The genus Pilocarpus (Rutaceae) includes the only known species able to synthesize pilocarpine, an imidazole alkaloid largely employed in glaucoma and xerostomia treatments. Pilocarpus microphyllus Stapf ex Wardleworth has a broad geographical distribution in the Northeast Brazilian territory, produces a substantial amount of pilocarpine and constitutes the only known natural source of this metabolite. Intensive extractivism and deforestation over recent decades have led to substantial declines in yields, plant populations and genetic diversity of P. microphyllus. Currently, it is recognized as a threatened species, and the few remaining areas harboring large populations (and potentially high genetic diversity) experience substantial farming and mining pressures. In addition, extractivism in these areas is still occurring by local communities because of the high market demand for pilocarpine. Conservation programs and sustainable management are urgently needed to maintain the long-term viability of this species in natural areas. Here, we highlighted the socioeconomic importance of this species, showing the massive reduction in the harvesting of raw materials and the consequences for the local people. We identified new potential areas of natural occurrence, including locations beyond the Brazilian borders, using species distribution modeling. We showed well-synchronized patterns of vegetative and reproductive phenophases, which may facilitate the management of this species. Finally, we emphasized the importance of cultivating P. microphyllus in a sustainable and productive way, involving the local communities and companies as a conservation strategies.
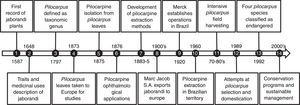
Jaborandi is the common name for Pilocarpus species, which comes from the Tupi-Guarani language ya-mbor-endi, meaning “what causes slobbering”. Traditional knowledge related to jaborandi plants is assumed to be one of the most precious inheritances of Amazonian indigenous culture, where this plant is historically used for medicinal purposes. Users have observed that infusions of jaborandi leaves have the property of stimulating the production of sweat and salivation, and they are applied in shamanic rituals for fever treatment, stomatitis and as an antidote for poisons and toxins (Holmstedt et al., 1979). This knowledge spread through the generations and cultures, resulting in the wide use of this plant in popular medicine by native people and the people who came from abroad after the XVI century (see the short description of Pilocarpus records in Fig. 1).
Brief chronological records related to the Pilocarpus genus. (1) Jaborandi mentioned as a medicinal plant in the Soares de Souza (Portuguese) annotations. These manuscripts were written during the 17 years he lived in the Brazilian territory (1570–1587). (2) Plant traits and usages by locals described by the naturalist George Marcgrave (German) in the first official publication dedicated to the Brazilian native plants. (3) Pilocarpus defined as a genus by the description of Pilocarpus racemosus plants. (4) The physician Symphronio Coutinho (Brazilian) took samples of jaborandi leaves to be studied in Europe. By this time, jaborandi leaf extracts were used as diaphoretics and sialagogues. (5) Pilocarpine isolation as crystalline salts from the leaves of two Pilocarpus species in independent works performed in France (Hardy, 1875) and England (Gerrard, 1875). (6) Pilocarpine used by Adolf Weber (German ophthalmologist) to reduce intraocular pressure and eventually in glaucoma therapies. (7) Development of economically viable methods to extract pilocarpine from jaborandi leaves (Louis Merck thesis). As a consequence, the Merck Company began pilocarpine salt extraction in Germany from imported leaves (Brazil). (8) Marc Jacobs S.A. exports dried Pilocarpus leaves from Brazil. This company, built by French immigrants on the Brazilian northeast coast (Piauí State), exported 200kg packages of dried Pilocarpus leaves collected in natural conditions (forest). (9) The Merck Company establishes operations in Brazilian territory prospecting for plant substances with medicinal properties. (10) The Brazilian company PVP begins pilocarpine nitrate extraction. (11) Period of maximal leaf harvesting in natural conditions. Combined with habitat loss due to Amazon deforestation, this period was marked by significant Pilocarpus population declines. (12) Merck starts a Pilocarpus domestication program by establishing a field area with approximately 3million plants. Accessions selected from several natural populations were planted over 300ha. (13) Pilocarpus microphyllus and three other Pilocarpus species are included on the Brazilian list of endangered plant species because of reductions in expressive natural genetic diversity. (14) Beginning of conservation programs and sustainable management of Pilocarpus microphyllus in protected areas.
The stimulation effects observed derivate from the imidazole alkaloid pilocarpine. The active ingredient extracted from jaborandi leaves acts as a cholinergic parasympathomimetic agent, i.e., it stimulates secretions in sweat, lachrymal and salivary glands (Fox et al., 2016; Sidhu, 2014). Since its isolation (Gerrard, 1875; Hardy, 1875) and the discovery of its power to reduce intraocular pressure (Sneader, 2005), pilocarpine has constituted the base of several medicaments, especially those applied in glaucoma treatment. More recently, it has also been used to reduce xerostomia induced by head and neck radiation therapy and for the treatment of Sjogren's syndrome (dry mouth) (Gornitsky et al., 2004; Sidhu, 2014).
