We present a synthesis of the first 10 years of Long Term Ecological Research project in Amazonian Forest. We elucidate the natural dynamics of forest ecosystem processes and associated biota, and its changes caused by distinct pressures of selective timber extraction and forest fragmentation. We found that, for both plants and animals, densities of individuals and distribution of species assemblages are spatially heterogeneous at the mesoscale, even in relatively undisturbed forests, and that associations with topo-edaphic variables allow prediction of a considerable part of this variation. For biological groups whose dynamics were studied in the short-term, levels of change in species composition and densities were relatively high, and these changes were generally in tune with spatial environmental variation. The impact of selective logging on assemblages and ecosystem processes was normally moderate, and around 19 years were needed for recovering forest biomass and tree size distribution. Continued studies are needed to determine the time required for recuperation of timber stocks and pre-logging floristic composition. Selective logging appears to be compatible with the biodiversity conservation, but reduction and better planning of road access may be more important than planned logging intensities. Habitat-loss’ impact on organisms and ecosystem processes is large and long-lasting, since it induces the loss of many taxonomic groups and species, higher tree mortality and accelerated forest dynamics. There was a negative synergy between the impacts of habitat loss and climatic changes, and a better understanding of these processes can only be obtained through long-term research.
Growth of human populations and economic activities are transforming tropical forests into a mosaic of altered environments, pastures and isolated forest fragments, with severe consequences for biodiversity (Bierregaard et al., 1992; Laurance et al., 2011). The destruction of Amazonian forests grew exponentially during the 1970s and 1980s (Fearnside, 1987, 1990), and continues to impact dynamics of fauna, flora and ecosystem processes.
Human activities in tropical forest areas range from selective timber extraction with moderate environmental impacts to complete deforestation for agro-pastoral and industrial activities (Laurance and Vasconcelos, 2009). Understanding how and to what extent tropical forest ecosystems respond to different anthropogenic activities provides us an important base for environmental planning, management and monitoring, all essential for the promotion of sustainable development of tropical environments, such as Amazonia.
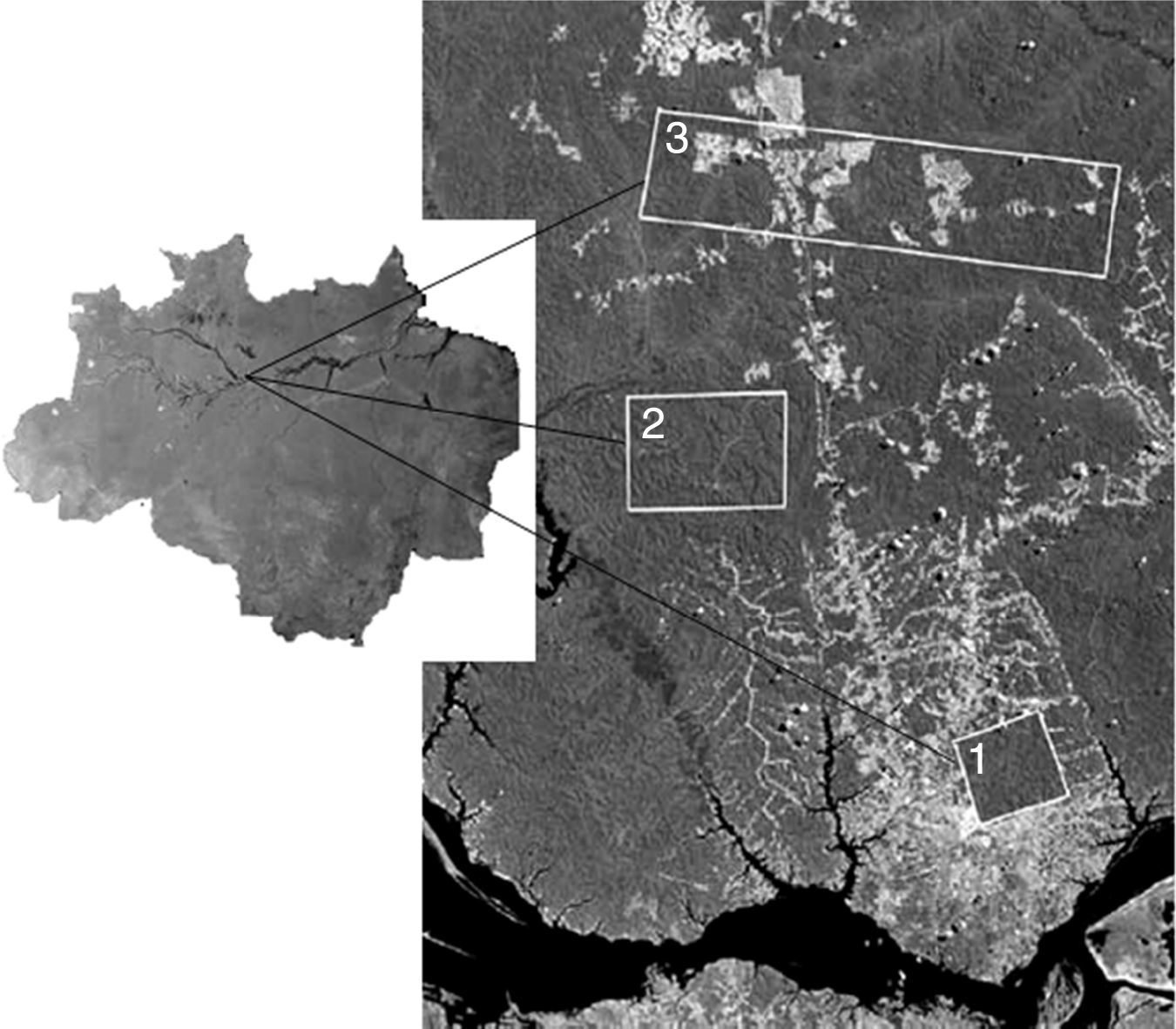
In response to these challenges, researchers of the National Institute for Amazonian Research (INPA) included Central Amazonia in the Brazilian program for Long Term Ecological Research – LTER (hereafter referenced as LTER-Site #1) established by the Brazilian National Research Council (CNPq). The aim of the program is to collect and organize data on the structure and functioning of the principal Brazilian ecosystems in order to generate a knowledge base to evaluate biological diversity, and subsequently propose plans for sustainable use and conservation. The LTER-Site #1 (Fig. 1) is the oldest Amazonian site in the Brazilian LTER program, and includes three forest reserves managed by INPA that differ in land use intensity. All sites are covered by evergreen dense terra-firme forest.
