Niche theory predicts that species abundance should increase with environmental suitability, but this relationship is highly variable across species. Understanding the causes of this variation is key to guide conservation actions, especially for threatened species. Here we test whether two potentially important but rarely considered factors, protection status and density-dependence, mediate the abundance-suitability relationship in the threatened and overexploited palm Euterpe edulis. We obtained population density data of E. edulis from 50 sites across the Atlantic Forest and estimated environmental suitability from niche modelling. We classified palm populations as low- or high-density, and as protected or unprotected based on the location of the populations (inside or outside protected areas, respectively). The overall abundance-suitability relationship was positive (R2 = 0.32). Inclusion of protection status increased the explanatory power of the models (R2 = 0.46). The abundance-suitability relationship was positive in low-density and in unprotected populations, but neutral in high-density and in protected populations. These patterns suggest that density-dependent factors mediate the abundance-suitability relationship in E. edulis. Our findings show, for the first time, that protection status is an important factor regulating the abundance-suitability relationship, and suggest that unprotected populations may be more vulnerable to future changes in environmental suitability due to climate change. Explicit inclusion of density-dependence and protection status in explanatory models in future studies may increase understanding of abundance variation across species’ geographical ranges, particularly for overexploited species.
Understanding the factors that determine abundance variation across species’ geographical ranges is a key issue in Ecology and Conservation Biology (Sagarin et al., 2006; Krebs, 2008). Estimating and predicting local abundance is crucial to understand ecological interactions and to guide conservation actions, since abundance is a good predictor of long-term species persistence (Ehrlén and Morris, 2015). Several factors may influence abundance patterns, such as environmental conditions, resource availability, biotic interactions, dispersal barriers, and natural or anthropogenic disturbances (Gaston, 2003; Boulangeat et al., 2012; Jiménez-Valverde et al., 2021). Based on niche theory, it is expected that local abundance may reflect the response of populations to environmental conditions, such as local climate, through demographic processes (e.g., survival, growth, and fecundity; Brown, 1984; Brown et al., 1995; Soberón, 2007; Osorio-Olvera et al., 2019). Environmental suitability across species geographical ranges can be estimated using ecological niche modelling (ENM), based on occurrence records and environmental variables (e.g., climate, soil, topography; Elith and Leathwick, 2009). The assessment of environmental suitability from these models could be a useful proxy to predict local abundances across geographical ranges, with higher abundances generally occurring in more suitable sites, potentially due to a better population performance (VanDerWal et al., 2009; Weber et al., 2017).
However, empirical evidence has shown that abundance-suitability relationships are highly variable, evidencing that environmental suitability alone may not always be a good predictor of local abundance (see Dallas and Hastings, 2018). Many studies showed a positive relationship between local abundance and suitability (e.g., VanDerWal et al., 2009; Kulhanek et al., 2011; Oliver et al., 2012; Weber and Grelle, 2012; Bean et al., 2014; Weber et al., 2017; de la Fuente et al., 2021), but others showed negative, weak or non-significant correlations (Pearce and Ferrier, 2001; Nielsen et al., 2005; Elmendorf and Moore, 2008; Filz et al., 2013; Dallas and Hastings, 2018; Sporbert et al., 2020). Such mismatches between local abundance and environmental suitability could be caused by several factors, such as biotic interactions, dispersal barriers, and stochastic events (Araújo and Luoto, 2007; Boulangeat et al., 2012; Braz et al., 2020). Therefore, to better understand the drivers of variation in species abundance, it may be necessary to consider other factors beyond environmental suitability alone.
One potential factor causing variation in abundance-suitability relationships is the density-dependence of some population parameters, caused by intraspecific competition, which can limit population abundance regardless of environmental suitability (Leach et al., 2016; Lewis et al., 2017). As individuals of the same species have similar resource requirements, intraspecific competition effects are likely to increase as local abundance increases, reaching the maximum intensity when populations approach their carrying capacities (Krebs, 1995, 2002). Near the carrying capacity, increases in environmental suitability may not lead to population size increases, as a stronger intraspecific competition can reduce the intrinsic growth rate through demographic processes (e.g., survival, growth and fecundity rates; Thuiller et al., 2014). Consequently, the predicted positive abundance-suitability relationship may be present only in low-density populations (i.e., populations far from attaining carrying capacity), but not in high-density populations. However, most studies investigating abundance-suitability relationships have focused only on density-independent factors, such as climate and soil, to explain abundance variation across species ranges (Anderson, 2016; Leach et al., 2016; Lewis et al., 2017; Mpakairi et al., 2017; Jenkins et al., 2020; but see Boulangeat et al., 2012).
In addition to environmental suitability and density-dependent effects, anthropogenic impacts can also alter the local abundance of many populations (Condit et al., 1996; Stouffer et al., 2006; Palmeirim et al., 2020), potentially causing further variation in abundance-suitability relationships. For example, the overexploitation of some species for commercial and/or sociocultural purposes can directly decrease population size (Asner et al., 2005; Burgess et al., 2017; Fois et al., 2018; Souza and Prevedello, 2020), potentially keeping populations far below carrying capacity, thus reducing the intensity of density-dependent effects on demographic processes (Freckleton et al., 2003). Such anthropogenic impacts, including overexploitation, are frequently less common inside protected areas (Joppa et al., 2008), which may thus harbor higher population densities of many species (Geldmann et al., 2013; Souza and Prevedello, 2020). Therefore, protection status may be a plausible proxy for other environmental factors affecting abundance, including anthropogenic impacts, and thus may mediate the magnitude or even the direction of the abundance-suitability relationship, especially for overexploited species. To date, however, no study has explicitly tested whether the abundance-suitability relationship differs between protected and unprotected populations, which is key for the conservation of threatened and overexploited species.