The genus Pilocarpus (Rutaceae) is the only natural and economically viable source of pilocarpine currently known (Abreu et al., 2007). Despite the occurrence of pilocarpine in several Pilocarpus species, it is essentially obtained from Pilocarpus microphyllus Stapf ex Wardleworth because of the high pilocarpine concentration in the leaves of this species (Pinheiro, 2002) and due to its supposedly broad geographic distribution (Kaastra, 1982). The habitat loss resulting from Amazon deforestation and the uncontrolled extractivism of jaborandi leaves (especially in the 1970s and 1980s) led to a depletion of the plant population, with an estimated reduction of natural populations by approximately 50% (Martinelli and Moraes, 2013). Therefore, since 1992, P. microphyllus has been included on the List of Endangered Brazilian Plant Species (IBAMA n° 37, 1992), along with two other Pilocarpus species (P. jaborandi Holmes and P. trachylophus Holmes). More recently, another Pilocarpus species (P. alatus C. J. Joseph ex Skorupa) was also added to the Brazilian red list, since its populations have also undergone a significant reduction (Martinelli and Moraes, 2013). In addition, jaborandi leaf harvesting under natural conditions constitutes an important income source for communities living in poor regions of Brazil. Here, we compile our understanding of the genus Pilocarpus, including knowledge gaps related to its natural occurrence, genetic variability, pilocarpine concentrations, pilocarpine synthesis pathway and domestication/cultivation, as well as socioeconomic aspects of the species P. microphyllus.
Pilocarpus microphyllus: plant description, occurrence and potential distributionThe genus Pilocarpus was firstly described by Martin Vahl in 1797 (Vahl, 1797) from the species Pilocarpus racemosus Vahl. Despite its importance as a widely used medicinal plant, most complete taxonomic studies of this genus were only latterly performed by Kaastra (1982) and Skorupa (1996), who identified 16 Pilocarpus species. Currently, we found 17 described and recognized species in the repository The Plant List (www.theplantlist.org) and 43 scientific plant names proposed for the Pilocarpus genus, involving synonymies and potentially new species (Supplemental Table 1). These species present a widespread distribution in Latin America, occurring from southern Central America (Mexico) to southern South America. The north and northwest portions of South America contain a large part of these species, especially in the Brazilian territory, which harbors 13 species, with 11 being endemic to Brazil (Skorupa, 2000). As a consequence, this part of the continent is recognized as the center of genetic diversity of Pilocarpus species (Oliveira, 2007).
Pilocarpus microphyllus is a tree reaching 1–6m in height, with a terminal raceme 15–40cm in length as the most common inflorescence type (Fig. 2). Leaves are composite and glabrous, and the leaflets vary in shape and color, are commonly elliptical, exhibit opposite lateral insertion, are 1.5–6×1–3.5cm and sessile and have an emarginated apex (Skorupa, 2000). We can observe substantial morphological variation in the leaf traits of different accessions growing under the same environmental conditions (Fig. 3), suggesting potentially large genetic variability in this species, at least for some traits (Moura et al., 2005b).
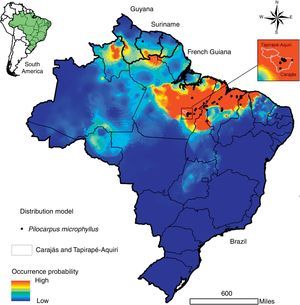
Described to naturally occur mainly in the northern region of Brazil (comprising the states of Pará, Maranhão and Piauí) and some locations in Suriname (Santos and Moreno, 2004; Skorupa, 1996), the potential distribution of P. microphyllus may be larger than currently observed. For the present study, we designed a potential distribution model considering the public data available from speciesLink (http://www.splink.org.br/) and the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/). In the construction of this model, we used the eight least correlated bioclimatic layers with 30 arc-seconds obtained from the Worldclim variables (Hijmans et al., 2005) and the Maxent algorithm (Phillips et al., 2006). The ROC-AUC (area under receiver-operating curve), whose values near 1.0 indicate good results (Fielding and Bell, 1997), was calculated. Additionally, to describe the main environmental features where P. microphyllus was recorded, we used the same dataset of occurrence points to calculate the frequency distribution considering three topo-climatic variables: altitude, annual mean temperature and annual precipitation using ArcGIS (Esri Inc.). As expected, the model shows that Piauí, Maranhão, and Pará contain the most representative jaborandi areas (Fig. 4). It also shows that northern areas of Ceará and Tocantins are suitable for the occurrence of this species. Additionally, the model suggests that some areas in the south of French Guiana, Suriname and Guyana as well as the Brazilian states of Amazonas, Roraima and Amapá are also suitable for the occurrence of P. microphyllus. For some of these locations (Guyana, Amazonas, Roraima, Amapá, Tocantins and Ceará), there is no record of P. microphyllus so far, and these areas should be checked through field studies. Particularly in the Brazilian territory, these areas have little biodiversity data available.
