Species invasions are severe drivers of environmental change. Invasive plants may affect soil dynamics, interactions, and ecosystem functioning, leading to environmental and economic losses. Although species invasion success has been explained by niche conservatism, recent studies have demonstrated that niche shifts may also play a key role in this process. In this study, we tested whether niche shift has occurred during the range expansion of the Yellow Bells, Tecoma stans (Bignoniaceae) and predicted its global risk of invasion. We used Reciprocal Ecological Niche Models techniques and multivariate analyses to test our hypothesis and produce a worldwide invasion risk for this species. Niche spaces of African, Australian, and American exotic populations did not differ substantially from the natural one, although the reciprocal models we fitted for exotic and native occurrences poorly predicted each other. The predictions of the models indicated that T. stans is prone to invade new areas where it has not been recorded yet. Given its competitive abilities, preventive programs in prone-to-be-invaded areas are highly recommended.
Biological invasion is the second most severe threat to biodiversity and it is one of the biggest challenges for biology conservation in the 21st century (Tylianakis et al., 2008). Generally, species invasion has four stages: (1) species introduction within a new range, (2) establishment of populations outside their native range; (3) naturalization of established populations; and (4) spread to new localities (Richardson et al., 2000). Once established, invasive plants have the potential to affect soil dynamics, biological interactions, and ecosystem functioning (Müller-Schärer and Steinger, 2004). Predicting biological invasions is an important measure for protecting biodiversity.
Based on how niche properties and spatial constraints determine species range, two theories explore species invasion success in reaching new areas. According to the pre-adaptation theory, a species may overcome historical constraints and invade previously inaccessible areas, whereas in the post-adaptation theory, invasion success depends on the ability of individuals to undergo new local adaptations, leading to niche shifts of invasive populations (Müller-Schärer and Steinger, 2004). Until this moment, most studies have shown evidence of niche conservatism in invasive plant species (e.g. Broennimann et al., 2007; Petitpierre et al., 2012).
In this study, we evaluated the invasive expansion of the Yellow Bells, Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) and the eventual role of niche evolution to explain its success while invading new ranges (the known invasion history of the species is described in the Supporting Material). We tested whether niche conservatism or niche shift has driven the invasive expansion of T. stans using both Reciprocal Ecological Niche Modeling (rENM) and multivariate analyses, also providing a global invasion risk assessment for this species.
Materials and methodsOccurrence and environmental variables datasetsUsing literature and on-line databases (see Supporting Material), we compiled 535 unique occurrences for T. stans. We defined native and exotic ranges based on previous literature (see Supporting Material). We found 348 native and 187 exotic occurrences located in: Brazil (67), Africa (58) and Australasia (62). We re-scaled all variables to 0.5° grid resolution and used a Factorial Analysis (see Supporting Material) to select five bioclimatic variables from the WorldClim database (www.worldclim.org/download) and two topographic variables from US Geological Surveys Hydro-1K database (edcdaac.usgs.gov/gtopo30/hydro) to test for niche conservatism/shift and predict the invasion potential of T. stans on new areas.
Niche differentiation, multivariate analyses, rENM, and evaluationWe used seven variables (see Supporting Material) to predict the environmental space of T. stans. With those variables, we used Broennimann et al.’s (2012) approach to measure niche equivalency and similarity between the native and the exotic ranges of T. stans. This approach calculates an observed measure of niche overlap and compares it to randomized niche overlap measures. Among the four methods tested by Broennimann et al. (2012), PCA-env was considered the best one to perform these analyses. A PCA is calibrated using the set of all studied areas considered in the comparisons. Then, the available environmental conditions for the species within the full studied background are compared to those conditions in areas that are effectively occupied by the species in each one of its ranges (native vs. the exotic). Later, it measures the niche overlap between native and exotic ranges using Schoener's D metric (Schoener, 1970). This metric varies from 0 to 1, representing totally dissimilar or completely overlapping niches, respectively (Broennimann et al., 2012). In the similarity test, one of the niches (i.e. native or exotic) is randomized (here we used 100 times) and, in each time, a randomized niche overlap is measured and compared to the observed value. On the other hand, in the equivalency test, both niches are randomized at the same time. Therefore, we can test the hypothesis of niche similarity and equivalence between each range by checking whether the niche overlap differ from random.