To test whether the abundance-suitability relationship may be regulated by protection status and density-dependent factors, we studied 50 populations of the threatened palm Euterpe edulis, spread across an entire biodiversity hotspot, the Atlantic Forest. This species is currently listed as Vulnerable in Brazil (Martinelli and Moraes, 2013), especially due to overexploitation, which causes significant variation in local abundance across the Atlantic Forest (Souza and Prevedello, 2020). In addition, the population dynamics of E. edulis has been shown to be affected by density-dependent factors (Silva-Matos et al., 1999). Therefore, extensive overexploitation and density-dependence make this species a suitable model organism to test whether protection status and density-dependent factors may drive variation in the abundance-suitability relationship.
Materials and methodsStudy speciesThe palm E. edulis has a wide geographic distribution, occurring in Brazil, Argentina and Paraguay (Henderson et al., 1995). Most of the native distribution range is in the Brazilian Atlantic Forest, where the species has a wide latitudinal distribution, but the species also occurs naturally in the Brazilian Cerrado (Henderson et al., 1995). Euterpe edulis is considered the most important non-timber forest product of the Atlantic Forest, as its palm heart has been extensively exploited for human consumption (Silva-Matos et al., 1999). This economic activity eliminates adults and reduces seed production, thus decreasing population density and growth (Freckleton et al., 2003; Souza and Prevedello, 2020). Also, this species has important ecological functions, as its fruits are considered a keystone resource consumed by a large variety of animals, especially birds and mammals (Galetti et al., 1999).
Population density estimatesDensity estimates of E. edulis were obtained from an extensive literature search on different databases (Web of Science, SciELO, Scopus, and Google Scholar), and from Melito et al. (2014) (see Souza and Prevedello, 2020 for details). We only included density estimates when precise coordinates were provided or could be obtained based on sampling sites description. We retained information from studies that measured the density (individuals/ha) separately for different ontogenetic stages of E. edulis. We did not use density estimates from studies that only sampled E. edulis individuals above a minimum size (e.g., diameter at breast height >10 cm). For studies reporting density values for different years in the same sampling site, we averaged density across the whole period, thus including a single density estimate per site in our analyses. Similarly, for studies with nearby sampling plots (<1000 m) in the same site, we calculated the average density across plots. Some studies showed density estimates for sites spread across a broader region, which were considered as independent estimates (e.g., Oliveira et al., 2014). With these procedures, we obtained density data for a total of 50 sites spread across most of the Atlantic Forest (Fig. S1a; see also Souza and Prevedello, 2020 for further details). The large geographical area covered by these population data includes large variation in latitude and climatic conditions, allowing a robust test of how variation in environmental suitability affects abundance.
From each study, we estimated E. edulis density for three ontogenetic stages separately: seedlings, adults and intermediate-class individuals. The seedling stage included stemless individuals with palmate leaves. The adult stage included reproductive individuals, i.e. individuals with flowers and/or fruits (Souza et al., 2018). The intermediate stage included sapling and immature individuals; finer subdivisions of this stage were not possible because different studies used different classification criteria. We also measured per capita seedling density (Comita et al., 2007) in each population, as the ratio between seedling density and adult density.
Density-dependent factors and protection statusA previous study has shown the occurrence of density-dependence effects on E. edulis, with a lower probability of seedling growth and survival in higher densities of seedlings and adults, which in turn can promote the regulation of population growth (Silva-Matos et al., 1999). Therefore, we considered “density class” as a predictor variable in our analysis, to check a possible density-dependent effect on the abundance-suitability relationship. To do so, we classified each of the 50 E. edulis populations as either “low-” versus “high-density” populations, based on the median density observed across all populations (see “Data analysis”, below).
We also recorded the protection status of each population as a second predictor variable. We included protection status as a proxy for anthropogenic disturbance, in particular harvest occurrence, since E. edulis populations inside protected areas are less subjected to palm-heart exploitation (Souza and Prevedello, 2020). In addition, protection status may also be a proxy for forest cover and seed disperser diversity, since both factors tend to be higher inside protected areas than outside. In addition, a previous study showed that E. edulis adult density is higher inside protected areas (Souza and Prevedello, 2020). This study, however, has not tested for differences in the other ontogenetic stages and per capita seedling density, neither investigated abundance-suitability relationships.
Ecological niche modellingWe obtained occurrence records of E. edulis in South America from the literature (Henderson et al., 1995; Sales et al., 2021) and from online databases: GBIF (https://www.gbif.org/), REFLORA (http://reflora.jbrj.gov.br), Project JABOT (http://jabot.jbrj.gov.br), and Steere Herbarium (http://sweetgum.nybg.org/science). For modelling, we only kept occurrence records spaced at least 10 km apart (ten times the pixel size of the environmental layers – 1 km; see below) to maximize their independence. With these procedures, we obtained a total of 335 valid occurrence records for E. edulis (Fig. S1b).