The Ducke Forest Reserve (RFD) is located 26km north of Manaus (2° 55′ 47.80″S; 59° 58′ 30.34″W), and covers 10.000ha (10×10km). This area does not suffer environmental impact within its borders, but is surrounded by urban sprawl from Manaus city. All studies were conducted in permanent plots regularly distributed across the landscape. The Experimental Forest Management Station (ZF2) is located about 90km northeast of Manaus (2°38′S, 60°11′W), and was submitted to selective logging treatments in 4-ha plots, arranged in three blocks. Each block has one control unlogged plot, three plots logged in 1987, and one plot logged in 1993 to assess the effect of time after timber extraction. The logged plots experienced different intensities of timber extraction. All these plots were established in the upland flat terrain (“plateau”), to minimize topographic effects. The reserves of the Biological Dynamics of the Forests Fragments Project (PDBFF) are situated 80km from Manaus and span about 1.000km2. The study area includes 11 forest fragments (five of 1ha, four of 10ha, and two of 100ha), and expanses of nearby continuous forest that serve as experimental controls. In the early 1980s, the fragments were isolated from nearby intact forest by distances of 80–650m by clearing and burning the surrounding forest. Most studies performed in PDBFF assessed the effect of fragmentation components – size of fragments and edge effect – on biodiversity, rather than evaluating fragmentation as a landscape process as proposed by Fahrig (2003, 2013).
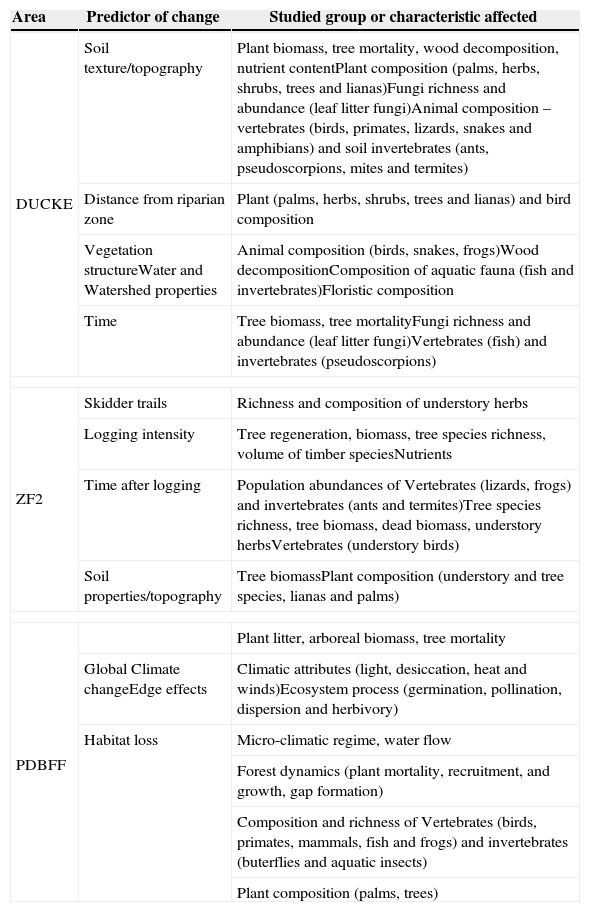
Since these areas are similar in terms of experimental designs (samples collected with standardized protocols in permanent nonflooded evergreen rain forest plots) we were encouraged to synthesize the information carried out during the last 10 years of research by the Brazilian LTER-Site #1 (1999–2009). Here, we present a synthesis of the main studies undertaken in this period, covering relationships between ecosystem processes and associated biota with different land uses, such as changes in natural forest landscapes, impacts caused by selective logging, and intense forest fragmentation. The biological groups studied and environmental characteristics affected by each predictor of change evaluated in each area are summarized in Table 1.
Table summarizing LTER-Site #1 areas with its respective studied groups and environmental characteristics affected by each predictor of change evaluated.