Following Petitpierre et al. (2012), we only used the similarity test between the random exotic niche and the observed native niche to test for the niche conservatism/shift during the invasion. By doing this, we can compare the current invasion pattern in the niche with one expected by random invasion. The rejection of the similarity hypothesis indicates that the niches are more similar than random and confirms the niche conservatism hypothesis, while the rejection of the equivalency hypothesis indicates that the niches are not equivalent or identical (Strubbe et al., 2013). Finally, we also measured the proportion of the exotic niche of T. stans that was stable (i.e. niche overlap), unfilled (i.e. non occupied niche in the exotic range), when compared and expanding (i.e. new niche in the exotic range) compared to its native niche, and considering different proportions of non-marginal environment, as proposed by Petitpierre et al. (2012) and Guisan et al. (2014).
We also used rENM to test for environmental niche differences between natural and exotic populations. This approach assumes that native and exotic models should be able to predict each other's occurrences if environmental niche is conserved. Reciprocal prediction failure of native and exotic ranges would indicate niche differences. We randomly divided the native and exotic occurrences into ten training/testing subsets (70%/30% of the occurrences, respectively) using a bootstrap sampling (Fig. S1). The global distribution model for the species was also evaluated considering the same data partitioning and evaluation procedures.
We used DOMAIN (DOM; Carpenter et al., 1993), Mahalanobis Distance (MHL; Farber and Kadmon, 2003), and BIOCLIM (BIO; Nix, 1986) to predict the potential distribution of T. stans. The performance of each model was evaluated using AUC values (Area Under the Receiver-Operator Curve; Fielding and Bell, 1997). We used the Repeated Measures ANOVAs to test the difference between the pairs of the training and the testing subsets of the rENM (e.g. native training vs. exotic testing and vice versa) using AUC and normalized suitability as response variables. Considering the ecological features of T. stans we restrained both multivariate analyses and rENMs to buffers between 120 and 275km surrounding all its known occurrences, as the available background for the allocation of the pseudoabsences (see Supporting Material). This approach increases the discriminatory power of both methods and allows conservative inferences regarding potential niche shifts of T. stans in new ranges. All analyses were run using dismo and ecospat packages in R 3.1.1 (R Core Team 2014). Further explanations on the occurrence data and methods we employed are given in the Supporting Material.
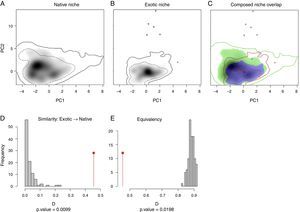
ResultsMultivariate niche analysesThe two niches are more similar than expected randomly (similarity test=0.0099; Fig. 1D), therefore the niche conservatism hypothesis was not rejected, although both niches were not identical (equivalent test=0.0198; Fig. 1E; observed Schoener's D=0.456). The niche centroid of T. stans shifted from its native range to the exotic in the same direction of the background centroid (Fig. 1A–C; Fig. S2). The niche expansion was 0.01%, niche stability was 99.9% and niche unfilling was 31.6% (Fig. 1C; Table S2).
Niche overlap between the native and exotic ranges of T. stans obtained through Petitpierre et al. (2012) and Broennimann et al.’s (2012) framework. (A) Niche occupied by T. stans in its native range. (B) Niche occupied by T. stans in its exotic range. (C) Composed niche overlap of both ranges (native vs. exotic). (D) PCA-env performed with the seven environmental variables obtained from our Factor Analysis, considering a buffer between 120 and 275km around the known occurrences of T. stans in both native and exotic ranges used as the background in the analysis. (E) Niche similarity of the exotic range to the native one. (F) Niche equivalency between native and exotic ranges of T. stans. The solid and the dashed lines in (A), (B), and (C) correspond to 100% and 50%, respectively, of the available (background) environment for each range of T. stans considered in the analysis. The colors in (C) are red for niche expansion, blue for niche stability, and green for niche unfilling. The red arrow represents the centroid shift between the native and exotic niche and the blue arrow represents the centroid shift between the native and exotic backgrounds.
