Agriculture, cattle grazing, and human settlements negatively affect bird biodiversity, driving the loss of ecologically specialized species and favoring the dominance of generalists. Because ecological pressures define organisms’ success by acting on their intrinsic traits, biodiversity loss due to anthropization might cause directional trait shifts. Here, we use a trait-based approach to find empirical evidence of trait-shifts in bird communities across an anthropization gradient in seasonal forests in central Mexico. We performed point-count bird surveys within a region of tropical deciduous and seasonal oak forests considering three degrees of anthropization: primary forest, secondary growth, and human settlements. A multivariate analysis (PCA) showed similar trait-covariation patterns for both forest types; in the anthropized habitat the bird communities exhibited shorter life-cycles, higher fecundity, and broader ecological niches (diet, foraging habitat, and nesting resources) than those in the primary forests. Our finding of directional trait shifts resembles Evolutionary Ecological Strategies Theory (EES) predictions for successful organisms within highly disturbed anthropized habitats, which are known as a “ruderal adaptative strategy” in the EES framework. The use of trait-based approaches could improve ecological generalizations in bird communities, leading to a better understanding of avian biodiversity's responses to anthropization.
Anthropic activities like agriculture and urbanization drive habitat loss in terrestrial ecosystems, jeopardizing the world’s biodiversity (Vitousek et al., 1997; Corlett, 2015). Many studies have used bird communities as a model to understand biodiversity losses by assessing the shifts in abundance among species and trophic guilds in anthropized areas. Their results show that ecologically specialized birds decrease and become extinct, while generalist birds (frequently, invasive species) become dominant regardless of geographic location (McKinney and Lockwood, 1999; Davey et al., 2012; Lepczyk et al., 2017).
Given that ecological pressures affect organisms’ success by acting on their traits, and that anthropization changes ecological pressures in non-random ways, it is plausible that biodiversity loss under anthropization could follow specific patterns (Shipley, 2010; Grime and Pierce, 2012). However, the classical ecological framework is not capable to detect shifts in traits because it considers species’ abundances, but not the traits related to their responses to environmental change (Shipley, 2010). Animal ecologists have therefore increasingly adopted a functional ecology framework (Luck et al., 2012), which assesses changes in the representation of organisms’ intrinsic functional traits, such as life cycle development, resource attainment, and the organism’s effects on the ecosystem (Violle et al., 2007; Salgado-Negret and Paz, 2016). Because these traits are the vehicle by which ecological pressures act on fitness, this functional approach provides a basis for making mechanistic generalizations about species’ responses to environmental changes and the consequences of biodiversity losses (Diaz et al., 2004; Shipley, 2010; Salgado-Negret and Paz, 2016).
Trait-based studies focusing on birds in anthropized environments have detected the loss of species with large bodies, “slow” strategies (long lifespan, long development times, and low reproductive output; Cooke et al., 2019), and traits related to ecological specialization (Newbold et al., 2013). Recent studies using comprehensive global bird and mammal biodiversity databases have shown that the same trait patterns were favored in both taxa when communities faced simulated extinction scenarios due to anthropization (Cooke et al., 2019). This suggests that the responses underlying community assemblage within anthropized habitats may be broadly generalizable across taxa (Shipley, 2010).
These findings also resemble aspects of Ecological Evolutionary Strategies theory (EES, Grime and Pierce, 2012), which proposes that natural selection favors general strategies of response to a given set of ecological conditions. Specifically, EES predicts that disturbed and unstable environments favor rapid resource attainment, short lifespan, small biomass, and high reproductive output, which are known as a “ruderal” strategy, exemplified by annual herbaceous plants in anthropized areas (Grime and Pierce, 2012). Trait shifts within anthropic bird communities seem to mirror EES predictions, potentially demonstrating that taxa other than plants could follow trait-based pathways shaped by ecological pressures. However, field studies providing empirical evidence are lacking (Grime and Pierce, 2012).
In this study, we use a functional ecology approach to assess whether anthropization favors traits considered advantageous in disturbed environments. We study the resident bird communities of two seasonal forests types in central Mexico: tropical dry forest and oak forest. Both of these ecosystems are imperiled by habitat loss from agriculture and cattle grazing. While several studies in the seasonal tropics have tackled changes in bird communities due to anthropization (MacGregor-Fors and Schondube, 2011; Maya-Elizarrarás and Schondube, 2015; Vázquez-Reyes et al., 2017), they followed the classic species-abundance framework. These works have found losses of species richness and communities dominated by generalist species. In addition, it has been suggested that anthropization drives trait shifts in Neotropical birds (Newbold et al., 2013, 2014). However, whether bird communities exhibit dominance of a trait-based ruderal ecological strategy in response to anthropization remains unknown. Using empirical evidence, we tested whether there was a directional shift in functional traits that could favor birds' success across a gradient of anthropization in seasonal forests of Central Mexico. We hypothesized that anthropization could favor trait patterns that resemble a ruderal strategy in bird communities; thus, we expected that bird traits related to short life cycles, high fecundity, and broader ecological niche requirements would increase in dominance in the more anthropized habitats.