| Area | Predictor of change | Studied group or characteristic affected |
|---|---|---|
| DUCKE | Soil texture/topography | Plant biomass, tree mortality, wood decomposition, nutrient contentPlant composition (palms, herbs, shrubs, trees and lianas)Fungi richness and abundance (leaf litter fungi)Animal composition – vertebrates (birds, primates, lizards, snakes and amphibians) and soil invertebrates (ants, pseudoscorpions, mites and termites) |
| Distance from riparian zone | Plant (palms, herbs, shrubs, trees and lianas) and bird composition | |
| Vegetation structureWater and Watershed properties | Animal composition (birds, snakes, frogs)Wood decompositionComposition of aquatic fauna (fish and invertebrates)Floristic composition | |
| Time | Tree biomass, tree mortalityFungi richness and abundance (leaf litter fungi)Vertebrates (fish) and invertebrates (pseudoscorpions) | |
| ZF2 | Skidder trails | Richness and composition of understory herbs |
| Logging intensity | Tree regeneration, biomass, tree species richness, volume of timber speciesNutrients | |
| Time after logging | Population abundances of Vertebrates (lizards, frogs) and invertebrates (ants and termites)Tree species richness, tree biomass, dead biomass, understory herbsVertebrates (understory birds) | |
| Soil properties/topography | Tree biomassPlant composition (understory and tree species, lianas and palms) | |
| PDBFF | Plant litter, arboreal biomass, tree mortality | |
| Global Climate changeEdge effects | Climatic attributes (light, desiccation, heat and winds)Ecosystem process (germination, pollination, dispersion and herbivory) | |
| Habitat loss | Micro-climatic regime, water flow | |
| Forest dynamics (plant mortality, recruitment, and growth, gap formation) | ||
| Composition and richness of Vertebrates (birds, primates, mammals, fish and frogs) and invertebrates (buterflies and aquatic insects) | ||
| Plant composition (palms, trees) | ||
The tropical forest is characterized by ample natural variation generated by differences in soil characteristics, topography, climate and vegetation structure. Studies in RFD at LTER-Site #1, Central Amazonia, have shown that within mesoscales (10.000ha), soil and topography gradients are the principal factors responsible for differences between landscape elements.
The local topography, consisting of plateaus, slopes and stream valleys, clearly influences soil texture and nutrient distribution. Soil clay content is high on plateaus and low-lying areas have sandy soils (Araújo et al., 2002; Luizão et al., 2004). The nitrogen concentration (N) in leaves, plant litter and the upper soil stratum were found to be lower in valleys than on slopes and plateaus. The carbon concentration (C) did not vary with topography, resulting in high C/N ratios in valley bottoms. The low quantities of N circulating in valleys are likely attributable to sandy soils and seasonal flooding (Araújo et al., 2002; Luizão et al., 2004).
Levels of nitrogen mineralization and nitrification were significantly lower in soils of valleys, with N immobilization mainly observed during the rainy season (Luizão et al., 2004). The production of fine plant litter (maximum stem width ≤2cm) and its decomposition were slightly greater in plateaus as compared to valleys (Luizão et al., 2004). Therefore, a thick layer of leaves accumulates over sandy soils due to the scarcity of nutrients found in these soils, which then results in relatively slower decomposition rates. The local topography (plateau, slope and valley) clearly influences the litter and soil nutrient distribution. These differences should be considered in the construction of models predicting carbon emission rates that use data on decomposition in their estimates.
Some authors demonstrated that at mesoscales, the heterogeneity of soil granulometry and topography are primarily responsible for differences in structure and above-ground live biomass accumulation. Castilho and collaborators (2006) have shown that arboreal plant biomass in RFD tends to be greater in clay-rich soils of plateaus while palm biomass is greater in sandy soils of slopes and valleys. In the PDBFF areas, tree biomass increases with the quantity of clay in soils as well as soil fertility, mainly due to nitrogen (Laurance et al., 1999). However, the correlation between biomass and nitrogen may be due to the contribution of leaves to soil nitrogen, and not caused by soil nitrogen content. Since soil variables are markedly influenced by local topography, these studies show the importance of taking into account topography to improve our current estimates of carbon stocks over large areas in the Amazon, and it can be easily assessed using satellite imagery.
The process of wood decomposition is largely independent of soil characteristics, topography, and vegetation structure in RFD (Toledo et al., 2009). The absence of a relationship between wood decomposition and variables influencing tree biomass and decomposition of plant litter indicates an asymmetry between productivity and decomposition, which should in theory bring about a greater accumulation of dead wood in valleys, where rates of tree mortality were reported to be greater (Toledo et al., 2012). According to Toledo et al. (2012), the greater proportion of tree death due to uprooting in valleys was likely to be related to restrictions on root establishment due to anoxic supersaturated soils, and the reduced need for fine roots because of the greater availability of phosphorous in valley soils. Together, these factors favor low investment in subterranean biomass in valleys.
Biodiversity distribution follows similar patterns to those observed for ecosystem processes. Studies demonstrate that the composition of plants in many functional groups studied in RFD (herbs, palms, shrubs, trees and lianas), as well as fungi, is strongly associated with edaphic conditions, changing gradually along the topographic gradient that extends from sandy, poor and poorly-drained soils of valleys to more clay-rich and well-drained soils of plateaus (Costa et al., 2005, 2009; Kinupp and Magnusson, 2005; Braga-Neto et al., 2008; Drucker et al., 2008; Schietti et al., 2013; Rodrigues et al., 2014). Similarly, studies from non-fragmented areas of the PDBFF reserves show that topography and soil fertility affect the distribution of understory plants (Harms et al., 2004), lianas (Laurance et al., 2001), arboreal successional species (Laurance et al., 2006a), and the composition of the tree community (Bohlman et al., 2008; Laurance et al., 2010).
Some animal groups, especially those assumed to have limited dispersal – such as amphibians (Menin et al., 2008) and soil invertebrates (Araújo, 2007; Franklin et al., 2007; Oliveira et al., 2009; Dambros, 2010; Souza et al., 2012) – displayed distributions to some degree associated with topography and soil characteristics. There is evidence that abundance and diversity of edaphic arthropods are associated with different levels of C, P, Na and Ca in litter and/or in soil. This pattern suggests that quality of substrate may limit the soil mesofauna in Ducke Reserve. Approximately half of the ant species were found solely in valleys or only on plateaus, suggesting that the majority are habitat specialists and depend of soil texture, which is related to moisture content (Oliveira et al., 2009). Pseudoscorpions, however, were not found to be sensitive to environmental factors, such as terrain topography, percentage of clay in soils, quantity of plant litter or soil pH, and as such, can be considered habitat generalists (Aguiar et al., 2006).