To generate the background area over which the ecological niche models (ENMs) were built, we created a 200 km buffer around the minimum convex polygon encompassing all occurrence records of E. edulis (as in Souza and Prevedello, 2021). We obtained 19 bioclimatic variables and elevation from WorldClim 2.1 (Fick and Hijmans, 2017) at 30 s resolution (i.e., a cell with ∼1 × 1 km), representing historical (∼1970–2000) conditions. To select the bioclimatic variables for modelling, we extracted their values at 1,000 random points within the background area and portrayed them into a correlation matrix. We only kept six bioclimatic variables with low correlation (r < 0.65), in addition to elevation: temperature annual range, mean temperature of wettest quarter, annual precipitation, precipitation seasonality, precipitation of warmest quarter, and precipitation of coldest quarter.
We also considered the Normalized Difference Vegetation Index (NDVI) as an additional explanatory variable in the ENMs. This variable may be a proxy of biotic conditions and habitat suitability for E. edulis, as individuals of this species only grow and survive inside forest areas (Gatti et al., 2014), and their seeds are dispersed by many forest-dependent species (Sales et al., 2021). NDVI was obtained by averaging monthly data (2001–2010) from the MODIS-Terra MOD13C2 v006 dataset with resolution of 0.05 min (Didan, 2015). After averaging, we resampled the original resolution to 30 s through a bilinear interpolation. The ENMs including NDVI returned similar results as the models without NDVI, but with slightly lower coefficients of determination for explanatory models (Table S1). Therefore, we present in the main text only the results for the ENMs without NDVI.
We estimated environmental suitability for E. edulis using five algorithms that encompass different modelling assumptions and approaches: BIOCLIM (Booth et al., 2014), GLM (Guisan et al., 2002), MaxEnt (Phillips and Dudík, 2008), Random Forest (Breiman, 2001), and SVM (Drake et al., 2006). For BIOCLIM, we used as training data only the occurrence records of E. edulis. For GLM, SVM and Random Forest, we used as training data the occurrence records plus 10 pseudo-absence points for each record, totaling 3,350 pseudo-absences. Pseudo-absence points were randomly chosen from pixels located within the background area, but outside the environmentally suitable area as estimated from BIOCLIM (following Lobo and Tognelli, 2011). For MaxEnt, we used as training data the occurrence records plus 10,000 background points randomly chosen within the background area.
For each algorithm, we used 10-fold cross-validation, splitting data into 90% training and 10% test, replicated 10 times (Elith and Leathwick, 2009). For testing, we used the 10% remaining presences and pseudo-absences for all algorithms. We estimated the predictive performance of each model through the true skill statistic (TSS, Allouche et al., 2006) and the area under the receiver operating characteristic curve (AUC), separately for each algorithm and iteration. We only retained as valid models those with both TSS > 0.70 and AUC > 0.85 (as in Souza and Prevedello, 2021). We then used each valid model to produce a separate map of predicted environmental suitability across the Atlantic Forest.
To ensemble the continuous maps produced by each algorithm, we first rescaled pixel values to vary between 0 and 1 for standardization, as the original output scale varied among algorithms (i.e. minimum = 0 and maximum = 1 for BIOCLIM, MaxEnt and Random Forest, but not for GLM and SVM). We then calculated a TSS-weighted mean across the maps, obtaining a single final map of continuous suitability. Since the choice of the algorithm is the greatest source of uncertainty in ecological niche modelling (Watling et al., 2015), we used in the statistical analysis only the suitability values from the ensemble map. For each site with E. edulis density data, we extracted the environmental suitability value from the final ensemble map (Fig. S1b). Algorithms were implemented in R using the packages ‘dismo’ (Hijmans et al., 2017), ‘randomForest’ (Liaw and Wiener, 2002) and ‘raster’ (Hijmans, 2017).
Data analysisWe first tested the overall relationship between population density and environmental suitability across the 50 sites combined, using a linear regression model. We applied a logarithmic transformation to density values (i.e. ln(density+1), to normalize model residuals and thus attend the assumption of linear modelling. We focus the main text on the results for total population density only, i.e. the density of the three ontogenetic stages combined, as the results were similar for the three stages separately (Figs. S2 and S3). We used data from the 50 sites, including one site with zero density, sampled in a landscape where other populations of the species were recorded (Pires, 2006). A separate linear model was used to test the overall relationship between ln(per capita seedling density + 1) and environmental suitability. This model was fit using only 43 populations, as five populations had zero density for adults, and information on seedling density was unavailable for two populations, precluding calculation of per capita seedling density. For all analyses, we assumed that the 50 sites provided independent information, based on the analyses of Souza and Prevedello (2020), which showed that pseudo-replication is unlikely in this dataset, as similar results were obtained using simple or mixed-effect linear models.
To test whether the relationship between density and suitability differed between low- versus high-density populations, we first classified each of the 50 populations according to its total density. “Low-density” populations corresponded to populations with total density lower or equal to the median total density observed across all populations (1,844 individuals/ha), whereas “high-density” populations corresponded to populations with density higher than the median density. We used the median as the cutting value to obtain similar sample sizes for low- and high-density populations. We then built two separate linear models for each dependent variable (population density or per capita seedling density, both as ln(variable + 1)), one model for each density class (i.e. low- or high-density populations), including environmental suitability as the explanatory variable. Similar results were obtained using an alternative analytical approach, in which we included environmental suitability, density class (low- versus high-density) and their interaction as explanatory factors in a single model for each dependent variable (Table S2a,b). This alternative approach showed significant interaction terms, which indicate that the effects of environmental suitability on the dependent variables differ between low-versus high-density populations, the same result shown by the separate linear models (see Results).