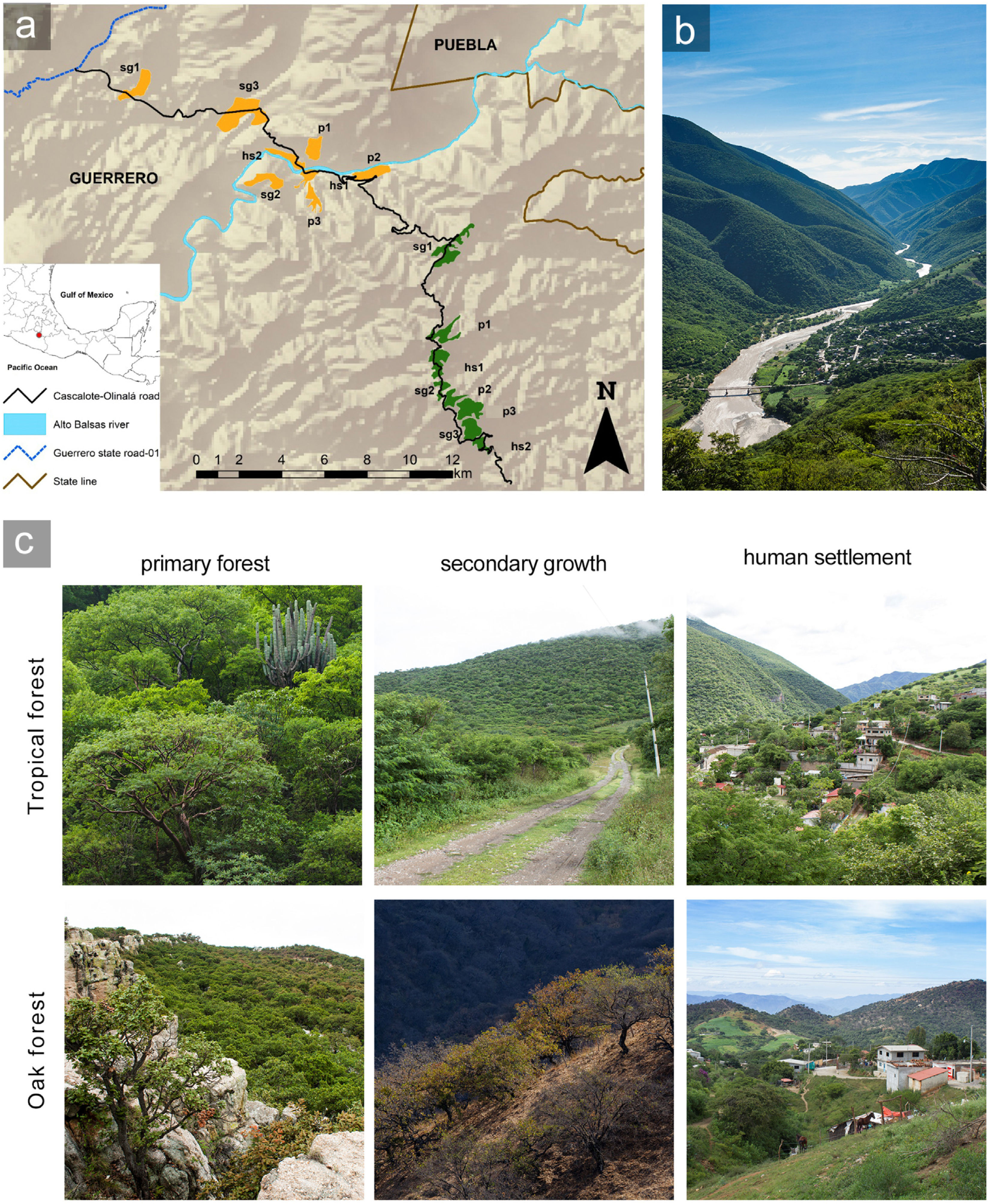
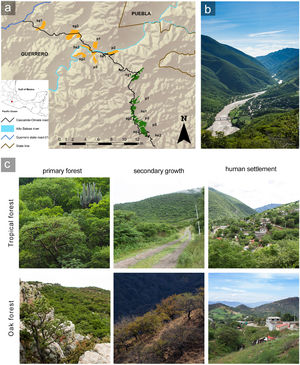
MethodsStudy siteWe conducted this study in the Alto Balsas river basin, in northeastern Guerrero state (18° 03′ – 17° 54′ N; 98° 49′ – 98° 59′ W), which has an area of approximately 225,000 ha (Fig. 1) and an altitudinal range of 650–1950 masl. The climate is warm semiarid with summer rains under the Köppen classification (Peel et al., 2007), and the annual mean precipitation is 780 mm (Meza and López-García, 1997). The dominant vegetation type is tropical deciduous forest from 650 to 1200 masl and oak forest from 1400 to 1950 masl (Rzedowski, 2005). The zone has been altered by human activities, generating a managed mosaic landscape, where we considered three well recognizable anthropization levels: primary forest, secondary growth, and human settlements (Vázquez-Reyes et al., 2017). We defined primary forest as forest where human influence was limited to occasional transit and hunting, vegetation structure was practically intact, and canopy clearings were small (1.5−3 m). Secondary growth was forest whose structure had been modified by agriculture and cattle about 20 years ago and tree cover had partially recovered; secondary growth in the study area has larger canopy clearings (5–7 m) and less complex vertical stratification (13 versus 20 strata) compared to primary forest (Vázquez-Reyes et al., 2017). We defined human settlements as sites where houses, streets, paths, orchards, crops, and livestock enclosures had replaced the original forest, with populations having at most 500 inhabitants. Human settlements retained some forest tree species, as well as a variety of exotic plants. We did not collect quantitative data on vegetation structure within human settlements to avoid potential conflicts with landowners, but the three anthropization levels were easily distinguishable and non-overlapping based on the qualitative characteristics described (Fig. 1).
Study zone in the Alto Balsas region of northeastern Guerrero state, Mexico.
(a) Patches of tropical deciduous forest are in orange and oak forest in green. The anthropization level of each patch is indicated with the following abbreviation plus the corresponding patch number: primary forest = p; secondary growth = sg, and human settlement = hs. (b) Landscape of the Alto Balsas region. The foreground is the Papalutla human-settlement. The background shows the tropical forest at lower elevations (650–1200 masl) and the oak forests at higher elevations (1400–1950 masl). (c) Pictures showing the habitat characteristics within each anthropization level in the study zone. Photos: LDVR – Bio Pic A.C.