Potential distribution of Pilocarpus microphyllus. Warm colors (close to red) indicate a high probability of occurrence and the most suitable areas for the species. Black points indicate sites where P. microphyllus plants were collected. Box on the right highlights the National Reserves of Carajás and Tapirapé-Aquiri.
Results from the model suggest that P. microphyllus has a great probability of occurrence in regions with low altitudes (0–100m), annual precipitation of approximately 1300–1700mm, and a mean annual temperature between 25.5 and 27°C (Supplemental Fig. 1). These results confirm previous studies (Santos and Moreno, 2004; Skorupa, 1996) suggesting that P. microphyllus is mainly found in the lowlands of Amazon Tropical Moist Forest, despite reported occurrences of this species in environmental conditions such as those in the Caatinga Dry Forest in the Northeast region of Brazil. Overall, our results suggest that P. microphyllus can be found growing outside the limits of the three Brazilian states (Maranhão, Pará and Piauí), supporting the hypothesis that P. microphyllus is not a Brazilian endemic species (Martinelli and Moraes, 2013). In addition, the occurrence of this species in the northwestern portion of the species potential distribution map needs confirmation through field surveys.
The two National Reserves highlighted in Fig. 4, Carajás and Tapirapé-Aquiri, likely contain the largest remaining P. microphyllus populations and may act as natural reserves of genetic diversity for this species. Both of these areas have undergone intensive deforestation pressure inside and outside their limits, causing a consequent threat to several species, such as P. microphyllus. Outside the reserves, cattle farms have expanded into large areas that currently reach the reserve borders (Souza-Filho et al., 2016). Inside, mining is also expanding into the areas where P. microphyllus frequently occurs, i.e., the borders of iron crust inselbergs in the Amazon Forest, called Canga ecosystems (Canga is a Brazilian common name used to designate the ecosystems associated with the superficial ironstone (Skirycz et al., 2014)). Because local people predominantly collect leaves of P. microphyllus in these areas, especially in the Carajás National Reserve, management programs and reforestation with this species are highly recommended.
Current status and prospects of genetic diversity analyses in Pilocarpus speciesKaryotype analyses have provided several tools for plant breeding programs that can be applied in different phases of the process to develop new cultivars from accessions of a germplasm bank (Singh, 2003; Sybenga, 1992). However, as generally observed for most of the angiosperm families with recognized economic importance (Guerra, 2008), only a few groups in the Rutaceae have cytogenetic data available, as is the case of Citrus and related genera (see, for instance, Carvalho et al., 2005; Mendes et al., 2011; Silva et al., 2015). For Pilocarpus species, however, the chromosome numbers of only eight species (out of the 17 spp. in the genus) are available in the literature (P. giganteus, P. grandiflorus, P. microphyllus, P. pauciflorus, P. sulcatus and P. trachylophus, with 2n=44, and the two putative tetraploids P. carajaensis and P. spicatus, with 2n=88) (Skorupa, 2000). There is a lack of information on the divergence/similarity levels among karyotypes, which may be important in better directing interspecific hybridizations in jaborandi breeding programs (see Chen et al., 2003).
In addition, there are few studies addressing the genetic diversity of jaborandi (see Moura et al., 2005a,b; Sandhu et al., 2006; Rocha et al., 2014), which is a noteworthy fact, considering the importance of the species. However, two out of the four published analyses (Rocha et al., 2014; Sandhu et al., 2006) sampled a very limited number of genotypes because their primary interest was to find molecular markers rather than conduct a more detailed characterization of natural populations or compile germplasm banks. Sandhu et al. (2006) attempted to describe RAPD (Random Amplified Polymorphic DNA) markers associated with contrasting levels of pilocarpine accumulation in the leaves of 20 individuals collected from a natural population in the Brazilian state of Maranhão, but they did not succeed in finding such relationships. More recently, Rocha et al. (2014) tested the applicability of 48 ISSR (Inter-Simple Sequence Repeat) primers for genetic diversity analyses among jaborandi populations and reported some promising markers with high levels of polymorphism indexes.
On the other hand, a considerably larger sample was used in a study with two aims, one to evaluate the discriminatory power of RAPD markers (Moura et al., 2005a) and the other to use morphological leaf traits to access the genetic diversity among different populations of jaborandi (Moura et al., 2005b). Although moderate genetic variability among the tested accessions was observed in both studies, the authors could not observe geographic structure among the collection plots in addition to observing higher molecular variance within (75.84%) than among populations (26.16%) in the RAPD assay (Moura et al., 2005a). Despite the studies recognizing the existence of enough diversity in natural populations for genetic breeding programs, there is not enough knowledge on the genetic diversity of the species to assure successful programs.