Similarly, to test whether the relationship between density and suitability differed between protected and unprotected populations, we built two separate linear models for each dependent variable (population density or per capita seedling density, both as log(variable + 1)), one model for protected and another for unprotected populations, with environmental suitability as the explanatory variable. Similar results were also obtained when considering environmental suitability, protection status and their interaction as explanatory factors in a single model for each dependent variable (Table S2c,d).
ResultsTotal population density of E. edulis varied from 0 (one population) to 55,840 individuals/ha (mean ± SD = 6,203 ± 11,692), whereas environmental suitability varied from 0.52 to 0.88 (0.67 ± 0.08), across the 50 sites (Fig. S1b). Per capita seedling density varied from 0.3 to 930.7 seedlings/adult/ha across the 43 sites with valid data (57.5 ± 144.4). The performance of the valid (retained) environmental suitability models was very good (AUC and TSS mean ± SD = 0.99 ± 0.01 and 0.94 ± 0.06, respectively).
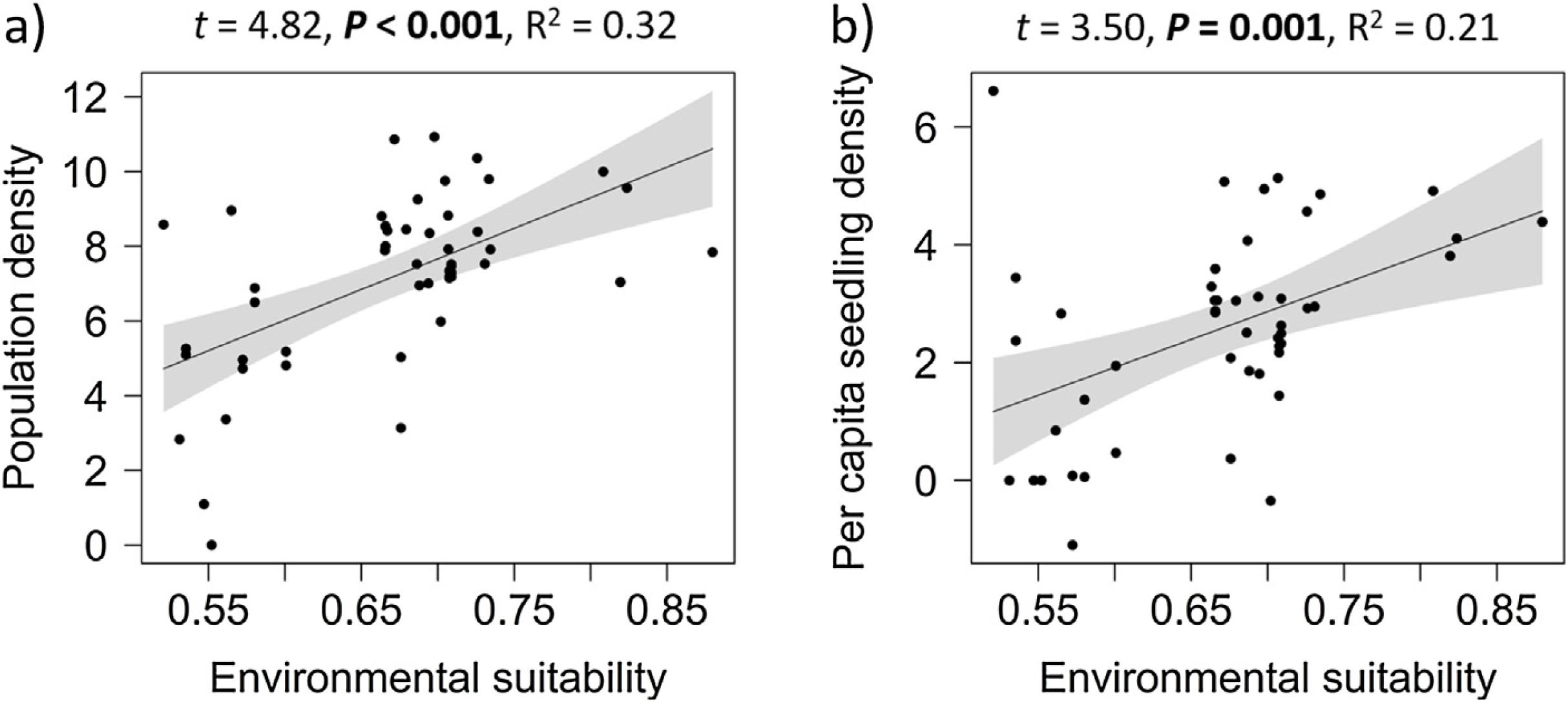
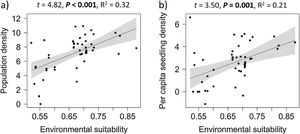
Overall effects of environmental suitabilityPopulation density increased significantly with environmental suitability across the 50 sites (df = 48, t = 4.82, P < 0.001; R2 = 0.32; Fig. 1a). Similarly, per capita seedling density increased significantly with environmental suitability (df = 46, t = 3.50, P = 0.001; R2 = 0.21; Fig. 1b).