We carried out four series of bird counts between February 2014 and May 2016 in eight sites in the tropical and oak forests. We surveyed three sites in primary forest, three is secondary growth, and two in human settlements in each forest type. The size of the patches surveyed ranged from 31 to 199 ha (mean 71.5 ± 40.5 ha). Patches within each forest type were at least 300 m apart and the tropical and oak forest stands were 8 km apart. This resulted in a total coverage of 175 ha in the tropical forest and 196 ha in the oak forest for primary forest; 355 ha in tropical forest and 211 ha in oak forest for secondary growth; and 97 ha in tropical forest and 109 ha in oak forest for human settlements. In each patch, during the early mornings of non-rainy days, we performed point-counts to record all birds seen or heard within a 25 m radius for 10 min, completing 6–8 points per patch during each of the four sampling events. To ensure that records from each point were independent, they were separated by at least 200 m (Ralph et al., 1995). We amassed a total of 204 point-counts in the tropical forest (76 in primary forest, 72 in secondary growth, and 56 in human settlements), and 202 within the oak forest (72 in primary forest, 69 in secondary growth, and 61 in human settlements). We excluded migratory species because their reproduction-related life-history traits are likely more strongly affected by conditions in their breeding range rather than their winter habitat in our study area (Komar, 2002). Supplementary Table S1 presents the raw abundance data at each recorded species by anthropization level.
Bird traitsWe searched bird ecological databases and literature to build a response-trait database for all recorded bird species, considering both life-history and ecological requirements (López-Ordóñez et al., 2016). We considered five continuous life history traits: body mass (grams), clutch size (eggs laid per clutch), hatching age (days), fledging age (days), and lifespan (years). We characterized niche width using three traits: diet (9 categories), habitat use (6 categories), and nesting substrate (12 categories). For each species, we calculated a continuous variable describing diet niche width by dividing the number of detected diet categories by the total number of categories that occurred among all of the communities surveyed. Thus, values close to 1 denote broader diets, while values close to 0 denote more specific diets relative to the communities surveyed (Luck et al., 2013). The same procedure was applied to calculate continuous descriptors for habitat use and nesting substrate. Table 1 lists all the 32 functional considered traits and their ecological significance. Supplementary Table S2 show the trait values for all recorded species.
Description of bird traits considered in the study. Data sources are listed in the Supplementary Table S2.
| Category | Trait | Trait description | Ecological significance |
|---|---|---|---|
| Life-history continuous traits | Body mass | Mass in grams. | Determines metabolic rate, longevity, displacement capabilities, home range, and foraging. Birds with large body mass tend to have ecological requirements associated with primary, unaltered forests. |
| Clutch size | Eggs laid per clutch | Birds with low fecundity are vulnerable to habitat perturbation. | |
| Hatching age | Days of embryo development period before hatching. | Longer incubation periods are associated with slower metabolic rates, usually present in forest specialist birds. | |
| Fledgling age | Age un days when fledglings leave the nest. | Longer fledgling periods are related with slow metabolic rates, which usually occur in large forest specialist birds. | |
| Lifespan | Longevity, in years. | Bird longevity is correlated with slow metabolic rates, which usually occur in large forest specialist birds. | |
| Ecological requirements niche width | Diet | Proportional representation of food items, according to reported diets for each species: (1) vertebrates, (2) carrion, (3) fruits, (4) seeds, (5) terrestrial arthropods, (6) aquatic arthropods, (7) nectar, (8) fishes, (9) anthropic food items. Index values range: 0.11–1. | Higher diet plasticity (niche width) provides more adaptability for birds facing ecological changes in the habitat; moreover, the food items consumed determine bird species’ roles in ecosystem function. |
| Habitat use | Proportional representation of habitat types used in our survey: (1) primary tropical forest, (2) secondary growth tropical forest, (3) tropical human settlement, (4) primary oak forest, (5) secondary growth oak forest, (6) oak human settlement. Index values range: 0.16–1. | Habitat specialist birds are more sensitive to ecological changes. | |
| Nesting substrate | Proportional representation of nesting substrates used for each species, according to specialized literature: (1) tree, (2) shrub, (3) sand bank, (4) cacti, (5) human structure, (6) herb, (7) rock cliff, (8) tree roots, (9) ground, (10) termite nest, (11) dead tree stump, and (12) variable nest substrate. Index values range: 0.083–1. | Higher nest site selection plasticity (niche width) may allow reproductive advantages in changing and perturbed habitats. |
We assessed the completeness of the surveys by computing an abundance-based coverage estimator (ACE) in EstimateS (Colwell, 2013). We calculated the relative abundance of each species in each community (forest type × anthropization level × survey event × patch cells) as the mean recorded abundance among the point-counts in each forest patch; we used the mean rather than the sum to avoid potential bias from repeatedly counting the same individuals (Ralph et al., 1995). We assessed the dominance of each functional trait in each community using Community Weighted Means (CWM, Casanoves et al., 2011), calculated as the sum of each species’ trait values multiplied by its relative abundance. Then, for each forest type (tropical forest and oak forest), we assessed changes in dominance of each functional trait across levels of anthropization by performing non-parametric permutational analysis of variance (PERMANOVA), which was robust to the lack of normality of our CWM values (Anderson, 2017), using anthropization level as the fixed factor. To account for the lack of independence among sampling events within each patch we also included patch-ID as a random factor. We did 9999 permutations, considering the null hypothesis of equality among communities, and considering a significance threshold of p < 0.05. PERMANOVA were computed using the software PERMANOVA+ for PRIMER-7 (Anderson et al., 2008).
In addition to single trait analyses, we used a multivariate approach to test the effect of anthropization on birds’ overall functional strategies. First, we did a principal components analysis (PCA) to summarize the patterns of multivariate trait covariation within each forest type; this allowed us to define the two main axes describing the continuous variables related to bird species’ strategies in the tropical and oak forests (Diaz et al., 2004). We perform the PCA using JMP-9 (SAS Institute, 2010). Similar to the single-trait CWM, we calculated the dominance of functional strategies (described by the PC1 axis) in each community as the sum across all species in the community of the species’ PC1 score multiplied by the species’ relative abundance. We did the same calculation using the PC2 score. Finally, we tested for changes in functional strategies across anthropization levels in each forest type by applying PERMANOVA to PC1 and PC2 as we described above.