Considering the current conservation status of wild jaborandi populations, the development and application of molecular biology tools to find genetic markers is mandatory. In contrast to the dominant nature of both RAPD and ISSR markers, i.e., there is not information on both alleles of a given locus, co-dominant marker systems (which assess both alleles) offer a better view of the genotypes by providing the assessment of a wider range of populational parameters (see Weising et al., 2005; Nybom et al., 2014). Favored by recent advances in sequencing technologies, the development of either single- or multi-locus co-dominant markers, such as the traditional and widely used SSR (or Simple Sequence Repeats, or microsatellites) and SNP (Single Nucleotide Polymorphisms) marker systems, respectively, has been significantly faster and more cost-effective over the last decade (Metzker, 2010; Nybom et al., 2014). Therefore, future investments in jaborandi breeding programs should be focused on developing such co-dominant markers for the species by planning large-scale genotyping of both natural populations and cultivated accessions in order to direct the first steps of the more efficient genetic improvement of cultivars with traits of interest, such as higher pilocarpine concentrations.
Phenology ofP. microphyllusMost of the plant phenology studies conducted in the tropics have been carried out at the community level, addressing specific life events, such as flowering and fructification (Croat, 1969; Frankie et al., 1974; Haugaasen and Peres, 2005; Morellato and leitao-Filho, 1996; Muniz, 2008). This was the case for P. microphyllus until recently, when (Muniz, (2008) determined the timing of flowering (April to May) and fructification (May to August) for this species in Northeast Brazil. These phenophases, shared with the other species also growing in the same forest understory, occur at the end of the wet season, contrasting with other species occupying the forest canopy (Croat, 1969; Muniz, 2008).
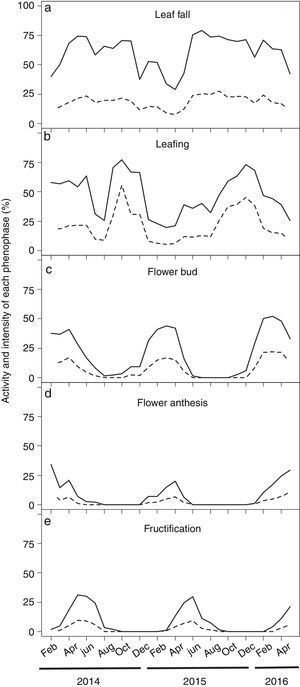
We recently performed a more comprehensive phenological study of P. microphyllus by monitoring vegetative (leaf fall and leafing) and reproductive (flower bud, flowering, fructification) events of 414 plants growing in the Carajás Natural Reserve (North Brazil) over 27 months (February 2014 to May 2016). In this study, the activity and intensity of each event were monitored monthly, following the methods proposed by (Bencke and Morellato, (2002) and (Fournier, (1974). We observed leaf fall in a large part of the plants over almost the entire period of evaluation (Fig. 5a). However, because the intensity of this event remained constantly at low values, P. microphyllus can be classified as an evergreen species (Rivera et al., 2002) in the studied site. Canopy renovation by leafing occurred continuously, with a peak of activity and intensity of this phenophase between September and January (Fig. 5b), a period coinciding with the transition of the dry to wet season in the Carajás National Reserve (Supplemental Fig. 2), aligning the leaf-growing period with the wet season. Because leaves are predominantly harvested in the forest during the dry season (June–September), most of these leaves have completed their growth, and the period of leafing can be avoided, which is essential in plant canopy recovery and leaf productivity.
Monthly time series of leaf phenology and reproductive events of Pilocarpus microphyllus plants growing under natural conditions (undisturbed areas) in the Carajás National Forest, Pará/Brazil. (a) Leaf fall, (b) Leafing, (c) Flower bud, (d) Flower anthesis and (e) Fructification. Data were recorded from 414 plants over 27 months. Solid and dotted lines represents the activity and the intensity of each phenophase, respectively.
The flowering (flower budding and flower anthesis) of P. microphyllus plants occurred during several months of the year, suggesting a possible annual pattern of long duration (Newstrom et al., 1994). Flower buds initially appeared in December, having maximal activity and intensity between February and April (Fig. 5c). We observed flower anthesis almost simultaneously (one month later) following a similar trend as described for the flower buds (Fig. 5d). The overall lower activity and intensity of this phenophase suggests possible leaf bud abortion, reducing the number of developed flowers or the duration over which the flowers remain open. Fructification also showed a regular annual pattern, starting in February, with a peak between May and June, and ending by July/August (Fig. 5e), when the dry period occurs in the region, which may favor seed dispersal because P. microphyllus has dry fruits with explosive dehiscence.