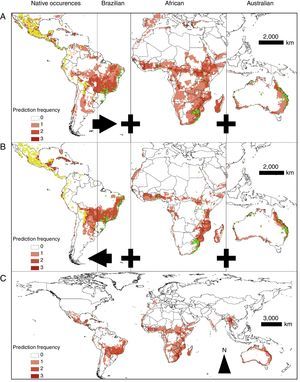
Models showed fair predictive power (AUC: 0.608±0.099; mean±standard error). The mean AUC values obtained with BIO, MHL, and DOM were 0.624±0.106, 0.602±0.095, and 0.598±0.095; mean±standard deviation, respectively. In general, the distributions produced with the native and exotic training subsets showed a better predictive power of their own test subsets rather than when they were used to predict each other's occurrences (Figs. 2A–B, 3A, and 3B). Conversely, models trained with the native occurrences of T. stans predicted its exotic occurrences in lower frequency (Figs. 2A–B and 3A), and the same was observed when the exotic occurrences of T. stans were used to predict its native ones (Figs. 2A–B and 3B). The rENMs fitted with exotic occurrences failed to predict many of the native occurrences of T. stans in Mexico, Central America, and South American countries (Venezuela, Colombia, Ecuador, Peru, and Bolivia). The normalized suitability of the models produced with native occurrences had high suitability both to native and exotic testing occurrences (Fig. 2B). However the models fitted with the exotic occurrences predicted high values for exotic testing occurrences and had the lowest values for the native testing occurrences, independent of the algorithm considered (Fig. 2B). The results which supports those results obtained with the multivariate niche analyses.
Cross-evaluations of the distribution of T. stans distributions. (A) AUC-ROC values (TH-ROC) and (B) normalized suitability values considering the three different modeling algorithms in the rENMs. Points represent the means and bars correspond to 95% confidence intervals. Exo. vs. Nat. refers to the evaluation of Exotic training subsets by their reciprocal Native testing subsets; Nat. vs. Exo. refers to the evaluation of the Native training subsets by their Exotic testing subsets; Exo. vs. Exo. refers to the evaluation of the Exotic training subsets by their own Exotic testing subsets; Nat. vs. Nat. refers to the evaluation of the Native training subsets by their own Native testing subsets. All vs. All refers to the evaluation of the distributions produced with the training subsets with all occurrences for T. stans by their own testing subsets, which also had both native and exotic occurrences of T. stans.
Models trained with all occurrences of T. stans had similar performances when compared to those in which native and exotic occurrences were used to predict their own occurrences (Figs. 2A–C and 3C). Tecoma stans shows a large invasive potential in many areas where it was not yet reported (e.g. central Brazil, southeastern Africa, India, southern Asia, and Indochina) and these areas should be considered as high invasion risk zones. MHL showed the most restricted distributions for T. stans in all rENM (Figs. S3A, S4A, and S5A), while DOM, and BIO showed wider but similar distributions (Figs. S3B–C, S4B–C, S5B–C).
DiscussionIn its exotic range, the niche of T. stans was highly conserved, once it reached high niche stability and little niche expansion when compared to its native range, as indicated by the niche similarity test. However, its niche in both ranges was not equivalent or identical to one another, since the exotic niche seemed as a subset of the native one. Therefore, if we only consider the exotic range, the species still did not reach all suitable environmental space it occupies within its native range. The centroid shift followed the trend in the background change and the pattern of niche unfilling. Finally, the high niche unfilling, niche centroid shift, and the non-equivalence were sufficient to cause the lower predictive power of the rENMs between ranges than within ranges. These findings are in agreement with the observation of other broad-scale studies indicating that niche shifts are rare among plant species (Petitpierre et al., 2012), invasive mammal, bird, amphibian, and fish species (Strubbe et al., 2015), as well as other groups (Peterson, 2011).