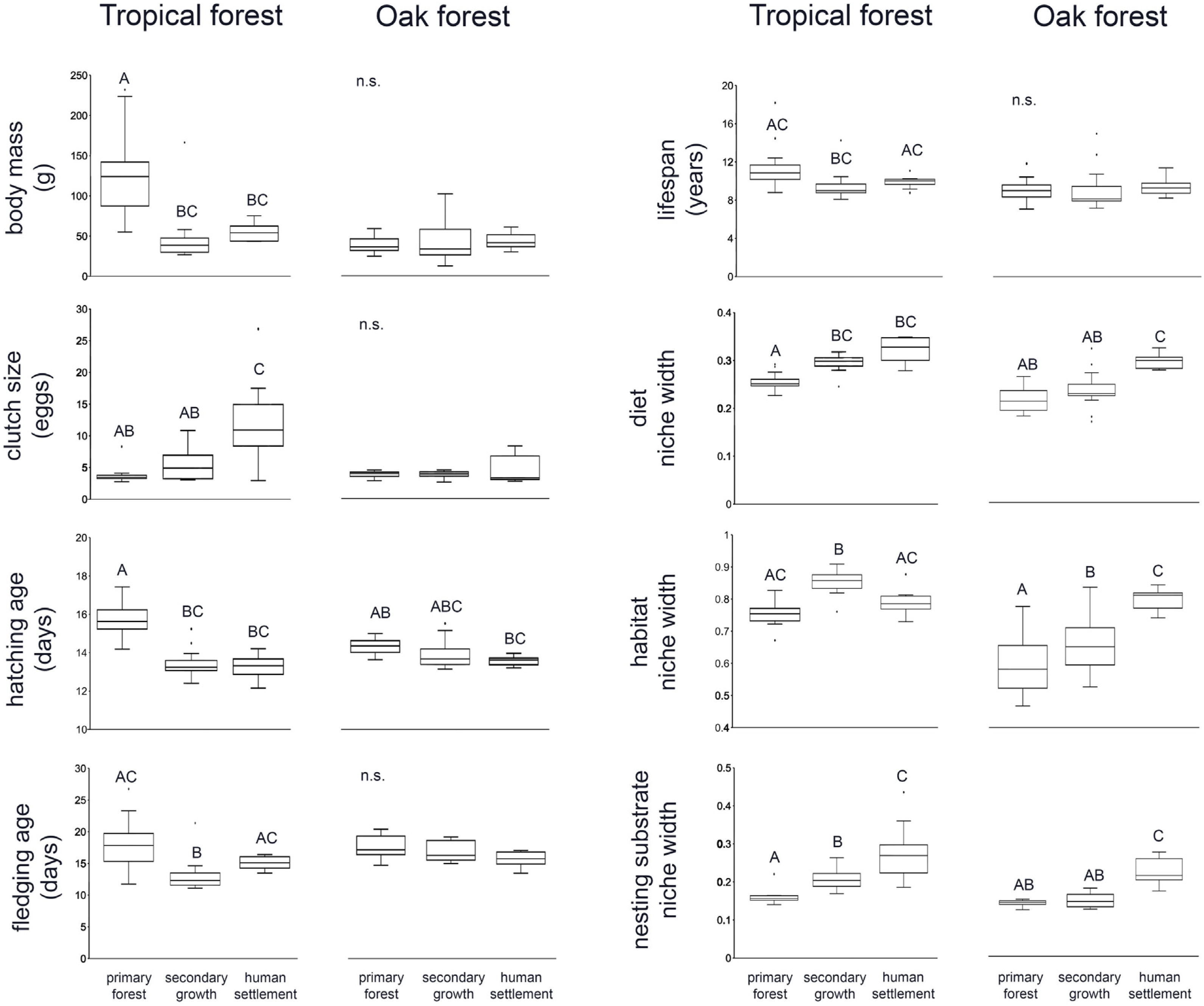
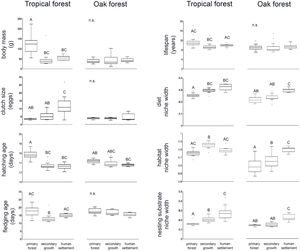
ResultsWe recorded a total of 83 bird species in our survey. In both tropical forest and the oak forest, most of the CWM values differ among anthropization levels, while patch effects were significant for only two traits (fledgling age and habitat use width within oak forest), suggesting that the overall functional dominance of the bird community showed low spatial variation (Table 2, Fig. 2).
PERMANOVA (9999 permutations) of bird traits’ CWM within the tropical and oak forest of the Alto Balsas region.
| Forest type | Trait | Anthropization level | Patch-ID | |||
|---|---|---|---|---|---|---|
| pseudo-F(2,3) | P | pseudo-F(2,24) | p | |||
| (a) | Tropical forest | Body mass | 11.55 | 0.0002 | 0.06 | 0.55 |
| Clutch size | 7.99 | 0.001 | 0.21 | 0.83 | ||
| Hatching age | 30.76 | 0.0001 | 0.05 | 0.94 | ||
| Fledgling age | 6.87 | 0.002 | 0.19 | 0.82 | ||
| Lifespan | 3.54 | 0.028 | 0.05 | 0.95 | ||
| Diet | 22.64 | 0.0001 | 1.07 | 0.35 | ||
| Habitat use | 16.58 | 0.0001 | 1.02 | 0.37 | ||
| Nesting substrate | 13.09 | 0.0001 | 0.26 | 0.78 | ||
| (b) | Oak forest | Body mass | 0.08 | 0.93 | 0.98 | 0.39 |
| Clutch size | 1.63 | 0.21 | 0.42 | 0.65 | ||
| Hatching age | 3.37 | 0.04 | 1.54 | 0.24 | ||
| Fledgling age | 2.13 | 0.14 | 3.58 | 0.03 | ||
| Lifespan | 0.03 | 0.97 | 0.69 | 0.52 | ||
| Diet | 12.35 | 0.0001 | 0.54 | 0.57 | ||
| Habitat use | 18.28 | 0.0001 | 14.98 | 0.0004 | ||
| Nesting substrate | 34.05 | 0.0001 | 0.61 | 0.56 | ||
Note: Due to the mixed model design that considered patch-ID as a random factor, the anthropization level was tested with d.f. = 3, while patch-ID was tested with d.f. = 24.