The earlier occurrence of reproductive events (flowering and fructification) we observed at the studied site compared to those described by Muniz (2008) is consistent with the suggestion that water availability drives most of the phenological patterns in the tropics (Fenner, 1998). While at both sites (500km distant from each other), flowering occurred in the middle of the wet season followed by fructification, which ends when precipitation declines, precipitation at the site where we performed our study started earlier (September–October) when compared to the Muniz site (November) (Supplemental Fig. 2). We could expect phenological patterns that are not synchronized with the changing of the seasons because P. microphyllus grows in the tropics. In these areas, it is common to observe continuous, sub-annual, annual, or supra-annual events (Newstrom et al., 1994; Sarmiento and Monasterio, 1983) that are normally associated with the diversity of pressures (biotic and abiotic) affecting these communities (Fenner, 1998). Nonetheless, the number of studies observing synchronism between plant events even when climatic seasonality is not obvious is increasing (Borchert et al., 2005; Caldeira et al., 2008; Menzel et al., 2006; Newstrom et al., 1994). In addition, observations of P. microphyllus phenological patterns in populations occurring in areas other than those that have been already studied will shed some light in regard to the behavior of such species.
Pilocarpine and other metabolitesAlkaloids constitute the most abundant secondary metabolites found in the leaves of Pilocarpus spp. (Kaastra, 1982). Their abundance may show seasonal (Abreu et al., 2007), developmental stage, and plant segment levels (Abreu et al., 2011; Andrade-Neto et al., 2002; Sawaya et al., 2011). The concentrations of the imidazole alkaloid pilocarpine can reach up to 1.04% of the leaf dry mass of P. microphyllus species (Costa, 2012). In our phenotyping study currently in progress (91 accessions of P. microphyllus), we identified accessions having pilocarpine leaf concentrations varying between 0.2 and 2.2%. This alkaloid is found in various concentrations in the leaves of six out of the seven Pilocarpus species analyzed so far (nearly zero pilocarpine was obtained from P. spicatus leaves, Sawaya et al., 2011). In this study, Sawaya et al. (2011) observed that the pilocarpine content may be higher in other Pilocarpus species (P. jaborandi and P. racemosus) than in P. microphyllus. Despite the similar total alkaloid content in these species (approximately 1% of the leaf dry mass), an overall lower pilocarpine content (less than 35% of the total alkaloids) was obtained from the two P. microphyllus accessions tested. A combination of events may have led to these results, including substantial genetic variability in this species (Sandhu et al., 2006), variation in the environmental conditions (plants cultivated similar to crops) (Sawaya et al., 2011) and plant/leaf age (Abreu et al., 2011). On the other hand, the considerable pilocarpine content found in P. jaborandi and P. racemosus enables them to be used as potential sources of pilocarpine.
Pilocarpine biosynthetic pathwayThe pilocarpine biosynthetic pathway remains unclear. A model proposes that l-histidine is at the base of this pathway (because of the imidazole ring) and that l-threonine or acetyl-CoA act as potential donors of the additional carbon atoms (Dewick, 2009; Sawaya et al., 2010), but experimental results are still lacking. A first result supporting this hypothesis emerged from the increase in pilocarpine production by the callus of P. microphyllus after the addition of histidine and threonine to the media culture (Abreu et al., 2005). Since then, a series of studies has been performed in an attempt to uncover this pathway because it represents an important step in improving pilocarpine yield. The main results obtained so far consist of the (i) selection of P. microphyllus cell lines in suspension culture producing only pilocarpine (Andreazza et al., 2009), which is a useful tool for discriminating the pilocarpine biosynthetic route from the pathway of synthesis of closely related alkaloids, such as pilosine; (ii) identification of alkaloid groups potentially acting as intermediary, parallel or competitive pathways in pilocarpine synthesis (Abreu et al., 2007); (iii) confirmation that pilosine (a possible competitive biosynthetic pathway) is only synthesized in mature plants of P. microphyllus, while pilocarpine is found at concentrations three times higher in juvenile rather than mature plants (Abreu et al., 2011); and (iv) verification that pilocarpine does not occur in the root system of P. microphyllus (Abreu et al., 2011). Because activity of the enzyme histidine aminotransferase (HAT) was detected in roots instead of leaves of P. pennatifolius Lem. plants (Santos, 2004), it was suggested that the pilocarpine biosynthetic route may occur in different plant parts, in which leaves may contribute to the final steps of biosynthesis (Santos and Moreno, 2013). The enzyme HAT is possibly involved in the formation of the first pilocarpine intermediary from l-histidine (Santos, 2004). A new series of studies applying recent tools is expected to be able to provide more accurate and integrative results to shed light on this question, following which we will be able to develop new potential ways to improve pilocarpine yield.