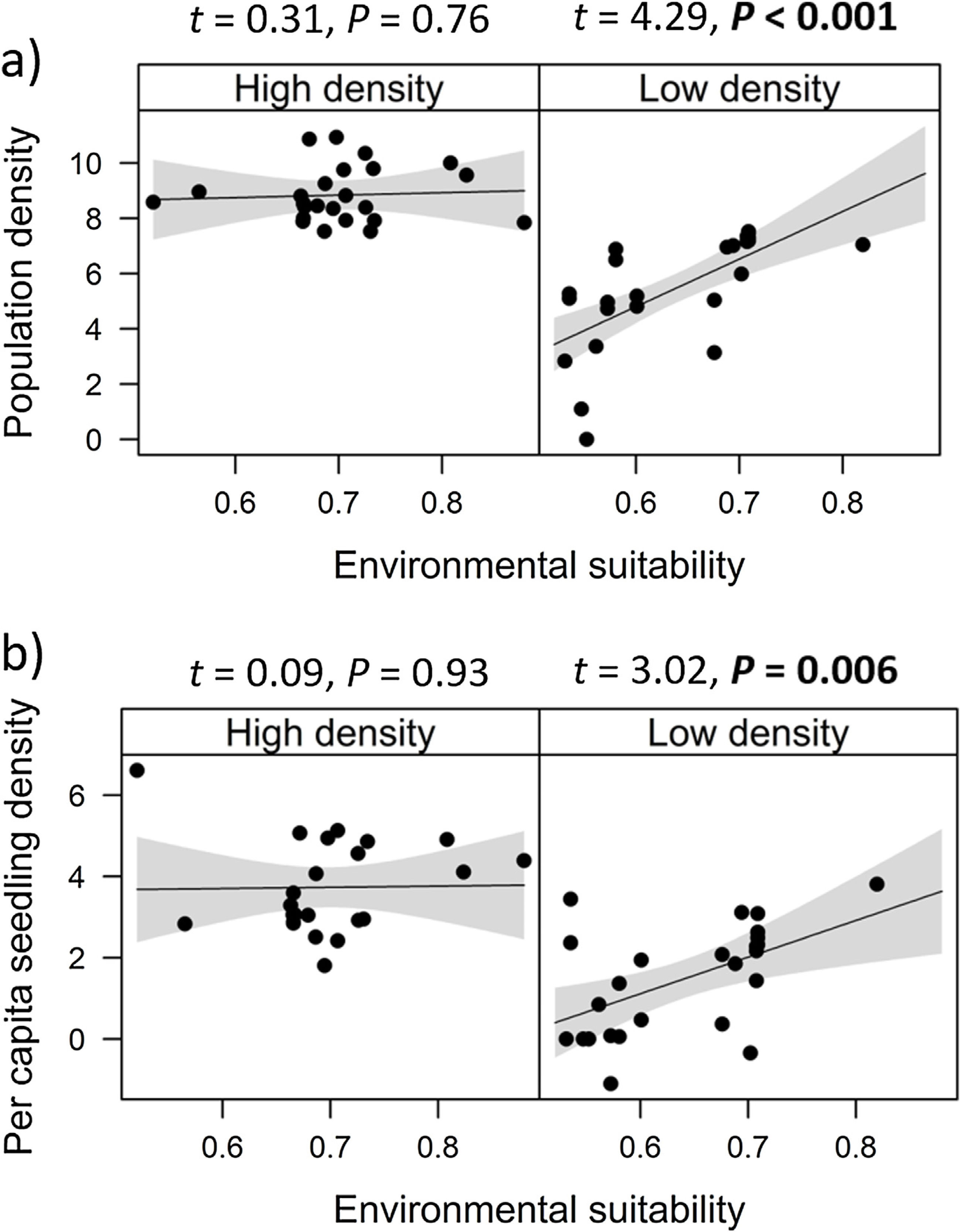
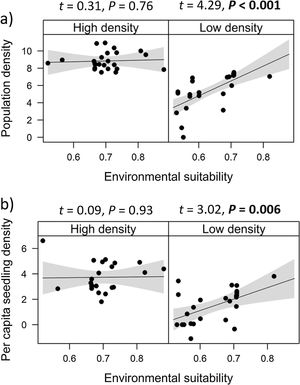
Combined effects of environmental suitability and density-dependenceEnvironmental suitability had different effects on population density in low- versus high-density populations. Environmental suitability had no significant effect on population density in high-density populations (df = 23, t = 0.31, P = 0.76), but a positive effect in low-density populations (df = 23, t = 4.29, P < 0.001; Fig. 2a). Similar patterns were observed for the three E. edulis ontogenetic stages analyzed separately (Fig. S2).
Effects of environmental suitability on (a) population density and (b) per capita seedling density in low-versus high-density populations of Euterpe edulis in the Brazilian Atlantic Forest. Low-density populations correspond to populations with density lower or equal to the median density observed across all populations; high-density populations had density higher than the median density. Density values are represented as ln(individuals/ha). Statistically significant relationships (P ≤ 0.05) are highlighted in bold.
Similarly, environmental suitability effects on per capita seedling density were different in low- versus high-density populations. Environmental suitability had no significant effect on per capita seedling density in high-density populations (df = 21, t = 0.09, P = 0.93), but a positive effect in low-density populations (df = 23, t = 3.02, P = 0.006; Fig. 2b).