In the tropical forest, bird communities in primary forest had higher body mass, incubation time, and age at fledging than in the secondary growth and human settlements, while clutch size, diet niche width, and nesting substrate width were smaller in the primary forest than in the anthropized habitat. Life span was longer in the primary forest and human settlements than in the secondary growth, while the habitat niche width was narrower in the primary forest and human settlements than in the secondary growth. In the oak forest, the niche width for diet, foraging habitat, and nesting substrate had lower values in the primary and secondary growth than in the human settlements. The hatching and fledging age were higher in the primary forest and secondary growth than in human settlements (Table 2, Fig. 2). Three traits—body mass, clutch size, and lifespan—were unaffected by anthropization level.
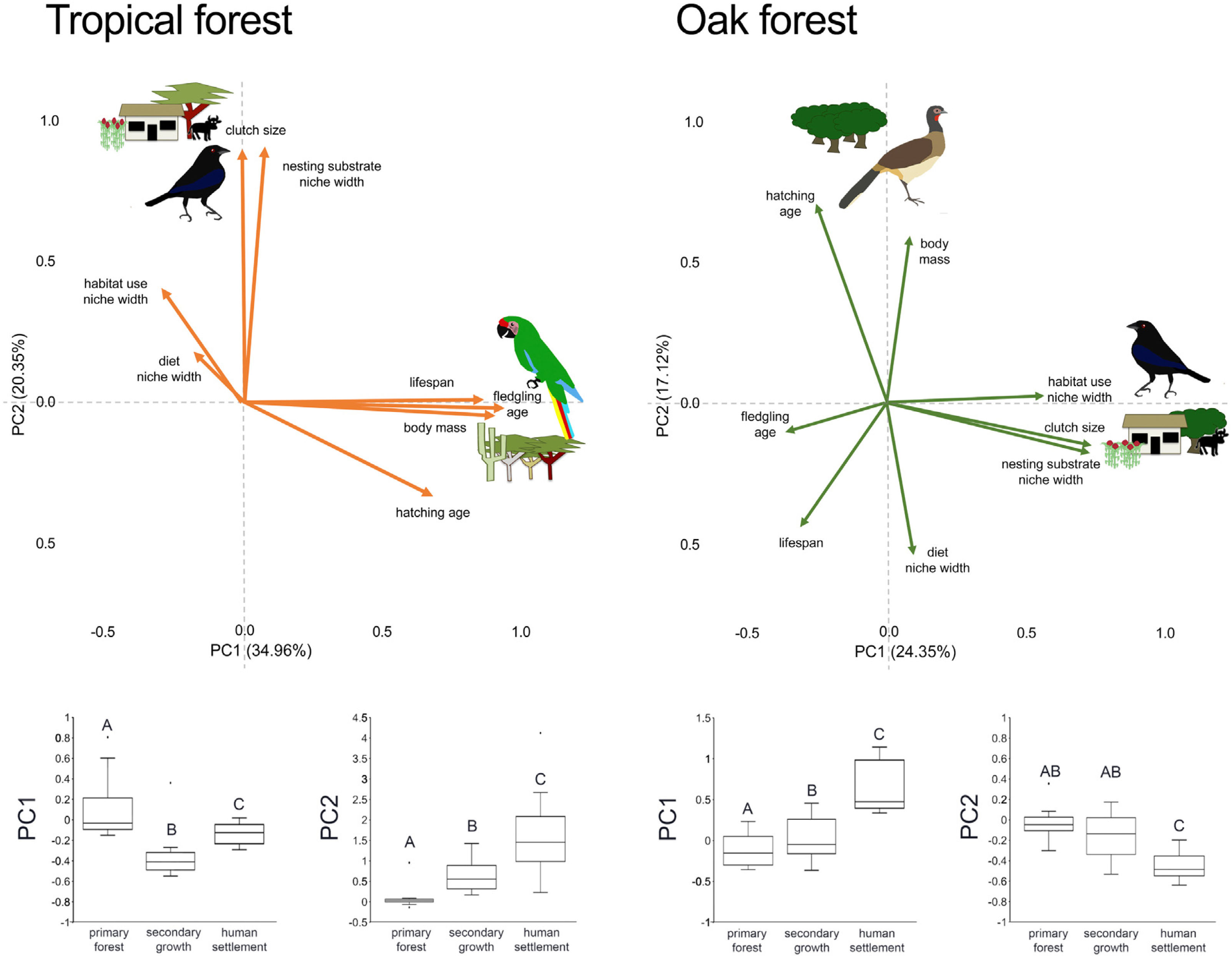
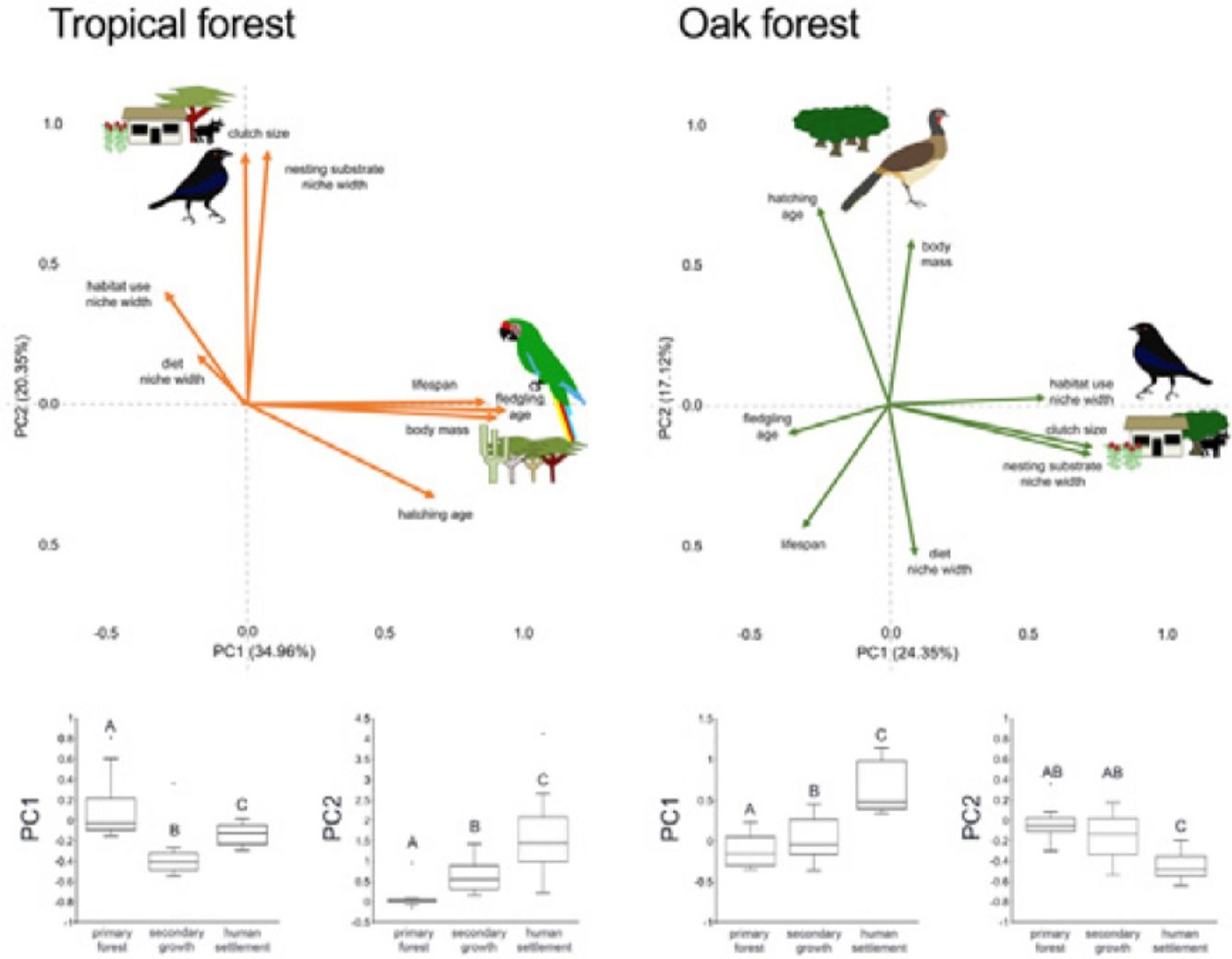
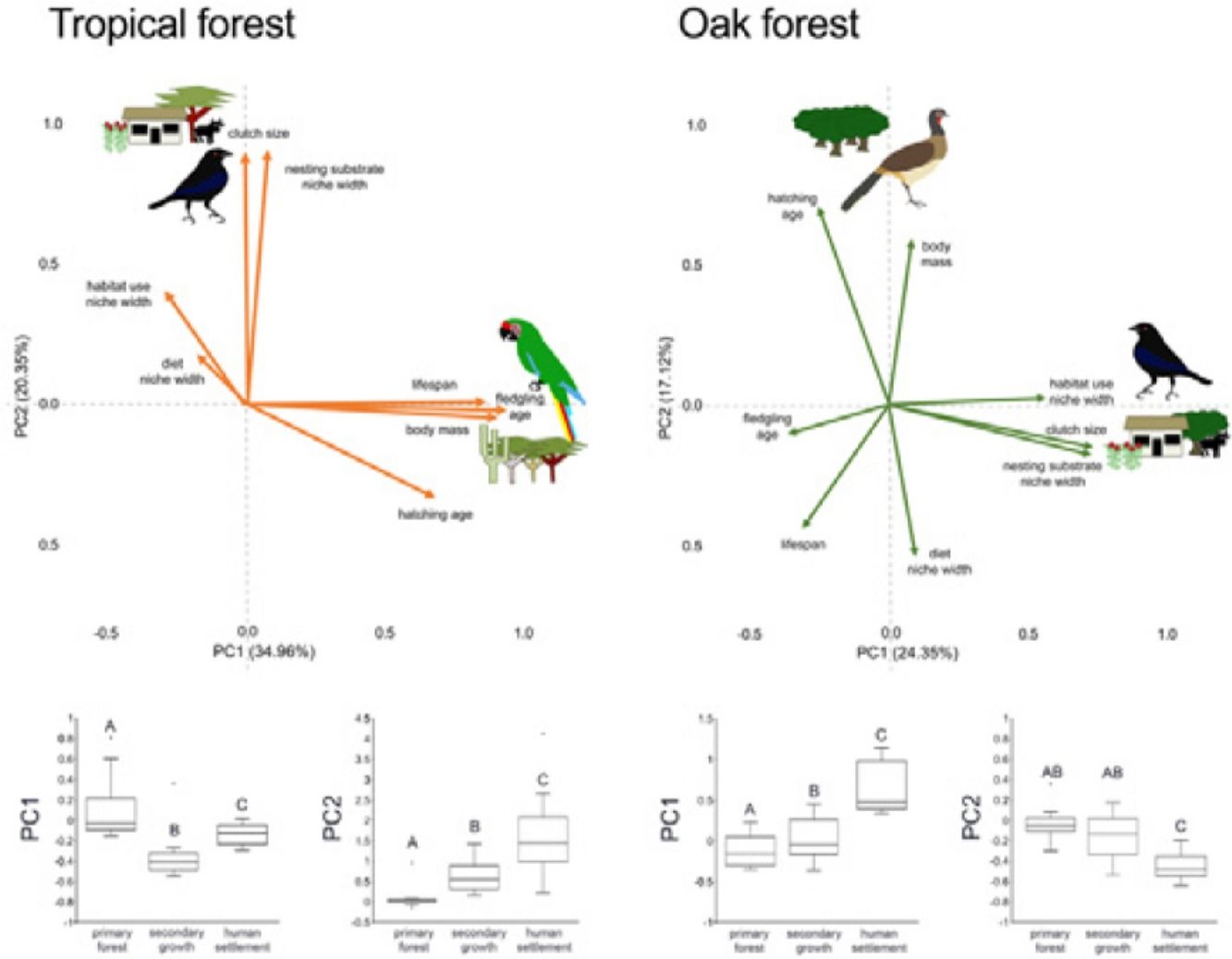
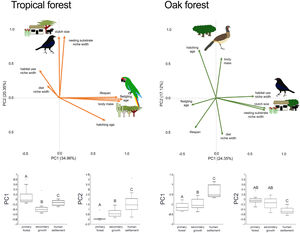
In the multivariate trait correlations, the first two components of the PCA (PC1 and PC2, respectively) explained 55.31% of the variance in tropical forest and 41.47% in the oak forest (Fig. 3, Table 3). In the tropical forest, PC1 indicated that larger body mass was associated with longer incubation times, older age at fledging, longer life spans, and narrower habitat and diet niche widths. PC2 showed that species with larger clutch size tended to have wider habitat use, diet and nesting substrate niches and shorter incubation periods. The results of the PCA in the oak forest showed similar associations among traits, but the order of the PC was reversed. PC1 indicated that species with larger clutches tended to have wider habitat use and nesting substrate niches, shorter incubation periods, younger age at fledging, and shorter lifespans (similar to PC1 in the tropical forest), while PC2 showed that birds with large body masses tended to have longer incubation periods and narrower diet niche width (similar to PC1). However, unlike the tropical forest, dominant birds within the primary oak forest tended to have shorter lifespans (Fig. 3, Table 3).