Alkaloids, coumarins, terpenoids and flavonoidsOther metabolites with potential applications in the pharmaceutical and cosmetic industries are also found in leaves of Pilocarpus spp., including coumarins, terpenoids, flavonoids and other alkaloids (an extensive compilation of these metabolites and their activities can be found in Santos and Moreno (2004)). Overall, pilosine, pilosinine, pilocarpidine, N,N-dimethyl-triptamine, dictamine, etc., are also alkaloids synthesized by Pilocarpus species. Some of them have well-known biological activities, such as N,N-dimethyl-triptamine, the most abundant alkaloid of P. organensis Occhioni & Rizzini (= P. pauciflorus A. St.-Hil.), which acts as a hallucinogen (Balsam and Voigtländer, 1978), and dictamine, common in P. grandiflorus and having antifungal effects (Souza et al., 2002). A large range of terpenes (approximately 75 types, including monoterpenes, diterpenes, triterpenes, sesquiterpenes, polyprenols and steroids) can be found distributed among different species of Pilocarpus. Playing an essential role in pollination (especially for insect/animal attraction–volatile oils), terpenes also exhibit antimicrobial, antibacterial and antihelminthic activities (Moon et al., 1999; Santos et al., 1997; Singh and Singh, 2003; Villaseñor et al., 2002). Approximately 27 coumarins have been isolated from Pilocarpus species. A wide range of uses are expected from this group of metabolites, including promising results in the treatment of some diseases (Mafezoli et al., 2000; Santos and Moreno, 2004) in addition to insecticidal (Calcagno et al., 2002), antifungal and bactericidal activities (Guerreiro et al., 2001). Finally, of the five flavonoids identified in one out of seventeen Pilocarpus species (P. trachylophus), four of them were found in the leaves (Bertrand et al., 2001) and one in the roots (Andrade-Neto et al., 1996).
Screening for other phytochemical products and developing economically viable extraction techniques are ways to detect new active ingredients, improving the valuation and conservation possibilities of such species, especially those that are threatened. In this way, synthetic procedures to obtain pilocarpine have been developed (Horne et al., 1993), but high costs make such production economically unviable when faced with the market for natural pilocarpine (Sawaya et al., 2010). Associated with the increased demand for products with more sustainable sources, the extraction of pilocarpine from Pilocarpus leaves will apparently continue and requires further investigations to enable sustainable harvesting in nature and the identification of cultivation conditions to produce high biomass and pilocarpine yields.
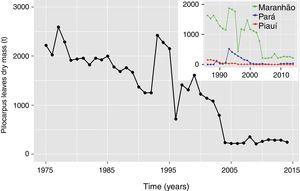
Socioeconomic aspects of P. microphyllus extractivismBrazil is the only known country commercially supplying pilocarpine (CNI, 2014), probably because of the natural occurrence and abundance of the species P. microphyllus. As a result of market demand, the harvesting of jaborandi leaves in nature was maximized between 1950 and 1990 (Berlinck, 2012), reaching its peak around the 1970s, when the state of Maranhão (major producer) alone provided more than 2500tonyear−1 of dried leaves (Fig. 6). A significant leaf yield reduction in these areas began in the early 1990s, when it was partially buffered by increasing harvesting in new areas, such as in the Carajás mountain range in the state of Pará (Grabher, 2015). The intense harvesting in these areas without a management program compromised the sustainability of this activity especially for the major P. microphyllus populations growing inside national forests (Carajás and Tapirapé-Aquiri), where environmental agencies started controlling and organizing the extractivism. Additionally, the establishment of mining operations in these areas also affected the mobility of leaf collectors and the availability of plants, since P. microphyllus usually occurs close to the mining areas in these National Forests.
Overall reduction in Pilocarpus microphyllus leaf harvesting in natural conditions (forest). Main panel: time series of the total leaves harvested in Brazilian territory and upper panel: time series of the leaves harvested in the Brazilian states with major production (data source: 1975–1985 compiled from Pinheiro; 1986–2014 from Brazilian Institute of Geography and Statistics-IBGE).
Motivated by the reduction in the raw material from nature and the need to supply pilocarpine demand, the Merck company, which holds a pilocarpine extraction patent and was the major pilocarpine supplier until recently, initiated a jaborandi domestication program in 1989–1990 by selecting accessions and establishing a jaborandi plantation with more than 3 million plants (300ha) in Maranhão. Currently, this farm has approximately 15 million jaborandi plants. It is owned by Centroflora Group, a Brazilian company devoted to extracting and processing compounds from jaborandi (and other species), especially those associated with the pharmaceutical and cosmetic industries. However, similar operations have not yet been established in other locations where P. microphyllus occurs.