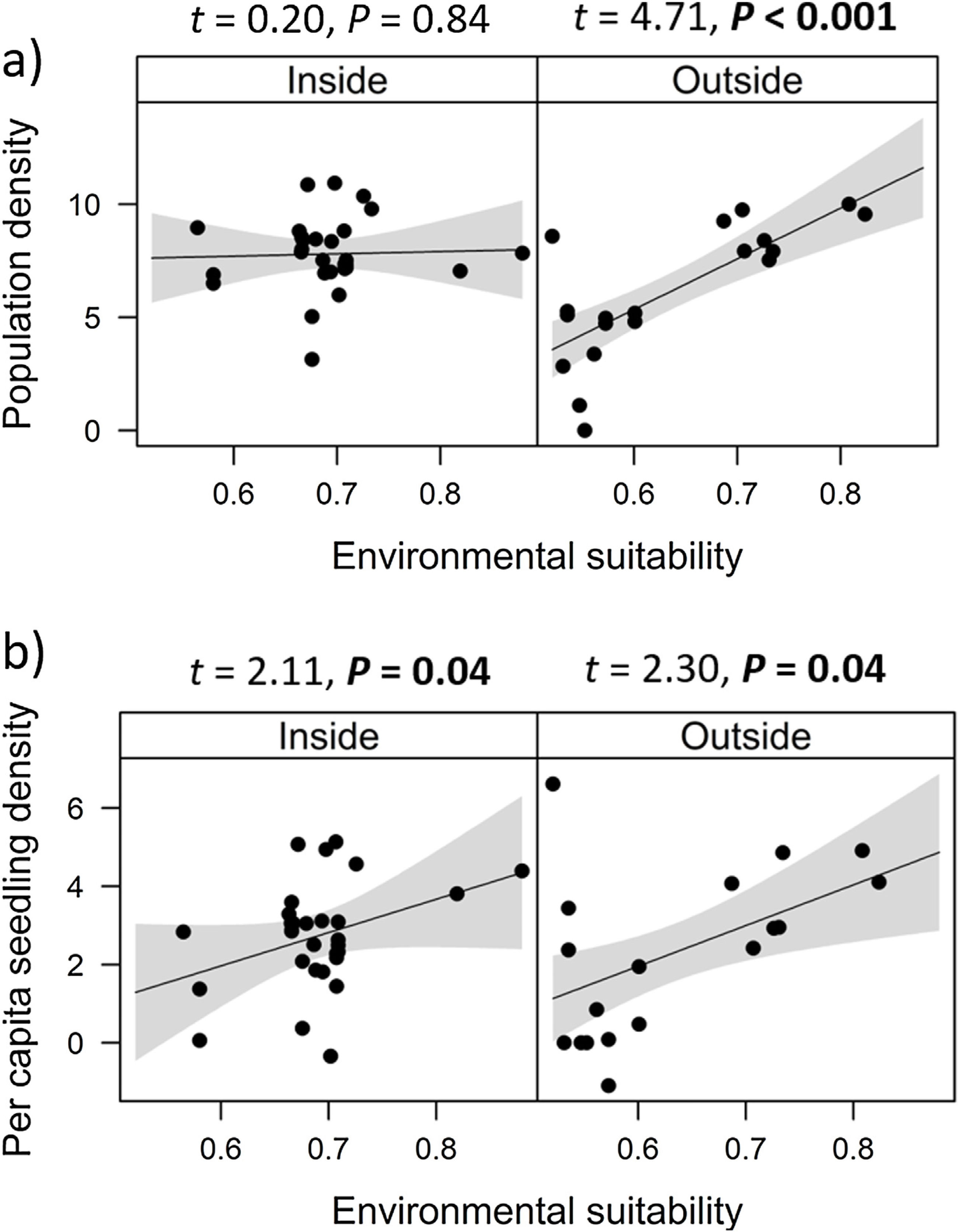
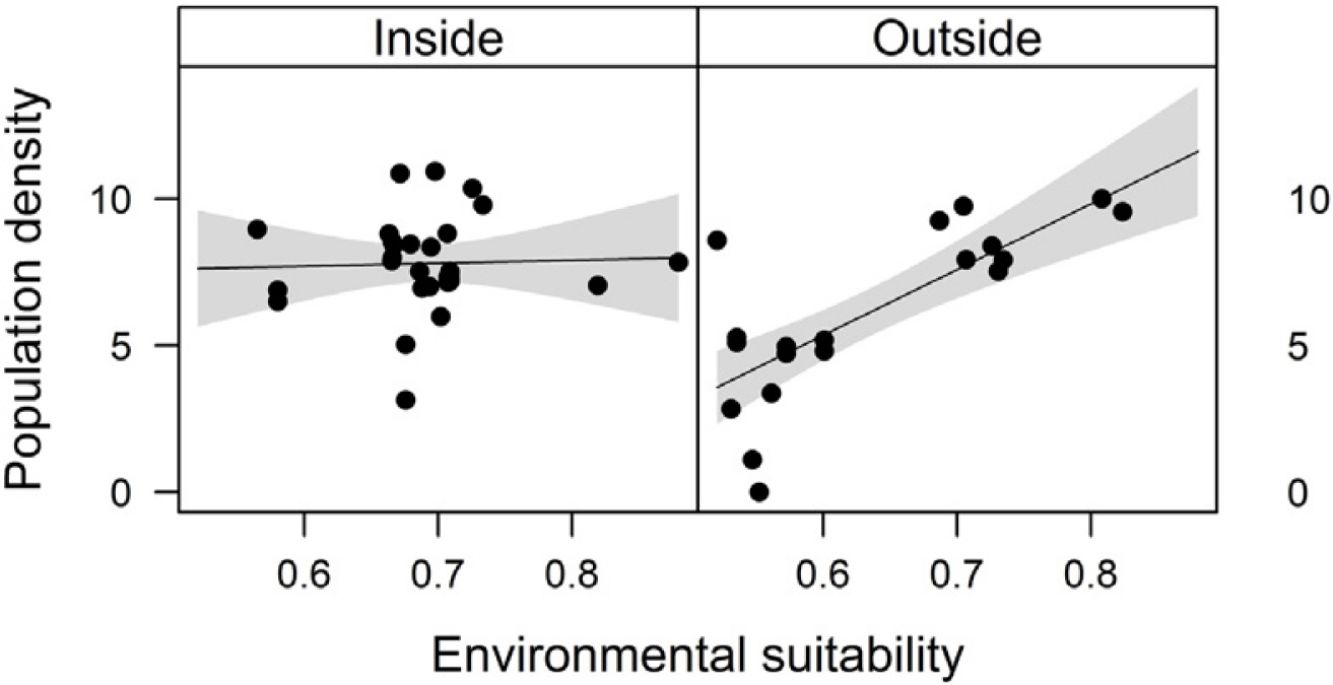
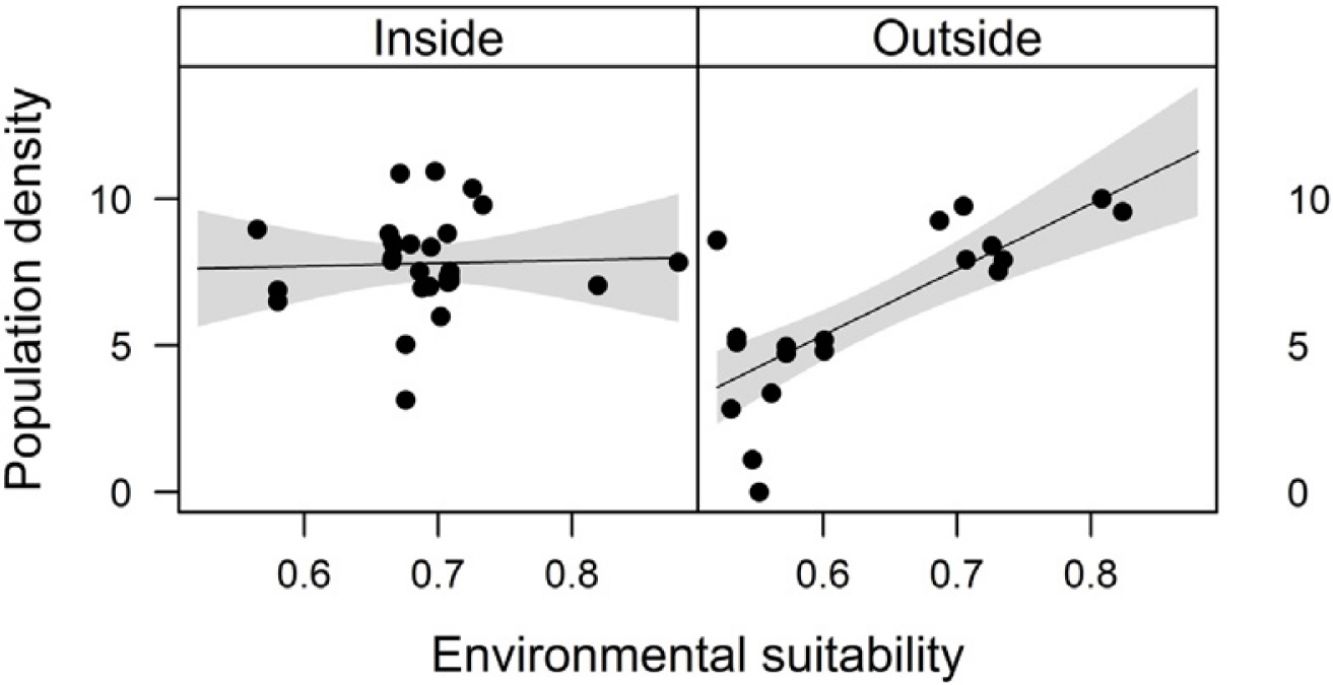
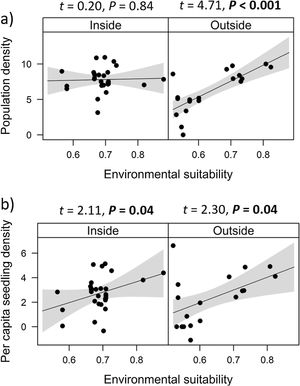
Combined effects of environmental suitability and protection statusThe inclusion of protection status (inside versus outside protected areas) increased the explanatory power of models for population density (R2 = 0.46) when compared to the model with environmental suitability only (R2 = 0.32; see above). Population density was on average 1.7 times higher inside (mean ± SD: 7,362 ± 13,885) than outside protected areas (4,312 ± 6,689 individuals/ha). Environmental suitability had different effects on population density inside and outside protected areas. Environmental suitability had no significant effect on population density inside protected areas (df = 29, t = 0.20, P = 0.84), but a positive effect outside protected areas (df = 17, t = 4.71, P < 0.001; Fig. 3a). Again, similar patterns were observed when different E. edulis ontogenetic stages were analyzed separately (Fig. S3).
Effects of environmental suitability on (a) population density and (b) per capita seedling density in populations of Euterpe edulis located inside and outside protected areas in the Brazilian Atlantic Forest. Density values are represented as ln(individuals/ha). Statistically significant relationships (P ≤ 0.05) are highlighted in bold.
Finally, environmental suitability had positive effects on per capita seedling density both inside (df = 28, t = 2.11, P = 0.04) and outside protected areas (df = 16, t = 2.30, P = 0.04; Fig. 3b).