Groups with ample geographic distribution, such as lizards (Pinto, 2008a) and snakes (Fraga et al., 2011) were also found to be little affected by topographic and soil variables present in the mesoscale context at RFD. Similarly, Vidal and Cintra (2006) showed that the topographic gradient characterized by plateaus – slopes – lowlands (60–140m of altitude) had little effect on the occurrence, size and group density of the primate Saguinus bicolor (Sauim-de-coleira, an endangered species). However, owls and bird species composition changes along the topographic gradient, and also in relation to leaf litter depth and distance to forest streams. Compositional differences were also found in the avian community in the eastern and western water basins that compose the reserve. These results suggest that although most bird species occur throughout the mesoscale, many species track differences in the landscape and forest structure (Barros and Cintra, 2009; Cintra and Naka, 2012).
Regarding aquatic fauna of RFD, Mendonça et al. (2005) showed that fish species composition varies between drainage basins, and that this difference is associated with variation in water chemistry, current velocities and substrate properties. The ridge that runs down the middle of RFD separates these two major water basins, with streams to the east flowing to tributaries of the Amazon River and those to the west to the Negro River (Mendonça et al., 2005). Some terrestrial plant groups also had species restricted to one or another water basin (Costa et al., 2005; Garcia, 2005; Kinupp and Magnusson, 2005). However, it is difficult to identify which factors account for this difference since there is little variation in soil and other environmental factors between drainage basins. The only important difference between watersheds is the available phosphorus content, which may be linked to the drainage patterns. Since these watersheds are separated by low ridges, they do not represent barriers to dispersal for some groups (Costa et al., 2005).
Studies have shown that, while extremely interesting for some questions, it is not generally economically viable to focus on exhaustive lists of megadiverse groups of invertebrates, such as oribatid mites and ants. For studies designed to detect environmental changes, it is better to evaluate changes in the presence of more common species (Moraes et al., 2011) or even use reduced-sampling techniques (Souza et al., 2009). For example, not sorting the less frequent species did not severely affect the observed pattern of similarity among sample plots, indicating that more abundant species can be used as substitutes for less abundant species in biomonitoring studies. In addition, the sampling effort reduction did not affect the capacity to detect the effect of soil pH for the community of ant genera (Souza et al., 2009).
These studies indicated that a large amount of the variation in species composition and ecosystem processes is determined by topography and soil characteristics on a landscape scale, even if dispersal limitation, history and biotic interactions still play a role in explaining the remaining variation. Therefore, models that extrapolate ecological processes, such as carbon sequestering and decomposition, measured on smaller scales may not provide useful predictions if they do not take into account the importance of the configuration of elements in the landscape. Species distribution models also need to consider these associations, as the ability of species to use local microclimates might be more relevant for future survival than their general associations with climate at large scales (Antonelli et al., 2010).
The importance of the riparian zoneDiverse studies in LTER-Site #1 have shown that species composition of distinct assemblages such as plants, frogs and tadpoles, snakes and birds, varies in relation to distance from water bodies (Drucker et al., 2008; Rodrigues et al., 2010; Fraga et al., 2011; Bueno et al., 2012; Schietti et al., 2013). In RFD, Drucker et al. (2008) showed that the floristic composition of understory herbs is distinct from that found up to approximately 100m horizontally from the edge of streams, or above about 70m above drainages, varying slightly with the size of the stream valley. As a result, the only distinct habitat recognized by most species appears to be restricted to areas around streams. Further away, differences are subtle and the pattern varies among species.
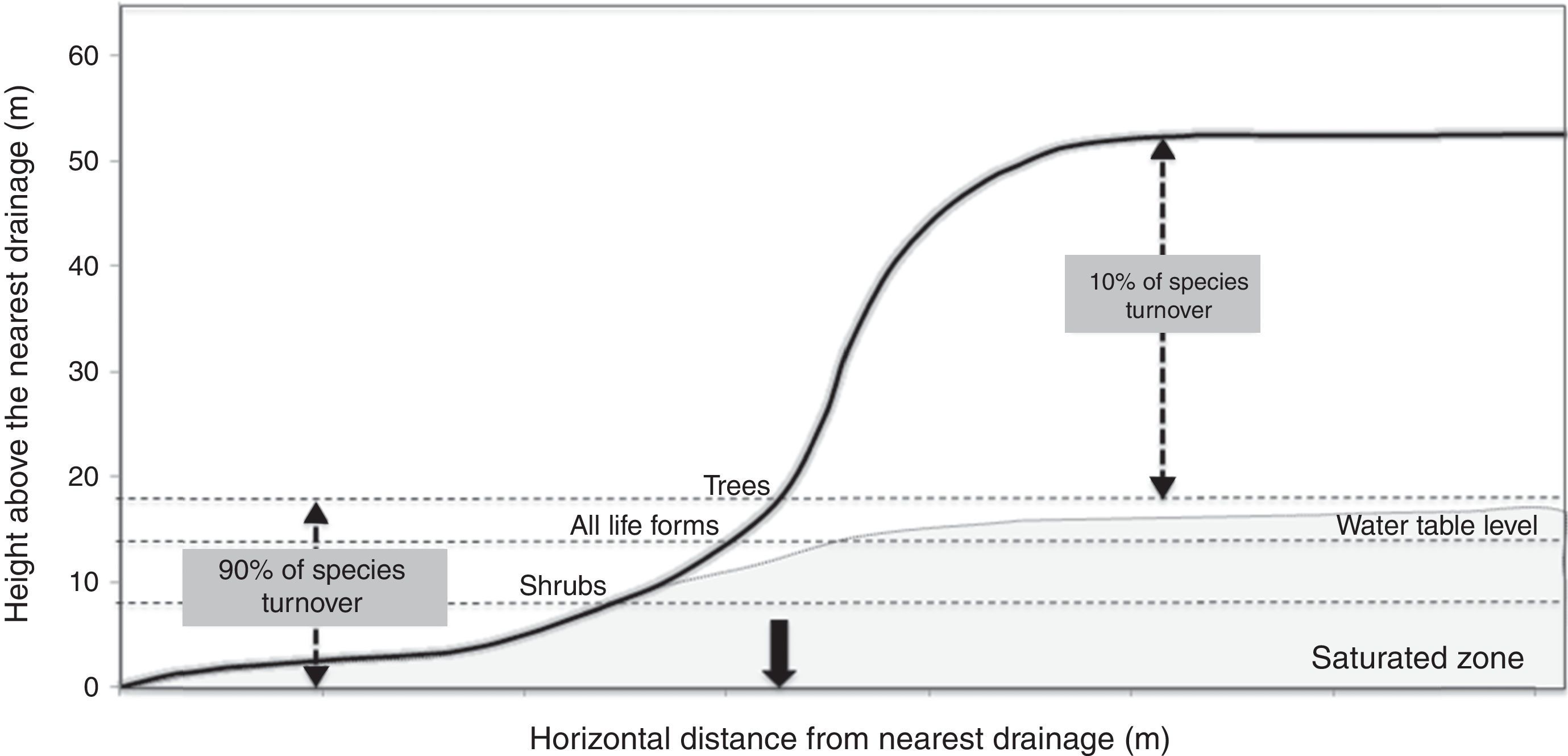