The jaborandi market has played an important economic role by creating job opportunities and providing important income by connecting collectors to the pharmaceutical industry. It has been suggested that jaborandi extractivism corresponded to major revenue for thousands of families in the Brazilian Northeast region during the peak of extraction (1970–1980), estimated to reach up to 25 and 1.2 thousand families in Maranhão and Pará, respectively (Homma, 2003). Despite the market fluctuations and the reduction in areas where it naturally occurs, jaborandi is still being exploited, mainly in wild areas. Overall, these activities remain economically feasible because the pilocarpine price has experienced a significant increase (up to 36%) in the last two decades, when the pilocarpine supply capacity declined while its demand did not (IBGE, 2014). Moreover, pilocarpine is currently the eighth most exported pharmaceutical substance from Brazil, reaching around U$ 6.8millionyear−1 (Grabher, 2015). Increasing the number of cultivated areas and establishing natural reserves to maintain genetic diversity and population viability may sustain this activity and preserve the threatened Pilocarpus species.
Conservation programs and management plans have been established for some species in the Carajás National Reserve (Pará, Brazil). More recently, a group involving the mining company Vale S. A. and institutions such as the environmental agencies IBAMA (Brazilian Institute of Environment and Renewable Natural Resources) and ICMBio (Chico Mendes Institute for Biodiversity Conservation) began promoting a series of conservational and social activities in this National Forest and surrounding communities. In regard to P. microphyllus activities, it includes (i) the establishment of a germplasm bank and rescue accessions, involving the reintroduction of thousands plants in areas designated for conservation and sustainable uses; (ii) a series of studies aiming to map populations inside the Carajás National Reserve, determine their growth dynamics, genetic diversity, and pilocarpine content and select genotypes for cultivation purposes considering efficient propagation protocols and nutritional requirements to maximize yield (pilocarpine and biomass); and (iii) helping collectors organize a cooperative (CoEx-Carajás) and perform sustainable management of P. microphyllus and other native species to generate income.
Cultivation of P. microphyllusSeveral approaches have been used in an attempt to improve the yield of crops and other plant species. In contrast to most common crops (maize, rice, wheat, etc.), for which environmental conditions favorable for growth and biomass production are translated into increased yield, improving the yield of medicinal plants is not necessarily linked to biomass accumulation. Most of the products extracted from these plants (secondary metabolites) often require some degree of stressful conditions to trigger and/or boost the biosynthetic process (Akula and Ravishankar, 2011; Avancini et al., 2003; Zhao et al., 2005). As an example, when P. microphyllus plant leaves were treated with methyl-jasmonate or salicylic acid, pilocarpine content increased up to 4-fold when compared to the control (null treatment) (Avancini et al., 2003). However, when the same Pilocarpus species is cultivated as a crop, with soil preparation, adequate nutrients and water supply via irrigation, the pilocarpine content of leaves can be reduced to approximately 0.5%, while we found a mean of 1.2% in the 91 accessions we have collected so far.
Techniques for cultivating P. microphyllus remain incomplete and have not yielded maximal productivity because of the few and fragmented studies conducted so far. A large part of these studies involved metabolite identification (Abreu et al., 2007; Santos and Moreno, 2004; Sawaya et al., 2011), taxonomy (Kaastra, 1982; Skorupa, 2003, 1996), and in vitro cell culture (Abreu et al., 2005; Sabá et al., 2002). Some studies provided information regarding propagation by seeds (Calil et al., 2008), micropropagation (Sabá et al., 2002) and potential relationships between low nutrients and pilocarpine (Avancini et al., 2003), but none have addressed genotype selection, nutrient requirements or culture management.
Propagation of P. microphyllusThe propagation of Pilocarpus plants is commonly carried out by producing seedlings from recently collected seeds, allowing germination to reach up to 85% and 96% for P. pennatifolius (Calil et al., 2008) and P. microphyllus (Meneses et al., 2007), respectively. This method can be used because species of the Pilocarpus genus can produce a substantial number of viable seeds and because some species may have continuous flowering/fructification (Lameira et al., 2014; Skorupa, 2000). This is also common in some P. microphyllus accessions when they are growing in a germplasm bank, where these plants normally receive supplemental fertilization. However, a peak in fructification and seed dispersal have been observed for this species, occurring between May and July under natural conditions and for most of the accessions collected so far (Muniz, 2008; Rocha et al., 2011). Despite the abundance of seeds during this period, Pilocarpus seedling production may be laborious because of a significant reduction in the viability of stored seeds. It has been suggested that the seeds of P. pennatifolius have a longevity of less than one year (Calil et al., 2008). Our preliminary results showed similar trends with P. microphyllus seeds stored for one year in a seed chamber (low temperate and air humidity). We found significant seedling recovery after pericarp removal and almost null germination when the seeds were maintained within the pericarp even after gibberellic acid was added to the culture media (Supplemental Fig. 4). Overall, these results suggest that Pilocarpus propagation requires recently collected seeds and the establishment of germplasm banks as nurseries and not as seed banks.