Bird trait covariation patterns (weighted-PC × bird abundance) within the tropical forest and the oak forest of the Alto Balsas region. Sketches show the bird species with the highest scores for each PC in the tropical forest (Military Macaw, Bronzed Cowbird) and oak forest (Bronzed Cowbird, West-Mexican Chachalaca). The anthropization level where those birds were mainly recorded are also sketched. The box-and-whisker plots below each PCA biplot show significant differences in the PERMANOVA post-hoc tests as letters between anthropization levels (p < 0.05; groups that do not share a letter differ significantly).
Eigenvalues of the principal component analysis on functional traits of bird communities within the Alto Balsas region. PCA was performed independently for the tropical forest and the oak forest.
| Tropical forest (55.31%) | Oak forest (41.47%) | |||
|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | |
| Explained variance | 34.96% | 20.35% | 24.35% | 17.12% |
| Body mass | 0.854986 | −0.042447 | 0.087524 | 0.614201 |
| Clutch size | −0.006321 | 0.846888 | 0.749003 | −0.155534 |
| Hatching age | 0.641124 | −0.313778 | −0.263723 | 0.73137 |
| Fledgling age | 0.884281 | −0.019365 | −0.37789 | −0.111098 |
| Lifespan | 0.814356 | 0.009429 | −0.320679 | −0.458407 |
| Diet | −0.168629 | 0.170766 | 0.099952 | −0.557062 |
| Habitat use | −0.276432 | 0.386382 | 0.590529 | 0.02364 |
| Nesting substrate | 0.071701 | 0.85538 | 0.758249 | −0.175918 |
The PERMANOVA results using the multivariate response variables reinforced the finding that the dominance of birds functional strategies differed among anthropization levels in both forest types (Fig. 3, Table 4). The weighted-PC1 for the tropical forest, and weighted-PC2 for the oak forest showed that dominant birds within the primary forest had larger body mass, longer incubation time, and older age at fledging, and wider habitat use and diet niches than the dominant birds in the secondary growth and human settlements. In the tropical forest, dominant birds in the primary forest also had longer lifespans than dominant birds in the secondary growth and human settlements. The weighted-PC2 for the tropical forest and weighted-PC1 for the oak forest showed that dominant birds in the primary forest had smaller clutch sizes and narrower habitat use than dominant birds in the secondary growth and human settlements (Fig. 3, Table 4).
PERMANOVA (9999 permutations) analyzing bird trait covariation patterns (PC weighted × bird abundance) within the tropical and oak forest of the Alto Balsas region.
| Forest type | PC x abundance | Anthropization level | Patch-ID | |||
|---|---|---|---|---|---|---|
| pseudo-F(2,3) | P | pseudo-F(2,24) | p | |||
| (a) | Tropical forest | PC1 × abundance | 9.85 | 0.0004 | 0.19 | 0.83 |
| PC2 × abundance | 10.45 | 0.0001 | 0.27 | 0.79 | ||
| (b) | Oak forest | PC1 × abundance | 20.29 | 0.0001 | 2.99 | 0.06 |
| PC2 × abundance | 9.26 | 0.001 | 0.91 | 0.41 | ||
Note: Due to the mixed model design that considered patch-ID as a random factor, the anthropization level was tested with d.f. = 3, while patch-ID was tested with d.f. = 24.
Our results indicated that large-bodied birds with slow developmental rates are negatively affected by the intensification of human activities, which has been well documented across different ecosystems (McKinney and Lockwood, 1999). The loss of large bird species in anthropized habitats has been explained by the scarcity of the available habitat and resources (McKinney and Lockwood, 1999; Newbold et al., 2013, 2014). The Military Macaw (Ara militaris) is an example of a large forest bird that was excluded from anthropized habitats (Cooke et al., 2019). Macaws are at the “slow” end of the continuum of life-history strategies exhibited by the bird communities studied here, as evidenced by life history trait values, substantially larger than the CWM in tropical forest (i.e.; body mass of 1100 g versus 125.7; life expectancy up to 40 years versus 11.5; incubation time of 26 days versus 15.7; age at fledgling of 96 days versus 18).