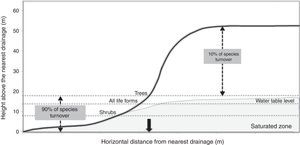
Schietti et al. (2013) showed that the floristic composition of herbs, shrubs, palms, trees and lianas vary considerably between valleys, but is relatively constant in areas of higher elevation (Fig. 2). Most changes in species composition (about 90%) occur up to distances between 8 and 18m in vertical drainage height, depending on the group of plants. This vertical interval is equivalent to a distance of approximately 60–250m horizontally from waterways. Similarly, frogs and their tadpoles (Rodrigues et al., 2010), understory birds (Bueno et al., 2012) and snakes (Fraga et al., 2011) studied in RFD also appear to recognize a riparian zone similar in width (approximately 100–200m) to that estimated for understory herbs and the other plant groups.
Representation of the positions where most changes in plant composition occurs in relation to the small forest streams. Water table fluctuation zone is shown in gray. The black arrow highlighted is the maximum extension of the riparian area (350m), given limits detected in plant species composition.
Under Brazilian law, the width of protected riparian vegetation depends on the width of the water body. This legislation mandates protection of areas within 30m for streams up to 10m wide, such as those that occur in the Ducke Reserve. Our studies showed that this riparian area is smaller than that recognized by most animal and plant groups, and that a width of 100–250m would be more appropriate for conservation purposes. The studies also showed that a minimum vertical distance from the closest drainage area would be a better criterion to protect biodiversity than protection based only on a horizontal distance. The critical vertical distance from drainage areas for conservations purposes should be defined by the zone of groundwater fluctuation, and this will vary among Amazonian landscapes depending on topographic variation, precipitation regime and soil properties. The differences in species composition between riparian areas in adjacent small watersheds also indicate that each small watershed has a novel contribution in terms of species to the regional diversity. These findings support our claim that narrow protected areas, as Brazilian law prescribes, will not be sufficient to protect the majority of species.
The finding that areas of highest compositional change and most distinct from others in the environment (about 200m horizontally on each side of stream, and 8–18m vertically from the riparian zone) are those directly affected by groundwater fluctuation also has implications for predicting the effects of climate change. If the climate becomes drier in Amazonia, water storage in the soil and groundwater levels will decrease. This will reduce the size of riparian areas and cause changes in species compositions due to differences in drought tolerance (Engelbrecht et al., 2007). The majority of tropical-forest species are not capable of dispersing over long distances (Clark et al., 2005; Colwell et al., 2008; Terborgh et al., 2011) and plants already confined to narrow zones near drainage areas will have little recourse to migrate to similar environments. These species may also become threatened by pressure from species of higher areas that will be able to invade areas around streams as the groundwater level drops (Schietti et al., 2013).
Spatial and temporal variation in biodiversity and ecosystem processesBiodiversity and ecosystem processes vary not only in space, but also in time, and this temporal dynamic can also be related to environmental characteristics. Understanding the variation of temporal dynamics in the landscape is important for modeling scenarios for climate change and conservation planning.
A study of fish assemblies in RFD streams showed that species composition and fish abundance vary between the dry and wet season, with richness (20%) and abundance (30%) being higher in the dry season. However, the composition tends to return to its original state at the onset of the next dry season, due to the commonest species that account for the stability of fish assemblies over the long term. Changes in species composition observed between seasons appear to be associated with lateral migration of fish to temporary pools and other micro-habitats during the flood period (Espírito-Santo et al., 2009, 2013). These observations are similar to lateral fish migration patterns associated with large Amazonian rivers (Junk et al., 1989).