Other techniques such as clonal propagation via stem cuttings or in vitro cell culture have not yet been applied to produce Pilocarpus seedlings, likely due to the limited information available or the high costs associated with them. In the only assay found using auxin (indole butyric acid, IBA) addition to promote root system development in shoot segments, few plants were recovered (Marques and Costa, 1994). In regard of explant development in media culture (Murashige and Skoog, 1962), apical explants were more efficient in shoot emission than nodal explants, having maximal growth when 6.66mM of cytokinin (6-benzilaminopurine) was added to the media (Sabá et al., 2002). The nonexistent adapted protocols to produce a large number of selected genotypes are highly recommended to standardize high producing areas. However, this works against conservation programs, as high genetic variability is recommended to maintain viable populations.
Field plantationsField planting is normally carried out at the beginning of the wet season with seedlings that are at least six months old (Marques and Costa, 1994). In the unique jaborandi commercial plantation at Vegeflora's farm, seedlings are currently planted into the field at high densities, reaching 50,000 plants per hectare. Part of this field receives supplemental irrigation and fertilization, where leaf harvesting can begin after 3 years. In these areas, it is possible to produce up to 3000kg of dried leaves over 4 or 5 harvests throughout the year when these plants achieve maximal productivity (Costa, 2012). As there are no specific recommendations in regard to nutritional requirements for growing Pilocarpus plants, all technical management is based on information from cultivated plants and may not provide ideal conditions for vegetative growth and pilocarpine production.
Apart from the high biomass yield, the pilocarpine content found in cultivated plants (0.5–0.6%) is largely inferior to that obtained from plants in natural areas (mean of 1.2%), and this remains an unsolved puzzle. The few results obtained from P. microphyllus plants suggest that a well-balanced nutrient supply is essential for maintaining pilocarpine production (Avancini et al., 2003). They also suggest that potassium (K) omission could increase pilocarpine content by 10x in young plants (five months old), as it does to caffeine in coffee seedlings. On the other hand, it has also been suggested that soil iron availability (Fe) may positively affect the pilocarpine content (Furtini Neto, personal information). This is particularly supported by recent (and current) observations of high pilocarpine concentrations in leaves of P. microphyllus accessions collected close to the iron ore mining areas in the Carajás National Reserve (data analysis in progress).
The application of phytohormones methyl-jasmonate and salicylic acid may be another possibility to increase pilocarpine biosynthesis. These molecules are usually associated with secondary metabolite accumulation in plant tissue, and Avancini et al. (2003) showed that pilocarpine production increased by 4× in P. microphyllus plants after methyl-jasmonate and salicylic acid application. Because the effects of the application of such phytohormones may vary considerably in dose-, time- and species-dependent ways (as they did in the (Avancini et al., 2003, study), a series of investigations still needs to be performed aiming to determine an adapted protocol for P. microphyllus.
In vitro cell cultureThe development of in vitro cell culture techniques devoted to producing specific plant compounds has received special attention because it offers the advantage of easy control of the environmental conditions. Techniques and protocols have been developed for a diversity of plant species, including Pilocarpus spp. A wide range of pilocarpine yields were observed when cells in a suspension culture (callus in a bioreactor) were fed with various concentrations of nutrients in the medium (nitrogen, phosphorus, potassium, histidine, and threonine) and in response to potential environmental stressors such as PEG (polyethylene glycol), NaCl and pH variations, as well (Abreu et al., 2005; Andreazza et al., 2009). Although there has been progress, pilocarpine production via in vitro cell culture remains economically unviable due to its high cost and low productivity. The limited information concerning the pilocarpine biosynthetic pathway remains one of the major difficulties in increasing its production.
Concluding remarksPilocarpus is a well-known plant genus, especially because of the medicinal importance of pilocarpine. As one of the most extracted and exported pharmaceutical constituents from Brazil, pilocarpine is an important source of income for many families depending on the extractivism of P. microphyllus leaves. Because of the absence of sustainable management for this natural resource, four (out of 17) of the Pilocarpus species (P. microphyllus included) were placed on Brazilian threatened plant species lists. Almost three decades later, limited progress has been made in terms of the continuous reduction of genetic diversity and population size of these species, causing the necessity for conservation programs.
Since the demand for natural pilocarpine remains high because there is no viable synthetic process and no efficient substitute active ingredient, sustainable management in natural areas and the cultivation of P. microphyllus are potential avenues for the conservation of this species. The establishment of a germplasm bank is one alternative that can be used in an effort toward preservation and as a source of genetic material for breeding. We suggest that conservation programs should pay attention to the development of adaptive management that will allow local families to cultivate this species. Such efforts will be expected to reduce the demand for the current unsustainable P. microphyllus harvesting in natural conditions. To do so, a sequence of multidisciplinary studies will be necessary, including those focused on genotype selection, propagation, the maximization of biomass and pilocarpine production, and the identification of nutritional requirements and environmental conditions that will improve pilocarpine yield.
FundingThis work was financially supported by Vale S. A. in cooperation with the Instituto Tecnológico Vale (Grant no. 4600034107).