DiscussionConsistent with many previous studies, our results showed a positive abundance-suitability relationship for the 50 E. edulis populations across the Atlantic Forest (Weber et al., 2017; de la Fuente et al., 2021; but see Dallas and Hastings, 2018). In addition, we observed that the direction and strength of the abundance-suitability relationship was affected by protection status and population density class (below/above median density), with a positive correlation only in E. edulis populations with low densities and outside protected areas. These results provide additional evidence that many factors other than climatic suitability alone can regulate variation in population abundance across species geographical ranges, such as dispersal limitation, local biotic interactions, and, in particular, anthropogenic impacts (Lewis et al., 2017; Mpakairi et al., 2017; Fois et al., 2018; Braz et al., 2020; Jiménez-Valverde et al., 2021). In addition, our findings reveal, for the first time, that the implementation of protected areas may not only affect population densities directly, but may also regulate the abundance-suitability relationship, particularly for exploited species. This result is especially important, considering that protected areas are a central conservation tool worldwide (Geldmann et al., 2013; Coetzee et al., 2014; Gray et al., 2016), and that overexploitation has been identified as a major driver of population declines across the globe (Maxwell et al., 2016).
The positive abundance-suitability relationship, observed across the species range and especially in low-density sites (i.e., below median density), was expected from niche theory, which predicts higher population performance (e.g., survival, growth and fecundity) when environmental conditions are more suitable, resulting in higher population density (Hutchinson, 1957; Brown et al., 1995; Soberón, 2007). Positive relationships with environmental suitability were consistently observed for total population density, for each ontogenetic stage separately (seedlings, intermediate-class individuals, and adults), and also for per capita seedling density of E. edulis. Such positive relationships are in accordance with many early predictions and studies, which suggested that local population abundance can be predicted by macroclimatic conditions (VanDerWal et al., 2009; Weber et al., 2017; de la Fuente et al., 2021).
In contrast, no clear abundance-suitability relationship was detected in high-density sites (i.e., above median density). This lack of relationship was consistently observed for the different dependent variables, i.e., when all ontogenetic stages were grouped, for each ontogenetic stage separately, and for per capita seedling density. This result suggests the occurrence of density-dependence regulation in E. edulis population growth, through demographic processes. Previous studies have found that population performance in plant species can be reduced due to negative density-dependence effects, such as intraspecific competition, which in turn might decrease or stabilize population size (McGill, 2012; Thuiller et al., 2014; Adler et al., 2018). Accordingly, at high E. edulis population densities, negative density-dependence effects could have precluded any positive effects from an increase in environmental suitability, resulting in stable populations despite significant increases in suitability (from 0.4 to 0.8) across sites (McGill, 2012).
In addition, the lack of correlation between per capita seedling density and environmental suitability in high-density populations (Fig. 2b) corroborates previous evidence that negative density-dependent effects can regulate E. edulis population growth (Silva-Matos et al., 1999). More specifically, Silva-Matos et al. (1999) documented lower seedling recruitment in sites with higher seedling density. This relationship can be explained by intraspecific competition, since similar resources requirement among E. edulis seedlings can result in lower seedling survival and/or seedling growth at higher densities. In addition, Silva-Matos et al. (1999) also detected a lower probability of survival and transition of seedlings to the next size class close to adult in E. edulis individuals. This pattern indicates negative effects of conspecific adults on seedling recruitment, potentially due to a higher attack of species-specific herbivores and pathogens beneath maternal plants (Janzen, 1970; Connell, 1971; Mangan et al., 2010). Also, this negative density-dependence can occur through the falling of palm leaves, which can physically damage seedlings and decrease light interception, resulting in a lower seedling recruitment beneath maternal individuals (Peters et al., 2004; Beck, 2007; Aguiar and Tabarelli, 2010).
The abundance-suitability relationships of E. edulis were clearly different inside and outside protected areas. Relationships were positive in unprotected populations, but neutral in protected populations. The most likely mechanism leading to this difference was differential palm harvest inside and outside protected areas. In a previous study (Souza and Prevedello, 2021), we showed that harvest occurrence is higher outside protected areas, resulting in lower E. edulis adult densities in unprotected populations (Souza and Prevedello, 2020). This higher frequency of palm harvest outside protected areas can maintain populations below carrying capacities, weakening negative density-dependence effects on demographic processes, thus allowing populations to grow with increasing environmental suitability. In addition to harvest, the higher forest cover inside protected areas may also increase E. edulis density, as this species is shade-tolerant and generally absent from large forest gaps (Reis et al., 2000; Gatti et al., 2014). Finally, a higher richness and abundance of pollinators and seed dispersers inside protected areas (e.g. Gray et al., 2016) may also affect positively E. edulis density.
Our findings have two main theoretical and applied implications, which can improve model predictions and conservation strategies. First, our analyses show that the inclusion of density-dependent effects on abundance-suitability models may increase understanding of abundance variation across species’ geographic ranges. As we have shown, this can be done by classifying populations a priori based on their density, to investigate abundance-suitability relationships for low- versus high-density populations separately. Secondly, our findings indicate that populations of exploited species located outside protected areas may be affected more strongly by future changes in environmental suitability. For instance, such unprotected populations could be more vulnerable to the widespread ongoing climate and land use changes, if these processes reduce environmental suitability. This possibility reinforces the need to implement new protected areas and to reduce overexploitation, whenever possible, across the distribution of threatened species, in particular overexploited species (Maxwell et al., 2016; Souza and Prevedello, 2020).
Conflict of interestNone declared.
This study was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (processes E-26/010.002334/2016 and E-26/ 010.000398/2016) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; process 424061/2016-3).