Contrary to the tropical forest, body mass, clutch size, and lifespan of the bird community did not differ among anthropization levels in the oak forest, and the PCA explained a lesser amout of variance. This could be the result of ecological and anthropic factors limiting the body size of the birds in the oak forest. For example, acorns do not have a fleshy mesocarp and are therefore not trophic resources for large-bodied birds (Ramírez-Bastida et al., 2015). This includes species such as the macaws, which consumes fleshy fruits as its main source of food (Contreras-González et al., 2009). This contrasts with the high diversity of trees with zoochoric fruits in the tropical forest. Secondly, in our study region, as in the rest of Mexico, even primary oak forests are impacted by cattle ranching and firewood extraction, which may negatively affect large-bodied birds (Maya-Elizarrarás and Schondube, 2015). Besides, the bird communities in the Alto Balsas basin are subject to loss of taxonomic differences due to anthropization (Vázquez-Reyes et al., 2017). In this process, certain tropical forest birds, such as the West-Mexican Chachalaca (Ortalis poliocephala) and the Russet-crowned Motmot (Momotus mexicanus), reach the highlands covered by oak forests. Finally, local inhabitants mentioned that the Band-tailed Pigeon (Patagioenas fasciata; body mass 360 g, CWM = 40.5 g) was present in the area, but was hunted in the region until 20 years ago. Notably, after a decade of survey, the lack of records suggests that this species is locally extinct (Vázquez-Reyes et al., 2018). Together, these factors may explain the lesser functional differentiation among the anthropization levels in the oak forests in comparison to the tropical forest.
The strong dominance of bird species with high fecundity and wider niches for both nesting substrate and habitat use in the human settlements suggests that those birds are favored by anthropization. For example, the Bronzed Cowbird (Molothrus aeneus) has an abundance up to 8 times higher than any other species within the human settlements. The hatching and fledging times (11 days each) of cowbirds chicks are shorter than the CWM values detected in the human settlement sites of both the tropical forest (13.2 and 15 days) and the oak forest (13.7 and 15.7 days). This is likely facilitated by the fact that cowbirds are interspecific nest parasites, which lay their eggs exclusively in the nests of other species, allowing them to invest most of their reproductive effort in egg production and minimizing parental effort (Mermoz and Ornelas, 2004). This allows cowbirds to be extraordinarily fertile, laying up to 40 eggs yearly (Billerman et al., 2021), placing them at the extreme “fast” end of the functional strategies’ axis described for birds (Cooke et al., 2019).
Nevertheless, having a fast-life pace is not the only biological factor that makes a bird successful in anthropized habitats. Having generalist strategies of resource use is also critical to adapt well to ecologically unstable conditions promoted by the frequent and intense perturbations within the anthropized habitats (Sol et al., 2017; Ducatez et al., 2018). Indeed, the dominance of generalist species is a well-documented pattern (Lepczyk et al., 2017). In our study, the most abundant species in the human settlements were generalist birds, such as the native Bronzed Cowbirds and Inca Doves (Columbina inca), but also the non-native House Sparrow (Passer domesticus) and Rock Pigeon (Columba livia). These species have wide substrate nesting niches and can use both natural and artificial resources for nesting, supporting the idea that generalist birds are more efficient in exploiting resources in anthropized habitats (Davey et al., 2012; Lepczyk et al., 2017).
Conversely, the Pale-billed Woodpecker (Campephilus guatemalensis) is a specialist bird that has been excluded from the human settlements. This species was recorded only in the primary tropical forest. Its exclusion may be due to the fact that they depend on large mature trees to excavate their nesting cavities (Monterrubio-Rico and Escalante, 2006). Similarly, in the oak forest, the Mountain Trogon (Trogon mexicanus) and the White-striped Woodcreeper (Lepidocolaptes leucogaster), both of which depend on forest cover for nesting and feeding (Ramírez-Bastida et al., 2015), were excluded from the human settlements.
The patterns of covariation of multiple traits favored by selective pressures can be defined as ecological strategies (Keddy, 1992; Diaz et al., 2004; Shipley, 2010). Therefore, identifying the dominant traits in anthropized habitats could suggest recognizable ecological strategies among birds (Cooke et al., 2019). The pattern of trait covariation detected in our study as a response to anthropization intensity is very similar to the ruderal strategy of herbaceous plants that are highly successful in anthropized habitats (Grime, 1977). Like the ruderal plants, successful birds in the anthropized habitats tend to have short life cycles and developmental times, high fecundity, and the ability to use a wide range of resources, analogous to high ecological plasticity among plants (Grime, 1977). Following the theory of Ecological Evolutionary Strategies, these ruderal traits may favor individual fitness in environments where disturbance is frequent and resource availability unpredictable (Grime and Pierce, 2012). Overall, similar functional responses between birds and plants to anthropized habitats suggest that the “ruderal strategy” could also be used by birds. Our findings show that functional traits-based ecology is a promising tool to assess patterns and to achieve generalizations on the biodiversity changes, under the rampant anthropization of the forest ecosystems of the world (Luck et al., 2012; Salgado-Negret and Paz, 2016).
FundingFieldwork was funded with grants from CONACyT (152060-B, AGNS and 220265, LDVR) and CONANP (PROCER/DRCEN/003/2015 and /06/2016, Naturam Sequi-LDVR). The Postdoctoral Fellowship (DGAPA-CTIC-UNAM) and SIJAC-UNAM programs (2019/AS/00212/857022) provided support to LDVR during the manuscript writing. HP thanks the sabbatical fellowship from DGAPA, UNAM.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the people of the communities of Papalutla, Mezquitlán, Tomatepec, and Xixila; Juan Esteban, Enrique Rosendo, MZFC-UNAM, Naturam Sequi AC, and BioPic AC for their valuable help during fieldwork. Ernesto Vega-Peña provided valuable suggestions for data analysis. Claudia Gutiérrez-Arellano designed the map in Fig. 1. Lynna Kiere reviewed the English. Víctor H. Jiménez-Arcos, César Ríos-Muñóz, Luis A. Sánchez-González, and three anonymous reviewers provided valuable comments on the manuscript.