellowship received from CAPES.