Braga-Neto and collaborators (2008) found that species of leaf-litter fungi respond to the interaction between edaphic conditions, which vary in space, and to precipitation, which varies in time. The number of fruiting morphospecies varies along an edaphic gradient only during the driest periods, being lower in clay-rich and drier soils. During the rainiest periods of the year, humidity is less limiting, resulting in a similar number of species fruiting along the edaphic gradient.
Comparisons between older studies (Adis and Mahnert, 1990, 1993) and more recent ones (Aguiar et al., 2006) on pseudoscorpions showed that these predators have a consistent pattern of abundance and diversity over decades. This result suggests that distributions of species in these assemblages are stable over the long term, contrasting with the variations observed in the short term for other organisms.
Castilho et al. (2010) showed that the short-term rate of biomass change in RFD was predominately positive in plots on clay-rich and more fertile soils, and predominately negative in plots with sandy and infertile soils during the first monitoring period (2001–2003), but not in the second (2002–2004) (Castilho et al., 2010). Therefore, in contrast to results of several recent studies, that did not take into account the interaction between climate and soils, that study suggests that large areas of Amazonia with sandy soils may lose biomass under the conditions predicted by most climate-change models. The persistence of this effect through time still needs to be verified.
The effects of soil and topography on tree mortality also vary temporally. Soil and topography explain about 25% of the spatial variation in small-tree (<30cm DBH) mortality during a period with heavy rain storms, while no effect was detected during a period of fewer storms (Toledo et al., 2012). The magnitude of the effect of soil and topography on tree mortality also increased after storms. In general, plots with less fertile soils on steep slopes or sandy soils in valleys had greater tree mortality than those on well-drained clayey soils on the plateaus (Toledo et al., 2012). In fact, the higher proportion of uprooted dead trees in valleys in Amazonia has been attributed to poor soil drainage, since water excess in these areas restricts root establishment, productivity of fine roots and rooting depth (Espeleta and Clark, 2007).
The dynamics of populations, species assemblages and ecosystem processes vary spatially on the landscape scale. Most of those studies reported that dynamics differ between different landscape elements, such as clay plateaus and sandy valleys. Knowledge of these variations in natural dynamics across space is crucial to interpret changes in dynamics due to anthropogenic disturbances. We demonstrate that local control plots that do not cover the landscape variation will fail to provide the baseline to which perturbations should be compared.
The effects of selective timber extraction on biodiversity and ecosystem processes are moderateExperimental timber extraction in ZF2 was conducted in plots that underwent different timber cutting treatments: low (25%), moderate (50%), high (75%) and a control treatment group that corresponds to the primary forests without wood extraction (Higuchi and Biot, 1997). The intensity of logging in each plot corresponded to the reduction in volume of all commercial trees species with DBH≥10cm.
The studies in the area of selective logging indicate that most of the tree biomass had recovered by 19 years after cutting. However, in this period, commercial species recovered only 66–88% of the original wood volume, suggesting that more time is needed for recuperation of the timber stocks after exploration (Pinto, 2008b). The composition of the forest still differs much from the original, with commercial species having lower basal area. The extraction of large trees may lead to changes in the long term, because establishment of many commercial hardwood species is slow. Logging significantly increased richness of the tree regenerating layer in relation to control plots (Magnusson et al., 1999) and affected the overall similarity in species composition among plots, but the magnitude of the effect was small compared to the difference between one control plot and all other plots (Magnusson et al., 1999).
Pinto (2008b) suggested that a cycle of 30 years is feasible for the forests to be managed again, if no external or internal disturbance occurs. A compilation of studies in the Brazilian Atlantic forest (Liebsch et al., 2008) estimated at least a hundred years to recover pre-disturbance proportions of animal-dispersed tree species (80% of the species), non-pioneer tree species (90%) and understory species (50%). Considering that the study at ZF2 was focused on commercial tree species and was carried out under controlled conditions of disturbance, we emphasize that this estimate of at least 30 years is applicable only to commercial plants and actions for timber management. More studies on long term effects of logging should be done to predict the time necessary for recuperation of the initial tree species composition.
Selective logging resulted in a large redistribution of biomass and nutrients in forest clearings formed during exploitation (Higuchi and Biot, 1997). Some areas in forest clearings especially near the center remained with exposed soils and little or no plant or litter cover. Other areas accumulated vegetative residues that decomposed in the years following cutting, contributing with carbon and nutrients to the soil, as well as CO2 to the atmosphere. The liberation of nutrients from coarse leaf litter and wood decomposition increased the concentration of potassium, calcium and magnesium available in the soils in the two years following logging. Necromass increased, and therefore the respiration rate also increased in the first five years and up to at least 15 years after cutting. Pinto (2008c) compared the nutrient content and stocks in trees of two secondary forests of different ages and history of land use. Trees in the secondary growth after logging (forest of 23 years age) had higher nutrient contents (N, K, Ca, Mg, Na) than trees in secondary forests developed after clear cutting (forest of 14 year age), showing the importance of use history and time after disturbance for the ecosystem processes.
Costa and Magnusson (2002) examined the effect of different logging intensities, time since logging and the skidder tracks on the composition and diversity of understory herbs. No overall differences were found in the composition of herbs between the different logging intensities, but there were local differences in plant composition associated with old clearings and along skidder tracks. The composition and richness of herbs did not vary with time since logging up to 10 years. Costa and Magnusson (2002, 2003) showed that selective cutting affects the reproduction of understory plants, but these effects were not necessarily negative. The abundance and number of flowering plant species was greater 4 years after logging and returned to control levels after 11 years. The intensity of logging had no impact on the reproduction of the understory-plant assemblages as a whole. However, for some species, reproduction was higher in areas with greater logging intensity. These studies demonstrate that managed forests with similar selective logging intensities can be used for the conservation of understory herb species. Additionally, skidder trails should be minimized since they cause greatest impact over the long term and permit colonization by invasive species.