A reliable explanation for the results we found is that T. stans is still not in climatic equilibrium inside its exotic range as we could see with the high niche unfilling measure (Fig. 1C; Table S2). Since modeling algorithms rely on target species’ known occurrences to generate its distribution, they may fail to capture the species’ whole environmental space (Araújo and Peterson, 2012; Jiménez-Valverde et al., 2011), generating the incompatible predictions we found between exotic and native ranges. Despite the extensive area that the propagules of T. stans reached, they may still have occupied only a small portion of the whole possible environmental niche of the species within its native range, especially if we consider that 99% of its exotic niche is stable with its native one and with just 0.01% of niche expansion. Therefore, T. stans undoubtedly colonized climatic zones close to those it occupies in its native range.
Another potential cause of niche conservatism among plant species is related to their range size: the bigger the species distribution is, the smaller the probability of niche shifts (Early and Sax, 2014). Species with bigger distributions tend to be generalists with big niche breadth and different environmental gradients and colonize only the analog climatic zones once their propagules reach exotic ranges. On the other hand, species with narrow distributions tend to show widespread climatic zones in their exotic ranges, once their propagules leave their native ranges (Early and Sax, 2014). Indirect evidence from studies evaluating species invasions from biogeographical perspectives suggests that the distribution of small-ranged plant invaders is less determined by climatic factors than wide-ranged ones (Baselga et al., 2012; Early and Sax, 2014; Jetz and Rahbeck, 2002). Once T. stans occupies a native range that is distributed along a wide variety of climatic zones from northern Mexico up to southern Argentina (Kranz and Passini, 1997; Pelton, 1964), its widespread exotic range was not a surprise and it may be simply colonizing those areas which bear analog climates found in its native range. Considering this perspective, we expect that T. stans continues expanding its distribution especially because of the high proportion of niche unfilling it exhibited when compared to its native environmental niche.
Such perspective is even more evident when we consider the potential distribution this plant species may become worldwide when all of its occurrences were considered in different modeling algorithms. Despite the uncertainty related to ENMs techniques, and the complexity of assessing alien species invasion risk (Jiménez-Valverde et al., 2011), we demonstrated that the invasive potential of T. stans is expected to expand in Central Brazil, Southeastern Africa, and South Asia. Special concerns regarding the colonization of new areas by T. stans are necessary because of its high invasive potential, and its high commercial and ornamental appeal (Kranz and Passini, 1997). Taking in account future scenarios of climate change (Tylianakis et al., 2008) and the preference of this species for warmer regions, its invasion potential may be even bigger than that shown here (Broennimann and Guisan, 2008). Therefore, new modeling procedures should be done as soon as new native and exotic occurrences are gathered, in order to improve future invasion assessment. Additionally, embargos to its use, sale, and transportation should be considered, as already implemented in a Brazilian state (i.e. Paraná; Kranz and Passini, 1997), and required in order to impede its establishment in climatically suitable but still not-colonized areas inside its exotic range.
In this study, we showed that T. stans’ exotic niche is conserved, having a great proportion of niche unfilling, which may explain the low predictive power of our rENMs predicting one another's occurrences. Just as proposed by Guisan et al. (2014), the standardization and continuous use of the framework employed here (rENMs+multivariate analyses) are effective to test niche shift/conservatism of invasive species.
Conflicts of interestThe authors declare no conflicts of interest.
We thank JAF Diniz-Filho, DB Provete, and two anonymous referees for the contributions to the final version of this manuscript. DPS received a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). FVF and RAC received fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). PDMJ has continuous support from CNPq productivity grants.