Low-impact selective logging caused moderate effects on both vertebrate and invertebrate assemblages. Guilherme and Cintra (2001) showed that the diversity, species composition and frequency of use by understory birds were not affected by different logging intensities, but were affected by time after logging. These authors showed that selective logging does not cause great impacts on bird assemblages, and that, in some situations, managed areas could be a better option for conservation in relation to other forms of land use. In contrast, Lima et al. (2001) found that selective logging affected three lizard species differently. The abundance of one species increased with selective logging while another species decreased. In contrast to the results for vertebrates, selective logging did not affect the number of species or total abundance of ants on the forest ground (Vasconcelos et al., 2000). However, differences in logging intensity caused changes in the density of various species of ants (Vasconcelos et al., 2000) and leaf-litter termites (Lima et al., 2000), and these effects lasted for more than 10 years after cutting.
In general, the effects of selective logging were moderate, and no species loss was recorded, even though the abundance of some species increased or decreased. However it is important to note that, in these studies, selective logging was undertaken by a research organization, did not experienced forest fires or other disturbances typically associated with illegal logging, and did not include an evaluation of the impacts of access roads. This form of selective logging appears to be compatible with biodiversity conservation, but reduction and better planning of access roads may be more important than planned logging intensities, a conclusion also reached in an area with commercial logging (Dias et al., 2010).
Forest fragmentation strongly affects ecosystem processes and associated biotaStudies on the composition and dynamics of the tree assemblages in fragmented areas of PDBFF at LTER-Site #1 are one of the largest in terms of spatial extent (1.000km2) and temporal scale (approximately 30 years) anywhere in the world. The impact of forest fragmentation on tree assemblages has a profound influence on the long-term persistence of animal and plant populations in fragmented forests. The micro-climatic regime in fragments differs from intact areas (Camargo and Kapos, 1995). Pasture or agricultural areas surrounding fragments have lower rates of evapotranspiration, and are hotter and drier. The effects of drying can penetrate from 100 to 200m into fragments, not necessarily following a linear pattern (Camargo and Kapos, 1995; Malcolm, 1998; Didham and Lawton, 1999). Water bodies in fragmented landscapes present greater temporal variation in flow than do those in forest, due to the lower rates of evapotranspiration (Trancoso, 2008). These differences promote localized flooding in the wet season and stream failure in the dry season. Together, these climatic and hydrological modifications have large effects on organisms and ecosystem processes, especially near forest edges.
Edge effects in fragments are variable. They include increase in desiccation, heat, light, and turbulence from winds among other climatic factors that directly affect ecosystem processes (Laurance et al., 1997, 2004a), such as seed germination and survival of seedlings (Davies et al., 1998; Bruna, 1999), pollination, seed dispersion, and herbivory (Harms and Dalling, 1997; Cordeiro and Howe, 2001, 2003; Terborgh et al., 2001), seed fall (Davies and Semui, 2006; Nascimento et al., 2006), and nutrient cycling (Klein, 1989). Fragmentation also alters forest dynamics, increasing mortality rates, tree damage and clearings formation (Ferreira and Laurance, 1997; Laurance et al., 1998a). One of the most important implications about the increase in tree mortality is the loss of live biomass in the first 100–300m of edge (Laurance et al., 1997, 1998a). Not only do more trees die in fragments, but the greatest proportion of these deaths is also constituted by large trees that make up the largest fraction of forest biomass (Laurance et al., 2000). Small increases in biomass of lianas and small trees, and coarse and fine plant litter do not compensate for the loss of biomass caused by increased tree mortality (Nascimento and Laurance, 2004). Mathematical models predict that the loss of biomass increases abruptly in fragments with areas between 100 and 400ha, depending on the shape of the fragment (Laurance et al., 1997, 1998a). These models also suggest that due to the rapid process of fragmentation that tropical forests are currently undergoing, live-biomass loss in these areas could be responsible for an annual emission of more than 150 million tons of carbon into the atmosphere (Laurance et al., 1998b).
Area reduction is commonly associated with the impoverishment of biodiversity in forest fragments. Reductions in the number of species with fragmentation were observed in a large number of taxonomic groups including primates (Gilbert and Setz, 2001; Boyle and Smith, 2010), insectivorous understory birds, (Stratford and Stouffer, 1999; Ferraz et al., 2007), palms (Scariot, 1999), and large herbivorous mammals (Timo, 2003), among others. However, smaller fragments do not always have fewer species than larger fragments (Laurance, 2008). For some taxonomic groups, such as butterflies (Brown and Hutchings, 1997), frogs (Tocher et al., 1997) and leaf-litter invertebrates (Didham, 1997), species numbers increase with fragmentation. In central Amazonia, the number of frog species increased as a result of the resilience to edge effects by the majority of forest species, and due to the influx of species coming from modified habitat surrounding fragmented areas (Tocher et al., 1997). Similarly, the number of butterfly species also increased after the isolation of fragments due to the invasion of species typical of open areas (Brown and Hutchings, 1997). In small fragments (plots of 1ha), the number of tree species did not decline within forest fragments, due to the recruitment of species from initial successional stages that normally have low frequency in continuous forests. This group of plants is favored by the process of forest fragmentation and proliferates in many fragments due to the mortality of large trees (Laurance et al., 2006b; Nascimento et al., 2006). The magnitude of floristic changes in seedlings is faster and more intense than that observed for adults (Benitez-Malvido, 1998; Sizer and Tanner, 1999), which may affect future generations.
Overall, the responses of different species or taxonomic groups to fragmentation varied considerably. Some species were favored by forest fragmentation, such as specialists on clearings formed by natural tree falls, which become more abundant in fragment borders. On the other hand, species sensitive to the effects of fragmentation generally do not tolerate habitat conditions surrounding fragments (Gascon et al., 1999), as they are vulnerable to edge effects and incapable of crossing deforested areas (Stouffer and Bierregaard, 1995; Laurance et al., 2004a), or have large home ranges (Rylands and Keuroghlian, 1988; Harper, 1989).
The main lessons learned are that small fragments (<100ha) are extremely sensitive and tend to degrade with time due to high tree mortality, but if protected from fires and other major disturbances, can begin to recover in just a decade or two (Laurance et al., 2011). Unfortunately, this is not the prevailing scenario in Brazilian ecosystems. In this way, we reinforce the importance of the long-term studies, including areas isolated for many decades, since fragmentation has long-lasting effects on biodiversity.
Anthropogenic disturbances and global changes interact synergistically on ecosystem processesSeveral hypotheses on the effects of global climate change on forest biomass and species substitution are being tested (Lewis et al., 2004a). It is still unclear if increases in temperature, CO2 concentrations, nitrogen deposition or changes in intensity of el Nino events are the most important drivers and what is the size of their impacts.
Laurance and Williamson (2001) showed that tree mortality and the decay of plant litter from trees under stress during periods of extreme drought in El-Niño years increase dramatically in already disturbed areas of fragment borders, as compared to areas inside fragments. The accumulation of combustible material and the greater penetration of light in borders could be the source of forest fires in drier years, especially under the scenarios predicted by most climate-change models.
Arboreal biomass increased over time in Central Amazonia, perhaps as a result of direct climate changes or the fertilization of the atmosphere with CO2 (Castilho et al., 2010). Biomass change averaged +5.6 Mgha−1ano−1, a level in accordance with studies in other Amazonian areas. Biomass change was found to be negatively associated with precipitation, suggesting that mortality rates increase in years with large storms and affect standing carbon stocks (Castilho et al., 2010). Similar results over a much larger area based on plots that include those from PDBFF at LTER-Site #1 were obtained by Phillips et al. (1998, 2004), Baker et al. (2004), Lewis et al. (2004b) and Laurance et al. (2004b), and those studies indicated that areas of intact forests could be significant carbon sinks.
In contrast, physiological models developed by Chambers et al. (2004) suggest that upland (terra firme) forests are not efficient in their use of carbon. Based on their models, carbon emissions via tree respiration and from organic material should be greater than the photosynthetic absorption by trees. This fact suggests that the forest growth probably does not respond to the increase in atmospheric CO2 concentration. If this is the case, the forest would not be behaving as a carbon sink. These differences between models and results regarding vegetation component show the importance of long-term investigation to the interpretation of results from short-term studies and physiological models.
ConclusionsThe results obtained in the first 10 years of Amazonian LTER (1999–2009) showed that densities of individuals and species assemblages distributions are spatially heterogeneous, even in relatively undisturbed forests, and that associations with topo-edaphic variables allow prediction of a considerable part of this variation. For the biological groups whose dynamics were studied in the short-term, levels of change in species composition and density were relatively high, and in general changed in tune with the environmental variation in space. Therefore, inclusion of environmental predictors such as topography, soil content and water attributes allows development of more precise models of future dynamics.
The impact of timber harvesting on assemblages and ecosystem processes is generally moderate, and temporal monitoring allowed for the determination of the length of time needed for the structural recuperation of logged areas. Nevertheless, continued studies are needed to determine the time required for the recuperation of timber stocks, and the recovery of pre-logging assemblages. In contrast, the impact of forest fragmentation on organisms and ecosystem processes is great and long-lasting. Studies indicate that there is negative synergy between the impacts of fragmentation and climatic changes, and that a better understanding of these processes can only be obtained through long-term research.
The results of the research in LTER-Site #1 indicate that conservation decisions regarding the Amazon forest are not going to be easy. Interventions, such as selective logging, are likely to affect different groups, such as commercially valuable trees and invertebrates, differently. Fragmentation has immediate obvious effects on biodiversity, but even in relatively undisturbed areas, subtle differences in landscape elements make large areas suitable or unsuitable for some taxa, which mean that it will often be necessary to undertake in situ studies to effectively plan reserve boundaries, at least for mesoscale reserves. Possible sources of funding for reserves, such as carbon trading, are based on static models of biomass distribution, but the interactions between edaphic features and climate currently make predictions imprecise, and the in situ measurements are not in accord with theoretical models. Systematic conservation planning will only be effective if we can generate the data necessary for models, and the experience from LTER-Site #1 indicates that we are far from having adequate understanding of the long-term processes.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful to financial and logistic support granted by Long Term Ecological Research Program (Programa de Pesquisa Ecológica de Longa Duração, PELD) through CNPq, the Program for Biodiversity Research (PPBio) through Ministério de Ciência, Tecnologia e Inivação (MCTI), Coordination for the Improvement of Higher Education Personnel (CAPES), Biological Dynamics of the Forests Fragments Project (PDBFF - ST 669) and Smithsonian Tropical Research Institute (STRI). CNPQ and CAPES provided many scholarships to the students involved. We also thank all the researchers, students, field and lab assistants involved in the studies, who are too numerous to name here.